Abstract
The objectives of this study were (i) to isolate and characterize of cultivable denitrifying bacteria using classic microbiological and molecular methods, (ii) to compare of 16S rRNA and nosZ genes as molecular markers, (iii) to determine bacterial community structure and diversity in soil samples using single-strand conformation polymorphism (SSCP) analysis. In this study, 49 bacterial isolates were cultivated and phylogenetic analyses grouped them into two phyla: Proteobacteria (37 species) and Firmicutes (12 species). Our study showed that the nosZ functional gen could be used to identify denitrifying bacteria abundance in environment but could not be used to identify pure bacterial cultures. In addition, the bacterial community structure showed significant differences among the various soil types. Phylogenetic analysis of community structure indicated that 51 clones could be divided into 2 phylotypes. Uncultured bacteria (80.4%) and Gammaproteobacteria (19.6%) were the dominant components of the soil bacterial community. For 16S rRNA, PCR products of 49 bacteria were obtained with 27F-1492R primer pairs. For nosZ, PCR products were obtained with primers 1F-1R (259 bp), 2F-2R (267 bp), and F-1622R (453 bp) of 39 bacteria that the single nosZ band provided on the agarose gel. The bacterial 16S rRNA gene clone library was dominated by Gammaproteobacteria and Bacilli. The nosZ clone sequences did not represent the bacteria from which they were obtained but were found to be closer to the environmental clones. Our study showed that the nosZ functional gene could be used to identify denitrification abundance in environment but could not be used to identify pure bacterial cultures. It was also found that the nosZ sequences showed uncultured denitrifier species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In nature, the nitrogen cycle takes place through the biosphere, the hydrosphere, and the atmosphere. Nitrogen enters the biosphere with the biological and chemical fixation of dinitrogen (N2) and is again removed by denitrification (Zumft 1997). The dominant gas in the atmosphere (78%) is N2 gas with high stability. Biological nitrogen fixation converts N2 gas to ammonia (NH3), the fast conversion form of nitrogen needed for plant growth. Nitrogen fixation is carried out only by soil and aquatic microorganisms which have acquired this property. Other living organisms cannot use static atmospheric N2 directly. However, they eliminate this deficiency through organic matter, plants, animals and microbial communities that accumulate in the soil (White and Scott 2006). The denitrification rate is influenced by the interaction of various physical, chemical, and biological conditions of the soil (Saggar et al. 2013). In many studies, researchers has been carried out to find out which ones have the best process from the factors involved in denitrification control. The most important regulators have been reported as soil texture, water content, pH, and denitrifier community structure (Skiba et al. 1998; Pihlatie et al. 2004; Morkved et al. 2007; Čuhel et al. 2010).
The nitrogen cycle is one of the most important nutrient cycles in the terrestrial ecosystem. This cycle includes four microbiological processes. We can list them as follows: nitrogen fixation, mineralization (decay), nitrification, and denitrification (Hayatsu et al. 2008). Denitrification is a microbial respiration process that uses nitrogen oxides as an alternative electron acceptor when oxygen is limited. In addition, denitrification is associated with the environment because it is a major biological process responsible for the emission of N2O, which is considered to be one of the six greenhouse gases by the Kyoto protocol, which results in a substantial loss of nitrogen, which is the most limited nutrient for harvest production in agricultural areas (Philippot et al. 2007).
Denitrification is a microbial process that reduces nitrate (NO3−) and nitrite (NO2−) nitrogen to nitric oxide (NO), nitrous oxide (N2O), and dinitrogen (N2). N2O may spread as a result of incomplete denitrification (Dandie et al. 2007). It is foreseen that the potential N2O emission will grow exponentially due to the accumulation of unstable-N in agricultural soils and consequently the increase in the use or capture of this accumulation at any point in time by the crop (Shcherbak et al. 2014; Cui et al. 2016). According to this information, it is reported that the reduction of N2O was done biologically by only N2O reductase (nosZ) enzyme to N2 (Richardson et al. 2009; Gaimster et al. 2017).
