Abstract
Fructooligosaccharides (FOS) are fructose-based oligosaccharides employed as additives to improve the food’s nutritional and technological properties. The rhizosphere of plants that accumulate fructopolysaccharides as inulin has been revealed as a source of filamentous fungi. These fungi can produce FOS either by inulin hydrolysis or by biosynthesis from sucrose, including unusual FOS with enhanced prebiotic properties. Here, we investigated the ability of Fusarium solani and Neocosmospora vasinfecta to produce FOS from different carbon sources. Fusarium solani and N. vasinfecta grew preferentially in inulin instead of sucrose, resulting in the FOS production as the result of endo-inulinase activities. N. vasinfecta was also able to produce the FOS 1-kestose and 6-kestose from sucrose, indicating transfructosylating activity, absent in F. solani. Moreover, the results showed how these carbon sources affected fungal cell wall composition and the expression of genes encoding for β-1,3-glucan synthase and chitin synthase. Inulin and fructose promoted changes in fungal macroscopic characteristics partially explained by alterations in cell wall composition. However, these alterations were not directly correlated with the expression of genes related to cell wall synthesis. Altogether, the results pointed to the potential of both F. solani and N. vasinfecta to produce FOS at specific profiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fructose-based polymers (fructans) and their oligomers called fructooligosaccharides (FOS) are carbohydrates used by many soil microorganisms as carbon sources. They lack nutritional value for humans since the required β-glycosidase activities for their hydrolysis in our digestive tract are absent. However, these molecules exhibit prebiotic activity as they selectively stimulate the multiplication and the activity of beneficial intestinal bacteria such as bifidobacteria and lactobacilli (Le Bastard et al. 2020; Swanson et al. 2020). These microorganisms, as well as the metabolites resulting from fructan fermentation, have been associated with positive effects on intestinal functioning (de Vries et al. 2019), plasma cholesterol and triglyceride levels (Liu et al. 2017; Atef et al. 2020), bone health (Zaiss et al. 2019) and immune and inflammatory responses (Peshev and Van den Ende 2014; McLoughlin et al. 2019), mimicking or improving the properties of conventional sugars, among other benefits. In addition, FOS are stable at high and low temperatures and are easily incorporated into food products, such as dairy, baked goods, cereals and beverages (Flores-Maltos et al. 2016).
FOS can be found in plants, bacteria and fungi but are industrially produced from sucrose through microbial fructosyltransferases (EC 2.4.1.9) or β-fructofuranosidases (invertases, EC 3.2.1.26) with transfructosylating activity. These enzymes cleave the sucrose glycosidic bond and use the energy released to link the free fructosyl moiety to either sucrose or longer fructose-containing molecules, yielding inulin-type FOS, mainly represented by 1-kestose (GF2), nystose (GF3) and β-fructofuranosylnystose (GF4) (Singh et al. 2017; Sánchez-Martínez et al. 2020). FOS are also produced by enzymatic hydrolysis of inulin, a linear β-(2,1) polysaccharide found as storage carbohydrate in many plant species but extracted on an industrial scale predominately from chicory roots. Microbial endo-inulinases (EC 3.2.1.7) act on inulin, randomly hydrolysing the internal linkages yielding FOS (also called oligofructose) with a degree of polymerisation ranging from 2 to 8 (Singh et al. 2017; Singh and Singh 2010; Sánchez-Martínez et al. 2020).
Studies of fructan-accumulating plants from the Brazilian biome Cerrado showed the presence of filamentous fungi in their rhizospheres, including Fusarium solani (Mart.) Sacc. and Penicillium janczewskii Zaleski, capable of hydrolysing inulin. Other fungi, such as Neocosmospora vasinfecta E.F.Sm. and Gliocladium virens Mill., Giddens & Foster, were capable of synthesizing FOS from sucrose (Cordeiro-Neto et al. 1997; Figueiredo-Ribeiro et al. 2007, 2014; Pessoni et al. 2009; Fialho et al. 2013). Penicillium janczewskii, isolated from the rhizosphere of Chrysolaena obovata (Less.) Dematt., exhibits exo-inulinase activity on inulin, releasing free fructose in the culture medium (Pessoni et al. 1999). Figueiredo-Ribeiro et al. (2007) reported high inulinase specific activity in F. solani and P. janczewskii, indicating their potential for FOS production. Subsequently, these authors also reported FOS production by N. vasinfecta, with the predominance of 1-kestose in both mycelium and culture filtrates (Figueiredo-Ribeiro et al. 2014). Despite the vast literature on this subject, there are no studies to date relating the influence of the carbon source on the FOS production by F. solani and N. vasinfecta.
FOS metabolism depends on the cell morphological integrity since it is performed outside the fungal cells by enzymes secreted or attached to the cell wall. The cell walls of filamentous fungi are complex structures composed mainly of β-glucans, consisting of β-1,3-linked glucose units, and chitin, a polymer of amino sugars, consisting of β-1,4-linked N-acetyl-glucosamine units. This glucan–chitin complex is covalently bound to other polysaccharides. Proteins are also essential cell wall components and are associated with carbohydrates such as mannose and galactose, resulting in glycoproteins (Patel and Free 2019; Garcia-Rubio et al. 2020). Modifications of the nutritional availability can induce changes in the cell wall structure, affecting the morphology and integrity of the fungal cells. Pessoni et al. (2005) reported cell wall structure and composition changes in P. janczewskii growing in different carbon sources. According to these authors, the growth in an inulin-based medium altered the fungal cell wall resulting in a thinner structure and increased chitin content than the growth in a sucrose-based medium. The enzyme secretion might also be one reason for cell wall changes. Indeed, strains of Trichoderma reesei Simmons genetically modified to increase protein secretion showed increased chitin levels and decreased glucose and mannose levels in the cell wall (Perlinska-Lenart et al. 2006). Deletion of mannosyltransferase genes in Saccharomyces cerevisiae resulted in modifications in the protein glycosylation and increased cell wall porosity, which is often associated with hyperproduction of secretion proteins (Tang et al. 2016).
Most studies related to FOS production have been focused on Aspergillus and Penicillium, which produce inulin-type FOS. However, prospecting novel FOS-producing fungi is relevant since they might produce uncommon FOS as levan and neolevan-type FOS. Compared to the traditionally commercialised inulin-type FOS, these FOS have enhanced prebiotic properties and chemical stability. Therefore, this study aimed to investigate the effect of different carbon sources on FOS production by F. solani and N. vasinfecta through synthesis from sucrose and/or inulin hydrolysis and its relationship with cell wall composition and gene expression related to cell wall synthesis. Based on a previous screening of the fungal ability of FOS production, we selected F. solani and N. vasinfecta isolated from the rhizosphere of native fructan-accumulating plants as promising fungi for this study.
Materials and methods
Fungal strains
Fusarium solani (Mart.) Sacc. (URM 3338) and Neocosmospora vasinfecta E.F.Sm. (URM 3329) were isolated from the rhizosphere of Chrysolaena obovata (Less.) Dematt. (syn. Vernonia herbacea (Vell.) Rusby) and Viguiera discolor Baker, respectively, two Asteraceae species from the Brazilian Cerrado (Cordeiro-Neto et al. 1997). The fungi are available in the Micoteca URM Culture Collection, Department of Mycology, Centre of Biosciences, Federal University of Pernambuco, Recife, Pernambuco state, Brazil, which is part of the World Directory of Collections of Cultures of Microorganisms (WFCC) under the registration number 604.
Culture conditions
The fungi were cultured on potato dextrose agar (PDA) medium for 7 days at 28 ± 2 °C. A 9-mm-diameter disk of agar containing mycelium was transferred to Czapek solid medium containing the following (g/L): NaNO3 (3), KH2PO4 (1), KCl (0.5), MgSO4·7H2O (0.5), FeSO4·7H2O (0.01) and agar (15). Fructose, glucose, sucrose, fructose + glucose (1:1) and inulin from chicory (Sigma-Aldrich®) at 10 g/L were used as carbon sources. The cultures were grown in the dark at 28 ± 2 °C for 14 days, and the colony diameter was measured every two days. For cultures in liquid media, three 9-mm-diameter disks with mycelium from PDA cultures were transferred to 250-mL Erlenmeyer flasks containing 50 mL of Czapek liquid medium with the same components described above except agar. The cultures were grown in the dark at 28 ± 2 °C with constant shaking (140 rpm). Every 5 days for 15 days, the mycelium was collected by filtration, thoroughly washed with deionised water and freeze-dried for dry matter determination, cell wall analyses and RNA extraction. The culture filtrates were used for pH measurement, sugars, extracellular proteins and enzymatic analyses. All experiments were performed using independent triplicates.
Quantification of sugars and extracellular proteins
Total and reducing sugars and extracellular proteins in the culture filtrates were determined according to Dubois et al. (1956), Miller (1959) and Bradford (1976), respectively. Glucose, fructose, sucrose and inulin were used as standards for sugar determinations and bovine serum albumin for protein analysis. All standards were purchased from Sigma-Aldrich®.
High-performance anion-exchange chromatography
For FOS analysis, 1 mL of culture filtrate samples was deionised by ion-exchange chromatography in columns containing cationic (Dowex® 50 W × 8) and anionic (Dowex® 1 × 8) resins (Sigma-Aldrich®). After deionisation, the pH was adjusted to 7.0 with 0.5 M ammonium hydroxide and the total sugar content was determined as mentioned above (Dubois et al. 1956). The samples were filtered using 0.45-µm nylon filters (Millipore®) and analysed by high-performance anion-exchange chromatography with pulse amperometric detection (HPAEC/PAD) on a Dionex ICS-3000 system with a CarboPac PA-1 column (4 × 250 mm), using a gradient of 500 mM sodium acetate in 150 mM sodium hydroxide (Shiomi et al. 1991; Vieira et al. 1995). The peak identification was performed by comparison with the retention time of the commercial glucose, fructose, sucrose, 1-kestose and nystose standards (Sigma-Aldrich®), as well as 6-kestose kindly provided by Dr. Norio Shiomi (Rakuno Gakuen University, Ebetsu, Hokkaido, Japan). Further confirmation of the peak identities was accomplished by co-eluting some of the samples with authentic standards.
Inulinase activity
The sugars in the filtrates of 5-day-old cultures (inulin-based medium) were removed by size exclusion chromatography using Bio-Gel P6-DG spin columns (Bio-Rad®) (Pessoni et al. 1999, 2007). The extract was assayed for enzyme activity using inulin from chicory (Sigma-Aldrich®) as substrate. The assay conditions consisted of a mixture of 100 μL of enzyme extract and 100 μL of 50 g/L inulin in 100 mM sodium acetate buffer (pH 6.5). The mixture was incubated at 45 ○C for 30 min, and the reaction was stopped by boiling for 5 min. The incubation mixture was filtered using 0.45-µm nylon filters (Millipore®), and the inulinase activity was detected by analysing the reaction products using HPAEC/PAD, as described above. One unit (U) of exo- and endo-inulinase activity was defined as the enzyme amount that releases one micromole of fructose and 1-kestose, respectively, from inulin per min under standard assay conditions.
Cell wall extraction
Samples of mycelia (50 mg) were grounded in a mortar-pestle with liquid nitrogen and added 1 mL of 10 mM Tris–HCl buffer (pH 7.8) containing 1 mM phenylmethyl sulfonyl fluoride (PMSF). The samples were vortexed for 1 min and subsequently incubated in an ice bath for 1 min. The procedure was repeated three times. After centrifugation, the supernatant was discarded, and the pellet resuspended in 1 mL of the same buffer and sonicated for 1 h. The residue containing cell walls was recovered by centrifugation and washed with 1 M NaCl containing 1 mM PMSF, and afterwards with deionised water with 1 mM PMSF, both three times each. The cell walls were suspended in 1 mL 50 mM Tris–HCl buffer (pH 7.8) containing 2% (w/v) SDS, 100 mM Na-EDTA, 40 mM β-mercaptoethanol and 1 mM PMSF and boiled for 5 min. After centrifugation at 3.9 g for 10 min, the supernatant was discarded, and this procedure was repeated. The cell walls were then washed six times with deionised water containing 1 mM PMSF, yielding protein interaction-free cell walls (Schoffelmeer et al. 1999) that were lyophilised and weighed.