Bacterial denitrification usually occurs only under anaerobic conditions. Denaturing enzyme activities are inhibited by oxygen (O2), and the expression of these genes is fully suppressed. However, many bacteria isolated from water, soil, and sediments have been reported to have the ability to perform denitrification in the presence of oxygen (Morley et al. 2008). Since the denitrificators does not have close phylogenetic relationships, the molecular methods included in the 16S rRNA are not suitable for detecting these physiological groups. Nitrite reductase (nir) genes are the first marker used in denitrifier diversity studies and continue as the most common molecular marker for denitrifier communities. Also, nitrate reductases (Nar and Nap) are not preferred for denitrification studies since they are also found in non-denitrifying bacteria. Apart from these, nitric oxide reductase (norB) gene has been used as a marker in these studies because of its ability to form N-N bonds (Wallenstein et al. 2006). It is stated that approximately 30% of denitrifying bacteria are missing the nosZ gene and thus contribute to the spread of N2O globally, especially from the soils under agricultural management (Philippot et al. 2013). Recently, studies on nosZ have been shown to be phylogenetically separated into two groups, and after these studies, nosZ has started to be called nosZI and nosZII (Sanford et al. 2012; Jones et al. 2013). According to the investigations on microbial genome, nosZI clade is more common in organisms with all of the denitrification metabolic pathways, whereas it is reported that the lack of other denitrification genes is much more frequent in the metabolic pathway of nosZII clade (Graf et al. 2014). In the light of all this information, the non-denitrifier nosZII clade was only responsible for the consumption of N2O in the soil communities in which it was dominant (Wittorf et al. 2016). Recent studies have shown that the nosZ gene has a high level of compliance with taxonomic classification based on 16S rRNA compared with other denitrification genes (Heylen et al. 2006; Dandie et al. 2007).
The aim of this study was (i) to obtain information about the structure of the community by using culture-independent molecular methods (ii) to determine the denitrifers that can be cultured from the same soil samples following the first step taken culture-independent (iii) the phylogenetic comparison of isolates according to 16S rRNA* and nosZ*.
Materials and methods
Sampling
Soil samples were taken from 20 stations from Sinop, Kastamonu, and Samsun provinces between August and December 2015 (Avşar 2018). The locations and property of the soil samples are given in Suppl. Table 1. Three replicate samples were collected for all sampling site. All samples were sieved through a 2-mm sieve to eliminate gravel and large organic debris and kept at − 20 °C until DNA isolation. In addition, soil samples were processed directly for culture-dependent bacterial isolation studies.
Cultivation of denitrifying bacteria
For the determination of microorganisms capable of denitrification, the enrichment medium was applied using standard denitrification MPN tubes. In 95 mL of sterile phosphate-buffered saline (PBS), it was mixed with 10 g of fresh soil blender. Soil suspensions were diluted in 10-fold to 10−7 in PBS and inoculated by injecting 1 mL of each dilution from 10−3 to 10−7 into sterile Hungate tubes containing nutrient broth (NB; Difco) with 5 mmol/L KNO3 and Durham tube. The tubes were incubated with Anaerocult® (Merck) in a desiccator for 17 days at 30 °C in order to test turbidity, gas formation, and nitrate output. Nitrate output was tested with Quantofix® nitrate-nitrite test sticks (Sigma-Aldrich) according to the manufacturer's recommendations. Bacteria from positive tubes were diluted in sterile PBS and inoculated on nutrient agar (NA) and allowed to incubate in the desiccator at 30 °C for 48–72 h. The steps described above have been repeated in order to verify whether the pure cultures are denitrifier (Dandie et al. 2007). After the classical microbiological tests of these isolated bacteria were performed, biochemical properties were performed according to BioMerieux API 20E and API 20NE identification kits based on the manufacturer protocols. Pure cultures were stored at − 80 °C in NB medium with 15% glycerol.
PCR amplification and sequence analysis of 16S rRNA and nosZ
Genomic DNA isolation from pure cultures was performed according to Sambrook et al. (1989). All genomic DNA patterns were diluted in ~ 10 ng/μL and used in the PCR reaction. The reaction compositions and PCR conditions for 16S rRNA and nosZ (Throbäck et al. 2004; Henry et al. 2006) are given in Suppl. Tables 2 and 3. Amplified products were sequenced (Atlas Biotechnology, Ankara). The sequencing data were edited using Chromas version 2.24 software (Technelysium Pty Ltd.). These sequences were aligned by using Clustal W (Version 2.1). Phylogenetically related bacterial 16S rRNA sequences were checked by the BLAST search in the NCBI GenBank database. Phylogenetic tree was constructed using maximum likelihood (ML) and neighbor-joining (NJ) method with bootstrap sample size 1000 by MEGA 7.0.