Quantification of sugars and chitin in cell walls
For quantification of total sugars, samples of lyophilised cell walls (2 mg) were resuspended in 2 mL of 2 M trifluoroacetic acid (TFA) in a water bath at 100 °C for 4 h (Schoffelmeer et al. 1999). The acid was evaporated, and the residue was resuspended in 1 mL of deionised water. The total sugar content was determined as mentioned above (Dubois et al. 1956), using glucose (Sigma-Aldrich®) as standard. Uronic acids were measured by resuspending 2 mg of the lyophilised cell wall in 2 mL of 81% H2SO4 for 1 h at room temperature (Schoffelmeer et al. 1999). The uronic acid content was estimated according to Filisetti-Cozzi and Carpita (1991), using glucuronic acid (Sigma-Aldrich®) as standard. For chitin quantification, 2 mg of lyophilised cell walls was hydrolysed with 1 mL 6 M HCl in a water bath at 100 °C for 8 h. The hydrolysates were evaporated and resuspended in 1 mL deionised water (Schoffelmeer et al. 1999). The glucosamine concentration was determined as Chen and Johnson (1983) described and modified by Pessoni et al. (2005). Aliquots of 1 mL of the hydrolysates were added to 0.25 mL of 4% (v/v) acetylacetone in 1.25 N sodium carbonate and heated at 90 °C for 1 h in sealed flasks. After cooling, 2 mL of ethanol was added and stirred to dissolve the precipitate. Posteriorly, 0.25 mL Ehrlich’s reagent (1.6 g of p-dimethylaminobenzaldehyde in 60 mL of a solution of ethanol:concentrated HCl (1:1)) was added, and the absorbance was measured at 530 nm. Glucosamine hydrochloride (Sigma-Aldrich®) was used as standard.
Analysis of cell wall monosaccharides
Cell wall samples (2 mg) were suspended in 2 mL of 2 N TFA and incubated in a water bath at 100 °C for 4 h (Schoffelmeer et al. 1999). The acid was evaporated entirely, and the hydrolysates were dissolved in 1 mL of deionised water. Monosaccharides were analysed by HPAEC/PAD on a Dionex ICS-3000 with CarboPac PA-1 column, using isocratic 16 mM NaOH. Fucose, rhamnose, arabinose, galactose, glucose and mannose (Sigma-Aldrich®) were used as identification standards.
Primer design for RT-qPCR analysis
The complete sequences of the genes encoding for β-1,3-glucan synthase (FKS1), chitin synthases (CHS1, CHS 2, CHS 4, CHS 5 and CHS 7) and the elongation factor 1-α (EF1-α) from Nectria haematococca (teleomorph of Fusarium solani) were obtained from GenBank (Table 1). Primers for qPCR were designed on target and reference gene sequences, using the software Primer-BLAST (NCBI), according to the following parameters: size of the primers 20 bp, Tm value of 60 °C and amplicon sizes between 50 and 150 bp. Primer pairs were analysed using the OligoAnalyzer 3.1 online tool (https://www.idtdna.com/calc/analyzer) to determine self and hetero dimers and hairpins. Primer pairs were submitted to match analysis with coding regions of target genes. They were then subjected to in silico PCR (Primer-BLAST) using N. haematococca genome as a template. The sequences of the primers are shown in Table 1.
RNA extraction and cDNA synthesis
Samples of fungal mycelium grown for 5 days in Czapek liquid medium supplemented with sucrose, fructose, or inulin as carbon source were grounded in liquid nitrogen to a fine powder. Total RNA was extracted using the RNeasy Plant Mini Kit (Qiagen) following the manufacturer’s recommendations. The quality of the RNA was verified on agarose gel and was quantified using the equipment NanoVue (GE Healthcare Life Sciences). Treatment with DNAase and cDNA synthesis was performed according to the manufacturer's instructions using the QuantiTect Reverse Transcription Kit (Qiagen).
qRT-PCR
qRT-PCR was performed in ABI Prism 7300 system (Applied Biosystems) using SYBR Green PCR Master Mix kit (Applied Biosystems), following the manufacturer’s instructions. The amplification conditions used were as follows: initial step at 50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. PCR reactions were performed with cDNA RT ( −) samples for each condition to determine genomic DNA contamination, and the results showed no detectable amplification in any condition. The Cq values (quantification cycle) were determined manually for each amplification. Relative expression levels were calculated according to the method of 2−ΔΔCq (Livak and Schmittgen 2001), in which transcription levels were normalised against sucrose 5 days as a control condition and EF-1α as a reference gene to normalise the levels of basal transcription. The values between 0 and 1 (down-regulated genes) were transformed into − 1/2−ΔΔCq. The formula: δ = σ / √n, where σ is the variance of the different Cq values obtained, and n is the number of Cq values was used to determine the standard error (δ) of the endogenous control gene and the target genes. The propagation of the standard error δΔΔCt = √(δ1)2 + (δ2)2, where δ1 is the standard error of the target gene, and δ2 is the reference standard error was employed to determine the error ΔΔCq. All analyses were performed in three biological replicates with two technical replicates.
Statistical analysis
The data obtained were subjected to the analysis of variance (ANOVA) and, when appropriate, means were compared using the Tukey’s test (P ≤ 0.05) using the SAEG program.
Results
Fungal growth and sugar consumption
The growth rate of F. solani and N. vasinfecta in the solid medium was slightly affected by the different carbon sources (Fig. 1). Regardless of the carbon source, N. vasinfecta reached the maximum colony diameter at 10 days of culture (Fig. 1B), showing a faster growth than F. solani, which presented the same diameter at 14 days of culture (Fig. 1A). Moreover, F. solani and N. vasinfecta colonies that grew on fructose- and inulin-based media had more hyaline and sparser aerial mycelium than on other sources (Supplementary Figs. 1, 2).
Mycelial growth of Fusarium solani (A) and Neocosmospora vasinfecta (B) grown for 14 days on Czapek solid medium containing different carbon sources at 10 g/L. Values represent the mean of triplicates ± standard deviation. The asterisks indicate significant differences between the carbon sources at the same time point by Tukey’s test (P ≤ 0.05)
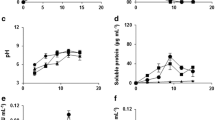
Fungal growth was also monitored for 15 days in liquid medium containing different carbon sources by the increase of the mycelium dry mass (Fig. 2). The final biomass produced by N. vasinfecta was 45% higher than F. solani (Fig. 2A, B). Both fungi produced higher biomass in inulin than in other carbon sources. The growth of F. solani was accompanied by the alkalinisation of the culture media, reaching pH 8 at the end of cultivation, independent of the carbon source (Fig. 2C). For N. vasinfecta, this pH increment was only significant in inulin (Fig. 2D). Fusarium solani secreted 41% more extracellular proteins than N. vasinfecta but without significant differences between the carbon sources evaluated (Fig. 2E, F).
Biomass production (A, B), pH of the culture medium (C, D) and extracellular proteins (E, F) in the culture filtrates of Fusarium solani and Neocosmospora vasinfecta grown for 5, 10 and 15 days in Czapek liquid medium containing different carbon sources at 10 g/L. Values represent the mean of triplicates ± standard deviation. The asterisks indicate significant differences between the carbon sources at the same time point by Tukey’s test (P ≤ 0.05)
Total sugar analysis of F. solani culture filtrates showed that about 98% of the carbon sources were consumed at the end of 15 days of growth (Fig. 3A). However, N. vasinfecta exhibited a slower sugar consumption profile, consuming 90% of the carbon sources (Fig. 3B). The reducing sugars in the culture filtrates were also monitored (Fig. 3C, D). In an inulin-based medium, both fungi consumed this polysaccharide without fructose accumulation. Conversely, in a sucrose-based medium, the disaccharide hydrolysis resulted in the accumulation of reducing sugars that decreased subsequently. On the fifth day of growth, the levels of reducing sugars in F. solani had a 4.6-fold increase while N. vasinfecta showed only a 1.4-fold increase under the same conditions.
Percentage of total (A, B) and reducing sugars (C, D) in the culture filtrates of Fusarium solani and Neocosmospora vasinfecta grown for 5, 10 and 15 days in Czapek liquid medium containing different carbon sources at the initial concentration of 10 g/L, which was considered as 100%. Values represent the mean of triplicates ± standard deviation. The asterisks indicate significant differences between the carbon sources at the same time point by Tukey’s test (P ≤ 0.05)
Conversion of sugars to FOS
The HPAEC/PAD analysis of the culture filtrates of F. solani grown in a sucrose-based medium showed the hydrolysis of sucrose into fructose and glucose by day 5, following the accumulation of reducing sugars. After 10 days, glucose was consumed entirely while residual fructose was high. After 15 days, no monosaccharides were detected in the medium. FOS production by F. solani was not observed under this experimental condition (Fig. 4A–D; Table 2). In the inulin-based medium, the hydrolysis promoted by the fungus resulted in a range of lower molecular weight molecules, including 1-kestose (maximum 92 mg/L), which were subsequently consumed (Fig. 4E–H; Table 2). The analysis of the culture filtrates of N. vasinfecta grown in a sucrose-based medium showed an increase in monosaccharides concurrent with the production of two FOS identified as 1-kestose (maximum 50 mg/L) and 6-kestose (4 mg/L on average) (Fig. 5A–D; Table 2). In the inulin-based medium, N. vasinfecta showed endo-inulinase activity, producing 1-kestose (9 mg/L in average) and fructans with a higher degree of polymerisation, as observed for F. solani (Fig. 5E–H; Table 2). Inulinase activity assays using the culture filtrates of the fungi grown for 5 days in an inulin-based medium showed hydrolytic activity on inulin, yielding fructans with a lower polymerisation (Fig. 6). For F. solani, the exo- and endo-inulinase activities were 13.9 and 15.9 U/mg protein, respectively, while N. vasinfecta presented about eight times less exo-inulinase activity than F. solani (1.5 U/mg protein). Under our enzyme assay conditions, endo-inulinase activity in N. vasinfecta was not detected, as indicated by the absence of 1-kestose as a product of the inulin hydrolysis (Fig. 6D).
HPAEC-PAD profile of the culture filtrates of Fusarium solani grown in Czapek liquid medium containing sucrose for 0 (A), 5 (B), 10 (C) and 15 (D) days, or inulin for 0 (E), 5 (F), 10 (G) and 15 (H) days, as carbon source at 10 g/L. G, glucose; F, fructose; S, sucrose; FOS, fructooligosaccharides; Ni, not identified. Peaks with retention times greater than 15 min correspond to long-chain inulin-type fructans
HPAEC-PAD profile of the culture filtrates of Neocosmospora vasinfecta grown in Czapek liquid medium containing sucrose for 0 (A), 5 (B), 10 (C) and 15 (D) days, or inulin for 0 (E), 5 (F), 10 (G) and 15 (H) days, as a carbon source at 10 g/L. G, glucose; F, fructose; S, sucrose; 1-K, 1-kestose; 6-K, 6-kestose; FOS, fructooligosaccharides; Ni, not identified. Peaks with retention times greater than 15 min correspond to long-chain inulin-type fructans
HPAEC-PAD profile of fructans obtained after incubation of the culture filtrates of Fusarium solani and Neocosmospora vasinfecta with inulin (25 g/L) as substrate at 45.○C for 0 (A, B) and 30 min (C, D). The culture filtrates were obtained by growing the fungi for 5 days in Czapek liquid medium containing inulin as a carbon source at 10 g/L. G, glucose; F, fructose; S, sucrose; 1-K, 1-kestose. Peaks with elution time greater than 20 min correspond to inulin-type fructans with a high degree of polymerisation
Characterisation of the fungal cell wall
Total sugar represented about 81% of the cell wall dry mass in F. solani and N. vasinfecta, whereas chitin and uronic acids represented about 15 and 3% of the cell wall composition in both fungi, respectively (Fig. 7). Overall, significant alterations in this composition were promoted by different carbon sources on the 5th day of cultivation. For example, F. solani cultivated in both glucose + fructose and inulin showed a 4.5% increase in uronic acid, while in sucrose, it dropped to the lowest level of 2% (Fig. 7E). In N. vasinfecta, 5-day-old cultures in a glucose-based medium had the highest cell wall content of total sugar (90%) and the lowest cell wall content of chitin (6%). In contrast, in a fructose-based medium, it showed the lowest content of total sugars (79%) and the highest content of chitin (17%) in the cell wall (Fig. 7B, D). On the other hand, the content of uronic acid in N. vasinfecta did not change significantly (Fig. 7F).
Relative proportion (%) of total sugar (A, B), chitin (C, D) and uronic acids (E, F) in the cell wall of Fusarium solani and Neocosmospora vasinfecta grown for 5, 10 and 15 days in Czapek liquid medium containing different carbon sources at 10 g/L. Values represent the mean of triplicates ± standard deviation. Means followed by the same letter do not differ by Tukey’s test (P ≤ 0.05). Uppercase letters compare the carbon sources within the same period, and lowercase letters compare the carbon source in different periods
Regarding the carbon source provided in the media, glucose (79.6%) was the most predominant monosaccharide in the cell wall of F. solani, followed by mannose (10.8%), galactose (6.8%), arabinose (2.3%), fucose (0.3%), and rhamnose (0.1%) (Table 3). The cell wall composition of N. vasinfecta differed from F. solani only in the higher arabinose content in the inulin- and fructose-containing media at day 5 (Table 4).
Fusarium solani exhibited changes in the monosaccharide levels during the cultivation time courses. Glucose decreased in the media with sucrose, glucose, or glucose + fructose while mannose increased. Rhamnose reached its maximal level upon 15 days of cultivation in glucose and glucose + fructose media. Deposition of arabinose in the fungal cell wall increased with ageing in all media, except for glucose + fructose (Table 3). On the other hand, N. vasinfecta cell wall had a reduced level of this pentose with culture ageing in all media, except for glucose + fructose (Table 4).