SSCP analysis of 16S rRNA gene regions for pure culture and culture-independent community structure
The soil microbial DNA was isolated using the FastDNA SPIN™ kit for soil (MP Biomedical, Santa Ana, CA) to at least 2 replicates per soil sample according to the manufacturer's instructions. Reaction compounds and PCR conditions for Com1 and Com2-Ph corresponding to a specific region of the 16S rRNA gene region to perform the SSCP analysis were obtained from the methods of Schwieger and Tebbe (2000) and Smalla et al. (2007). PCR products were purified using the Promega Wizard SV Gel and PCR Clean-Up System (Promega) purification kit. Approximately 700 ng of the purified PCR product was allowed to incubate for 1 h at 37 °C with 5U Lambda-exonuclease (Thermo Fisher Scientific, California) to cleave the phosphorylated chain. Single-chain DNA was purified using the Promega Wizard SV Gel and PCR Clean-Up System (Promega) purification kit. Four microliters of loading solution (95% formamide, 10 mmol/L NaOH, 0.025% bromophenol blue and 0.025% xylene cyanol) was added onto a 10-μL purified single chain DNA sample. The samples were denatured at 95 °C for 2 min and placed on ice quickly, and 5 μL was loaded onto the gel. For electrophoresis, a mixture of 0.6X MDE (Mutation Detection Enhancement, Thermo Fisher Scientific, Lonza) gel was prepared and carried out using Hoofer (SE400, USA) apparatus. The gel was run at 5 mA, 200 V, and 20 °C for 36 h. From the visualization of the DNA profiles from gel by means of Byun et al. (2009), silver staining method was applied according to four steps.
DNA extraction from polyacrylamide gel and sequence analysis of selected bands
After silver staining, gel image was taken for cultured bacteria and stored for analysis. For soil samples, dominant or single bands detected on polyacrylamide gel were cut with a sterile scalpel for subsequent analysis. The gel pieces were transferred to microtubes containing 100 μL of elution liquid (0.5 mol/L ammonium acetate, 10 mmol/L Mg2+-acetate, 1 mmol/L EDTA [pH 8.0], and 0.1% SDS). The tubes were allowed to incubate for 3 h at 37 °C and centrifuged at 12,000×g for 1 min at room temperature. A total of 80 μL of the supernatant was transferred to a micro test tube, and 2 volumes of cold ethanol (96%) were added to precipitate. After centrifugation at 12,000×g for 7 min, the DNA was dried for 30 min at 30 °C and dissolved in Tris-HCl (10 mmol/L, pH 8.0). Two microliters of this solution was used as the target DNA for PCR processing (Schwieger and Tebbe 1998). The PCR process and sequence analysis with Com1 and Com2 primers were performed as described above.
Results
Isolation of cultivable denitrifying bacteria
One hundred colonies were selected from soil samples and tested for turbidity, gas production, and nitrate or nitrite usage. In the next step, these 100 isolates were subjected to the same tests and 66 isolates were selected as denitrifier. Tests were repeated in these isolates, and 49 of them were selected as denitrifier, and work continued with these.
Identification of cultivable denitrifying bacteria
According to the results of Gram staining, the majority of isolates (75.5%) were Gram-negative bacillus, twelve (24.5%) isolates were Gram-positive, and only one of them had coccus morphology. As a result of nitrate reduction test, all isolates showed positive results. It was found that 15 (30.6%) isolates gave negative results for gelatinase activity, all isolates were positive for catalase, and 35 (71.4%) isolates gave negative results for oxidase test (Suppl. Table 4). In addition, API 20E and 20NE test kits were used to carry out biochemical tests of isolates. The API 20E V5.1 program was used to evaluate the API 20E test results, and according to the identification results, it was concluded that API 20E was insufficient to identify the Gram-positive bacteria isolated from environmental samples (Suppl. Table 5). Moreover, API 20NE V7.0 program was used to evaluate API 20NE test results. One of the isolates (3B1) was 99.7% to Rhizobium radiobacter; one isolate (5B2) to 99% Stenotrophomonas maltophilia; and the other isolates were similar to Aeromonas hydrophila and/or caviae, Pseudomonas luteola, Pseudomonas fluorescens, and Burkholderia cepacia at species level (Suppl. Table 6).