Relative expression of genes involved in cell wall biogenesis
The carbon source affected the expression of the FKS1 gene, which encodes β-1,3-glucan synthase, and CHS genes, which encode chitin synthases, enzymes related to cell wall synthesis. The amounts of CHS5 transcripts, and to a lesser extent of CHS2, in F. solani grown in fructose- and inulin-based media increased dramatically compared to a sucrose-based medium. Conversely, the amounts of CHS7 and FKS1 transcripts were reduced in a fructose-based medium (Fig. 8A). Regarding N. vasinfecta, the growth in an inulin-based medium increased the amounts of FKS1 transcripts. The CHS gene family (except CHS1 and CHS7) was also upregulated in the medium supplemented with inulin (Fig. 8B).
Relative expression of β-1,3-glucan synthase (FKS1) and chitin synthase (CHS) genes in Fusarium solani (A) and Neocosmospora vasinfecta (B) grown for 5 days in Czapek liquid medium containing fructose, inulin, or sucrose as a carbon source at 10 g/L. The dashed line indicates the normalisation of the transcription levels against sucrose as the control condition. Values represent the mean of triplicates ± standard deviation
Discussion
Interest in FOS has increased in the last decades due to their importance in the food and pharmaceutical industries. The carbon source and its concentration are the most studied variables to achieve the best FOS production (Sánchez-Martínez et al. 2020; Choukade and Kango 2021). In the present work, we tested the fungal growth and FOS production of Fusarium solani and Neocosmospora vasinfecta on sucrose and inulin. As the preliminary sugar analysis detected significant amounts of monosaccharides in sucrose- and inulin-based media before fungus inoculation, probably as a result of partial thermal hydrolysis during the autoclavation, we also tested FOS production in a mixture of glucose and fructose (1:1) and media containing these monosaccharides as single sugars for reference.
In relation to the carbon source, we observed increased pH and increased levels of extracellular proteins during the growth time course of both fungi (Fig. 2). Increased pH of the culture medium, followed by sugar consumption and protein secretions, has been already reported for other fungi such as Thermomyces lanuginosus (Chaudhuri et al. 1999) and Penicillium purpurogenum (Dhake and Patil 2007). Zaninette et al. (2019) also showed that the growth of P. janczewskii in sucrose resulted in medium alkalinisation. This increase in medium pH, associated with nitrate consumption, might play a key role in enzyme production and stability (Singh and Singh 2010). Indeed, Kurakake et al. (2020) reported medium alkalinisation (pH 8.7) by Aspergillus oryzae grown in 10 g/L sucrose and the effect of pH on the activity and stability of two β-fructofuranosidases produced by the fungus.
Differently from other fungi isolated from inulin-producing plants, such as P. janczewskii (Pessoni et al. 1999), F. solani and N. vasinfecta, preferentially grew on inulin instead of sucrose as a carbon source. Therefore, these fungi are well-adapted to metabolise plant-derived fructopolysaccharides (fructans). Cultivation in an inulin-containing medium also resulted in the highest secretion of extracellular proteins from both fungi on the 15th day. However, F. solani and N. vasinfecta showed different abilities in producing FOS. Neocosmospora vasinfecta produced the inulin-type FOS 1-kestose and the levan-type FOS 6-kestose from sucrose. Although in low amounts, the synthesis of 6-kestose detected in N. vasinfecta is uncommon in filamentous fungi but relatively well reported in yeasts such as Schwanniomyces occidentalis (Álvaro-Benito et al. 2010; Rodrigo-Frutos et al. 2019). Fialho et al. (2013) reported that the filamentous fungus Gliocladium virens, isolated from the rhizosphere of Viguiera discolor, could synthesise 6-kestose in a sucrose-based medium.
There are numerous patents on FOS production by different fungi, including species of Aspergillus, Penicillium citrinum and Aureobasidium pullulans, which obtained FOS yields from 30 to 90% in the case of mutant strains (Sánchez-Martínez et al. 2020). According to Nobre et al. (2019), P. citreonigrum was the most promising fungus in screening Penicillium strains for FOS production. After optimisation, P. citreonigrum was able to produce from 200 g/L of sucrose a FOS mixture consisting of 1-kestose (36 g/L), nystose (62 g/L) and fructofuranosylnystose (11 g/L).
Fusarium solani might not produce FOS from sucrose, probably due to its higher capacity to hydrolyse this disaccharide and consume the resulting monosaccharides compared to N. vasinfecta. In addition, sucrose at 10 g/L could not have favoured FOS production by F. solani. Gliocladium virens and P. janczewskii, also isolated from the rhizosphere of Cerrado inulin-accumulating plants, produced higher FOS amounts only when grown in high sucrose concentrations (Fialho et al. 2013; Zaninette et al. 2019). At 150 g/L, G. virens produced 3 g/L of 6-kestose. In contrast, P. janczewskii made 8 and 7.3 g/L of 1-kestose and neokestose, respectively, keeping high FOS levels in the medium until the end of the culture period. Therefore, the ability of F. solani to produce FOS in high sucrose concentration remains to be investigated. The low FOS content obtained in the present work compared to other studies might also be partially explained by glucose inhibition. A high amount of glucose is generated during the fermentation process, which results in transfructosylating activity inhibition, leading to low sucrose conversion yield. According to Castro et al. (2019), co-culture fermentation with Aureobasidium pullulans (used as FOS producer strain) and Saccharomyces cerevisiae (for small saccharides removal) resulted in 119 g/L of FOS and increased productivity compared to the control fermentation without co-culture.
Although many microorganisms can produce transfructosylating enzymes, it has been shown that only a few enzymes possess a high potential for industrial implementation. Notably, fungal transfructosylating enzymes have a greater FOS production capacity and are easier to handle than bacterial transfructosylating enzymes. Fructosyltransferases and β-fructofuranosidases from Aspergillus, Aureobasidium and Penicillium are currently the primary industrial sources for the mixture production of inulin-type FOS, such as 1-kestose, nystose or 1F-fructofuranosylnystose (Sánchez-Martínez et al. 2020). Typical β-fructofuranosidases such as invertases hydrolyse sucrose into glucose and fructose but, depending on its origin, might exhibit transfructosylating activity at high sucrose concentrations, probably due to a mechanism of reverse hydrolysis. In contrast, fructosyltransferases have low hydrolytic and high transfructosylating activities even at low sucrose concentrations (Choukade and Kango 2021). Therefore, considering that a low sucrose concentration (10 g/L) was used in the present study, the presence of fructosyltransferases in F. solani seems to be unlikely.