16S rRNA sequence analysis results and phylogenetic distributions of isolates
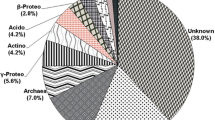
GenBank access numbers for 49 isolates were determined in the range MH269197 to MH269245. The similarity ratios obtained in the GenBank screening for the isolates are shown in Table 1 where the isolates show affinity through Blast. When the 16S rRNA sequence analyses of the same isolates were compared with the identification data obtained according to the API 20NE test results for Gram-negative isolates, it was found that they gave the same results at species level for 5B2 and 7S2 only at the genus level for 1B3, 1S2, 6K1, and 6K3. In phylogenetic tree analysis, Fig. 1a for ML and Fig. 1b for ML were found to be similar, but it was found that 16S rRNA gene regions of isolates were similar in both trees but Bootstrap values in nodes observed in ML phylogeny tree showed more reliable results than NJ trees. Table 1 and Fig. 1a and b were examined in a total of 49 cultivated denitrifying bacteria, 36 (73.46%) isolates Gammaproteobacteria, 12 (24.48%) isolates Firmicutes, and only 1 (2.04%) isolate from the Alphaproteobacteria filum was detected. This showed that denitrifiers do not belong to a specific group but are distributed among all bacterial groups.
SSCP analysis of cultivated denitrifiers
As a result of SSCP analysis, band profiles were evaluated based on 0.5% distance matrix in PyElph 1.4 program and 17 different band profiles were obtained. Of these, isolates 29 and 30 were found to exhibit 2 single chain band conformation unlike others. When the band profiles of the isolates identified according to 16S rRNA were examined in SSCP (Fig. 2a, b), the bands of the isolates identified as Bacillus and Staphylococcus from the Firmicutes phylum were found to be very close to each other. It was found that Bacillus subtilis used as positive control (P) was aligned with the bands (46 and 48) identified as Bacillus. At the same time, the bands expressing the isolates from the Gammaproteobacteria phylum are very close to each other (except bands 17, 34, and 40 identified as Citrobacter), and even in some of the bands 15, 27, 32, 41, 44, and 45 identified as Enterobacter and Klebsiella, they were aligned (shifts in the gel image were observed due to the combination of 3 different gel photographs and differences in the walking distance of the bands). Also, the bands 8 and 26, identified as Leclercia, and the bands 18, 35, 47, and 49, identified as Aeromonas, were found to give the same profiles among themselves. On the other hand, the band identified as Agrobacterium tumefaciens as the only isolate observed from Alphaproteobacteria and the band 23 identified as Enterobacteriaceae bacterium were found to have different profiles. It was also observed that the bands 31, 33, 42, and 43, which were identified as uncultured bacterium clone and identified as Gammaproteobacteria in the EzTaxon database, were aligned.
Phylogenetic comparison with nosZ and 16S rRNA
Although different PCR optimization conditions were tried, it was determined that 15 of the isolates were 1F-1R, 19 of the 2F-2R, and 5 of them gave single band with F-1622R primers and the sequence analysis was carried out with these PCR products. Although the majority of the nosZ gene regions obtained with the 1F-1R and 2F-2R primers were similar to the P. fluorescens Pf29Arp contig6 and P. chlororaphis UFB2 strains at about 88–90%, it was based on the nosZ clones showing the highest similarity in the Blast screen as shown in Table 2. For most of the previous studies for the nosZ gene region, Pseudomonas was preferred as the target species and the results of the sequence analysis performed for this region resulted in the closest similarity to the Pseudomonas species. Although the isolates 1, 9, 10, 11, 14, 19, 20, 21, 38, 39, and 46 were Gram (+), the affinity of the nosZ gene region sequence results to the Gram (−) Pseudomonas species supported the above claim. When Table 2 is examined, the sequence data of the 9 isolates with the primers 1F-1R and 2F-2R were successfully obtained and 7 of these isolates were similar to the similar clones but the nosZ gene regions obtained for 2 isolates (19 and 46) were found to be similar to the different clones. The 5 sequence results obtained by F-1622R primer did not give a direct affinity to the nosZ gene region in Blast screening (except for example 1) but instead resembled the whole genome of bacterial species. In phylogenetic tree analysis, ML for Fig. 3a and NJ for Fig. 3b, the nosZ gene region distributions of the isolates were similar in both trees but Bootstrap values in the nodes of the NJ phylogeny tree showed more reliable results than ML trees. For the isolate distribution, nosZ 1F-1R amplification group was found in cluster I in both trees. The nosZ 2F-2R amplification group was found to be present both in the cluster I and in the nosZ 1F-1R group and also in the cluster VI. Also, nosZ F-1622R group was found to be distributed in clusters I–III–IV and V.