When cultured in an inulin-based medium, F. solani and N. vasinfecta showed inulinase activity as demonstrated by the initial increase of polyfructans with a low degree of polymerisation and their subsequent consumption as the fungal growth proceeded. The presence of fructose in the inulin-containing medium at the beginning of fungal growth does not seem to interfere with enzyme activity, as fructose uptake could be observed early during the development in both fungi. The enzymatic assays using the culture filtrates demonstrated the presence of exo- and endo-inulinase activities in F. solani. Neocosmospora vasinfecta exhibited exo-inulinase activity but not endo-inulinase under our enzyme assay conditions, despite the fungus having produced FOS from inulin in the culture medium. These observations reinforce the importance of the optimisation of assays conditions. According to Sánchez-Martínez et al. (2020) and Choukade and Kango (2021), enzymatic FOS synthesis from sucrose seems more advantageous than inulin hydrolysis. Low product yield and high downstream processing cost are the major drawbacks of FOS extraction from inulin-rich plant materials. In addition, inulin hydrolysis leads to lower molecular weight FOS that possess better prebiotic and technological properties.
Both FOS synthesis and hydrolysis are performed outside the fungal cells, involving secreted or cell wall-attached enzymes. Hence, the efficiency of FOS metabolism is tightly related to the cell capacity to transport those enzymes, which depends on the cell morphological integrity. The maintenance of N. vasinfecta in the inulin-containing medium induced secretion of proteins and FOS production but resulted in a more hyaline mycelium; the same was observed when the fungus was cultured in fructose (Supplementary Fig. 2). The presence of fructose also caused differences in the growth of F. solani (Supplementary Fig. 1). Similar changes were previously observed in P. janczewskii either in the inulin-based medium (Pessoni et al. 2005) or in the presence of fructose (Pessoni et al. 2015), resulting in growth decrease, thinner hyphae and enzyme secretion. Therefore, free or polymerised fructose in the medium changes hyphae morphology, which appears to be an effect common to filamentous fungi. The mechanism by which fructose-containing polymers affects the cell wall structure is unclear. Still, it could be related to cell wall polysaccharide biosynthesis changes due to differential sensing of fructose and glucose during sugar uptake (Doehlemann et al. 2005). The increased protein secretion is also a factor affecting the fungal cell wall. Indeed, strains of Trichoderma reesei genetically modified to increase protein secretion demonstrated changes in cell wall composition and ultrastructure, presenting increased chitin levels and decreased glucose and mannose levels (Perlinska-Lenart et al. 2006). According to Tang et al. (2016), the deletion of mannosyltransferases in S. cerevisiae resulted in increased cell wall porosity, which contributed to the release of secretory proteins. Therefore, changes in protein glycosylation can modify the cell wall structure, which is often associated with the hyperproduction of secretion proteins.
Interestingly, we observed the highest arabinose proportion in the cell walls of N. vasinfecta grown on fructose- and inulin-based media and of F. solani on inulin. Although it is a minor component of the fungal cell wall polysaccharides, arabinose-containing polymers (arabinans) have been described as part of the highly mobile outer shell of the Aspergillus fumigatus cell wall (Kang et al. 2018). In plants, arabinans are plasticizing polymers that provide cell wall flexibility (Moore et al. 2013). Whether increased arabinose content could have contributed to structural cell wall changes that resulted in the more hyaline mycelium when fungi were cultivated in fructose- and inulin-based media remains to be investigated.
Chitin synthases play diverse roles in hyphal growth, conidiogenesis and appressorium development in yeasts and filamentous fungi. They are encoded by members of a large gene family, suggesting the possibility of both functional specialisation and redundancy (Yang and Zhang 2019; Garcia-Rubio et al. 2020). In F. solani, although the cultivation in fructose or inulin has induced the CH5 expression, no increase was detected in the chitin content. Inulin also upregulated FKS1, CHS2, CHS4 and CHS5 genes in N. vasinfecta, but the proportion of total carbohydrate and chitin did not increase. The multiplicity of CHS enzymes and the uncertain role of individual CHS genes in different fungi could explain these results. Indeed, as reported by Martín-Udíroz et al. (2004), no differences in the chitin content were observed in mutants of Fusarium oxysporum with disruption in the genes CHS1, CHS2 and CHS7 compared with the wild-type strain.
According to Patel and Free (2019), most cell wall polysaccharides are produced directly in response to environmental factors. The carbon source determines the quality and amount of cell wall polysaccharides synthesised by some microorganisms. Changes in the deposition of cell wall polysaccharides during hyphae cell elongation result in fungal biomass changes (Yang and Zhang 2019). We found a significant increase in N. vasinfecta biomass production grown in inulin, although no significant differences in the cell wall carbohydrate content were observed. Ene et al. (2012) reported that different carbon sources influenced the biomass production in Candida albicans but not the cell wall proportion of chitin, β-glucan and mannan. Changes in chitin architecture or chitin-glucan cross-links, rather than alterations in absolute cell wall carbohydrates levels, might explain these results.
Conclusion
Our results highlight the ability and the differences of N. vasinfecta and F. solani, two fungi isolated from the rhizosphere of fructan-accumulating plants from the Brazilian Cerrado, to produce FOS by converting sucrose into 1- and 6-kestose, or by hydrolysing inulin. We also demonstrated that the carbon source regulated the expression of cell wall genes differentially for both fungi and that inulin and fructose have a major impact on fungal growth and colony morphology. Modifications in the cell wall composition under these conditions could explain the changes observed in the fungal morphology, differences in protein secretion and FOS production capacity. However, a direct correlation with the expression of genes encoding for enzymes related to cell wall synthesis has not been observed. More investigations on nutritional factors in F. solani and N. vasinfecta, such as the effect of sucrose concentration on FOS production and the characterisation of their transfructosylating enzymes, are essential for future biotechnological applications of these microorganisms, which seemed to be well-adapted to metabolise fructose-containing carbohydrates. Additional studies related to the effects of fructose-containing sugars on cell wall structure are also necessary to better understand how these changes can be used to improve fungal enzyme secretion.
Abbreviations
- FOS:
-
Fructooligosaccharides
- HPAEC/PAD:
-
High-performance anion exchange chromatography with pulsed amperometric detector
- 1-K:
-
1-Kestose
- 6-K:
-
6-Kestose
References
Álvaro-Benito M, Abreu M, Portillo F, Sanz-Aparicio J, Fernández-Lobato M (2010) New insights into the fructosyltransferase activity of Schwanniomyces occidentalis β-fructofuranosidase, emerging from nonconventional codon usage and directed mutation. Appl Environ Microbiol 76:7491–7499. https://doi.org/10.1128/AEM.01614-10
Atef M, El-Matty DA, Habib DF, Nicola WG, Saleh S, Hanna H (2020) Ameliorative effects of inulin on non-alcoholic fatty liver disease associated with type 2 diabetes mellitus in obese women. J Innov Pharm Biol Sci 7:6–16
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Castro CC, Nobre C, De Weireld G, Hantson AL (2019) Microbial co-culturing strategies for fructo-oligosaccharide production. N Biotechnol 51:1–7. https://doi.org/10.1016/j.nbt.2019.01.009
Chaudhuri A, Bharadwaj G, Maheshwari R (1999) An unusual pattern of invertase activity development in the thermophilic fungus Thermomyces lanuginosus. FEMS Microbiol Lett 177:39–45. https://doi.org/10.1111/j.1574-6968.1999.tb13711.x
Chen GC, Johnson BR (1983) Improved colorimetric determination of cell wall chitin in wood decay fungi. Appl Microbiol Biotechnol 46:13–16. https://doi.org/10.1128/aem.46.1.13-16.1983
Choukade R, Kango N (2021) Production, properties, and applications of fructosyltransferase: a current appraisal. Crit Rev Biotechnol 20:1–16. https://doi.org/10.1080/07388551.2021.1922352
Cordeiro-Neto F, Pessoni RAB, Figueiredo-Ribeiro RCL (1997) Fungos produtores de inulinases isolados da rizosfera de Asteráceas herbáceas do Cerrado (Moji-Guaçu, SP, Brasil) (in Portuguese). Rev Bras Ciênc Solo 21:149–153
de Vries J, Le Bourgot C, Calame W, Respondek F (2019) Effects of β-fructans fiber on bowel function: a systematic review and meta-analysis. Nutrients 11:91. https://doi.org/10.3390/nu11010091
Dhake AB, Patil MB (2007) Effect of substrate feeding on production of fructosyltransferase by Penicillium purpurogenum. Brazil J Microbiol 38:194–199. https://doi.org/10.1590/S1517-83822007000200002
Doehlemann G, Molitor F, Hahn M (2005) Molecular and functional characterization of a fructose specific transporter from the gray mold fungus Botrytis cinerea. Fungal Genet Biol 42:601–610. https://doi.org/10.1016/j.fgb.2005.03.001
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Ene IV, Heilmann CJ, Sorgo AG, Walker LA, de Koster CG, Munro CA, Klis FM, Brown AJP (2012) Carbon source-induced reprogramming of the cell wall proteome and secretome modulates the adherence and drug resistance of the fungal pathogen Candida albicans. Proteomics 12:3164–3179. https://doi.org/10.1002/pmic.201200228
Fialho MB, Simões K, Barros CA, Pessoni RAB, Braga MR, Figueiredo-Ribeiro RCL (2013) Production of 6-kestose by the filamentous fungus Gliocladium virens as affected by sucrose concentration. Mycoscience 54:198–205. https://doi.org/10.1016/j.myc.2012.09.012
Figueiredo-Ribeiro RCL, Pessoni RAB, Braga MR (2007) Inulinases produced by microbes from the Brazilian Cerrado: characterization and potential uses. In: Shiomi N, Benkeblia N, Onedera S (eds) Recent Advances in Fructooligosaccharides Research. Research Signpost Press, pp 339–356
Figueiredo-Ribeiro RCL, Simões K, Fialho MB, Pessoni RAB, Braga MR, Gaspar M (2014) Potential of the filamentous fungi from the Brazilian Cerrado as producers of soluble fibers. In: Benkeblia N (ed) Polysaccharides natural fibers in food and nutrition. CRC Press, New York, pp 131–145
Filisetti-Cozzi TMCC, Carpita NC (1991) Measurement of uronic acids without interference from neutral sugars. Analyt Biochem 197:157–162. https://doi.org/10.1016/0003-2697(91)90372-z
Flores-Maltos DA, Mussatto SI, Contreras-Esquivel JC, Rodríguez-Herrera R, Teixeira JA, Aguilar CN (2016) Biotechnological production and application of fructooligosaccharides. Crit Rev Biotechnol 36:259–267. https://doi.org/10.3109/07388551.2014.953443
Garcia-Rubio R, de Oliveira HC, Rivera J, Trevijano-Contador N (2020) The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front Microbiol 10:02993. https://doi.org/10.3389/fmicb.2019.02993
Kang X, Kirui A, Muszyński A, Widanage MCD, Chen A, Azadi P, Wang P, Mentink-Vigier F, Wang T (2018) Molecular architecture of fungal cell walls revealed by solid-state NMR. Nat Commun 9:2747. https://doi.org/10.1038/s41467-018-05199-0
Kurakake M, Hirotsu S, Shibata M (2020) Relationship between pellet formation by Aspergillus oryzae strain KB and the production of β-fructofuranosidase with high transfructosylation activity. Fungal Biol 124:708–713. https://doi.org/10.1016/j.funbio.2020.04.002
Le Bastard Q, Chapelet G, Javaudin F, Lepelletier D, Batard E, Montassier E (2020) The effects of inulin on gut microbial composition: a systematic review of evidence from human studies. Eur J Clin Microbiol Infect Dis 39:403–413. https://doi.org/10.1007/s10096-019-03721-w
Liu F, Prabhakar M, Ju J, Long H, Zhou HW (2017) Effect of inulin-type fructans on blood lipid profile and glucose level: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr 71:9–20. https://doi.org/10.1038/ejcn.2016.156
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Martín-Udíroz M, Madrid MP, Roncero MIG (2004) Role of chitin synthase genes in Fusarium oxysporum. Microbiology 150:3175–3187. https://doi.org/10.1099/mic.0.27236-0
McLoughlin R, Berthon BS, Rogers GB, Baines KJ, Leong LEX, Gibson PG, Williams EJ, Wood LG (2019) Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. EBioMedicine 46:473–485. https://doi.org/10.1016/j.ebiom.2019.07.048
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Moore JP, Nguema-Ona EE, Vicré-Gibouin M, Sørensen I, Willats WG, Driouich A, Farrant JM (2013) Arabinose-rich polymers as an evolutionary strategy to plasticize resurrection plant cell walls against desiccation. Planta 237:739–754. https://doi.org/10.1007/s00425-012-1785-9
Nobre C, do Nascimento AKC, Silva SP, Coelho E, Coimbra MA, Cavalcanti MTH, Teixeira JA, Porto ALF, (2019) Process development for the production of prebiotic fructo-oligosaccharides by Penicillium citreonigrum. Bioresour Technol 282:464–474. https://doi.org/10.1016/j.biortech.2019.03.053
Patel PK, Free SJ (2019) The genetics and biochemistry of cell wall structure and synthesis in Neurospora crassa, a model filamentous fungus. Front Microbiol 10:02294. https://doi.org/10.3389/fmicb.2019.02294
Perlinska-Lenart U, Orlowski J, Laudy AE, Zdebska E, Palamarczyk G, Kruszewska JS (2006) Glycoprotein hypersecretion alters the cell wall in Trichoderma reesei strains expressing the Saccharomyces cerevisiae dolichylphosphate mannose synthase gene. Appl Environ Microbiol 72:7778–7784. https://doi.org/10.1128/AEM.02375-06
Peshev D, Van den Ende W (2014) Fructans: prebiotics and immunomodulators. J Funct Foods 8:348–357. https://doi.org/10.1016/j.jff.2014.04.005
Pessoni RAB, Braga MR, Figueiredo-Ribeiro RCL (2007) Purification and properties of exo-inulinases from Penicillium janczewskii growing on distinct carbon sources. Mycologia 99:493–503. https://doi.org/10.3852/mycologia.99.4.493
Pessoni RAB, Figueiredo-Ribeiro RCL, Braga MR (1999) Extracellular inulinases from Penicillium janczewskii, a fungus isolated from the rhizosphere of Vernonia herbacea (Asteraceae). J Appl Microbiol 87:141–147. https://doi.org/10.1046/j.1365-2672.1999.00805.x
Pessoni RAB, Freshour G, Figueiredo-Ribeiro RCL, Hahn MG, Braga MR (2005) Cell wall structure and composition of Penicillium janczewskii as affected by inulin. Mycologia 97:304–311. https://doi.org/10.3852/mycologia.97.2.304
Pessoni RAB, Simões K, Braga MR, Figueiredo-Ribeiro RCL (2009) Effects of substrate composition on growth and fructo-oligosaccharide production by Gliocladium virens. Dyn Biochem Process Biotech Mol Biol 3(Special Issue 1):96–101
Pessoni RAB, Tersarotto CC, Mateus CAP, Zerlin JK, Simões K, Fiqueiredo-Ribeiro RCL, Braga MR (2015) Fructose affecting morphology and inducing β-frutofuranosidases in Penicillium janczewskii. Springer plus 4:487. https://doi.org/10.1186/s40064-015-1298-7
Rodrigo-Frutos D, Piedrabuena D, Sanz-Aparicio J, Fernández-Lobato M (2019) Yeast cultures expressing the Ffase from Schwanniomyces occidentalis, a simple system to produce the potential prebiotic sugar 6-kestose. Appl Microbiol Biotechnol 103:279–289. https://doi.org/10.1007/s00253-018-9446-y
Sánchez-Martínez MJ, Soto-Jover S, Antolinos V, Martínez-Hernández GB, López-Gómez A (2020) Manufacturing of short-chain fructooligosaccharides: from laboratory to industrial scale. Food Eng Rev 12:149–172. https://doi.org/10.1007/s12393-020-09209-0
Schoffelmeer EA, Klis FM, Sietsma JH, Cornelissen BJ (1999) The cell wall of Fusarium oxysporum. Fungal Genet Biol 27:275–282. https://doi.org/10.1006/fgbi.1999.1153
Shiomi N, Onodera S, Chatterton J, Harrison PA (1991) Separation of fructooligosaccharide isomers by anion-exchange chromatography. Agric Biol Chem 55:1427–1428. https://doi.org/10.1080/00021369.1991.10870771
Singh RS, Singh RP (2010) Production of fructooligosaccharides from inulin by endoinulinases and their prebiotic potential. Food Technol Biotechnol 48:435–450
Singh SP, Jadaun JS, Narnoliya LK, Pandey A (2017) Prebiotic oligosaccharides: special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl Biochem Biotechnol 183:613–635. https://doi.org/10.1007/s12010-017-2605-2
Swanson KS, de Vos WM, Martens EC, Gilbert JA, Menon RS, Soto-Vaca A, Hautvast J, Meyer PD, Borewicz K, Vaughan EE, Slavin JL (2020) Effect of fructans, prebiotics and fibres on the human gut microbiome assessed by 16S rRNA-based approaches: a review. Benef Microbes 11:101–129. https://doi.org/10.3920/BM2019.0082
Tang H, Wang S, Wang J, Song M, Xu M, Zhang M, Shen Y, Hou J, Bao X (2016) N-hypermannose glycosylation disruption enhances recombinant protein production by regulating secretory pathway and cell wall integrity in Saccharomyces cerevisiae. Sci Rep 6:25654. https://doi.org/10.1038/srep25654
Vieira CCJ, Braga MR, Figueiredo-Ribeiro RCL (1995) Fructans in callus of Gomphrena officinalis. Mart Plant Cell Tiss Org Cult 42:233–238
Yang J, Zhang KQ (2019) Chitin synthesis and degradation in fungi: biology and enzymes. Adv Exp Med Biol 1142:153–167. https://doi.org/10.1007/978-981-13-7318-3_8
Zaiss MM, Jones RM, Schett G, Pacifici R (2019) The gut-bone axis: how bacterial metabolites bridge the distance. J Clin Investig 129:3018–3028. https://doi.org/10.1172/JCI128521
Zaninette F, Rocha GALM, Pessoni RAB, Braga MR, Simões K, Figueiredo-Ribeiro RCL, Fialho MB (2019) Production of inulin- and neolevan-type fructooligosaccharides by Penicillium janczewskii Zaleski CCIBt 3352. Biotechnol Appl Biochem 66:419–425. https://doi.org/10.1002/bab.1738
Acknowledgements
Thanks are due to Dr. Kelly Simões for the technical assistance with the HPAEC/PAD analyses.
Funding
This work was supported by FAPESP (2005/04139–7, 2012/16332–0), CNPq and CAPES DINTER-Novas Fronteiras. CNPq provided research fellowship to M.R. Braga.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Galvão, D.F.A., Pessoni, R.A.B., Elsztein, C. et al. A comparative study between Fusarium solani and Neocosmospora vasinfecta revealed differential profile of fructooligosaccharide production. Folia Microbiol 67, 873–889 (2022). https://doi.org/10.1007/s12223-022-00983-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-022-00983-4