SSCP genetic profile analysis for bacterial community
SSCP profiles of soil samples were found to be composed of 6–15 different bands with different densities. Most of these bands were produced by many soil samples and formed 28 different band profiles (gel image not shown). It has been determined that the structure of the bacterium community varies considerably. The UPGMA dendrogram analysis (Fig. 4) showed that 20 soil samples taken from 3 regions were separated into 2 basic clusters (X and Y). It is seen that there are soil samples taken from all 3 regions within X major cluster, 3 of these samples taken from Sinop are in cluster 1 and 2 samples taken from Samsun and Kastamonu is in cluster 2. In particular, the fact that 6 of these 7 specimens in the X-major cluster were soils from the rice field and that all of the rice soil samples were in this cluster showed that the bacterial communities in these soil samples were much closer to each other than the other soil samples. In the Y major cluster, it is seen that there are 3 samples from Kastamonu in cluster 3 and 5 in cluster 4 from Samsun region. Finally, 3 (S3, S5, and S6) were taken from Sinop in cluster 5 and 1 (K4) taken from Kastamonu; especially, 3 of these samples (S5, S6, and K4) were found to be samples taken from wheat soil.
Phylogenetic identification of bacterial community SSCP profiles
For the molecular identification of bacterial commune in soil samples, dominant and separating bands were selected from SSCP profiles of 20 soil samples and 51 different DNA single chains were cut as shown by plotting in Fig. 5 and sequence analysis was performed. When Table 3 is examined, only 10 (19.6%) SSCP bands were found to be similar to the isolates cultivated and identified at the species level (K. oxytoca and K. michiganensis). The remaining 41 (80.4%) SSCP band sequence data were found to be similar to non-cultured environmental clones. In addition, when comparing the sequence data of the bands cut off from the same or different positions on the SSCP gel, it was seen that they were similar to the same or different species or clones, whether in the same position or not.
Discussion
API 20E and 20NE test kits and 16S rRNA sequence analysis results for bacterial identification were compared. For Gram-positive bacteria, API 20E was insufficient, and for Gram-negative bacteria, API 20NE was consistent with 16S rRNA in 4 isolates at the genus and 2 isolates at the species level. These results showed that API 20E and 20NE kits were insufficient in the identification of Gram-positive and Gram-negative bacteria in environmental samples. In accordance with our data, Bosshard et al. (2004) compared the API assay and 16S rRNA sequence analysis to identify clinical isolates. The API 20 kit system for the majority of strains (96%) indicated that a reliable identification at the species level could not be performed and API test kits were inadequate for the detection of Gram-positive bacteria. Song and Leff (2005) reported that only one of the 20 bacterial samples isolated from the Mir space station was compatible with API 20NE and 16S rRNA. Awong-Taylor et al. (2008) compared the identification of bacterial samples obtained from environmental samples according to 16S rRNA, API 20E, and 20NE analyses and reported 74% inconsistency between API test kits and 16S rRNA sequence analysis. Furthermore, both API kits reported 86% of Gram-negatives, whereas only 33% of Gram-positives were identified; however, API 20NE was much more effective than 20E in identifying isolates from environmental samples. Belak et al. (2011) compared the identification of 47 bacterial specimens isolated from chicken meat with multiplex PCR and API kits and reported that API 20NE leads to misidentification at species level. Benga et al. (2014) reported in some cases a mismatch between 16S rRNA and API test kits and 16S rRNA sequence analysis as a very effective diagnostic tool for species identification. Carlson et al. (2017) the API test kits are limited to the bacteria growing in the culture medium, and also with the biochemical profiles obtained with the API test kits, 16S rRNA sequence analysis data is impossible to state because the genus level identification and biochemical profile estimates are very variable reported.
It was found that the SSCP technique was an effective method for classifying microorganisms within or between species, and it was found to be compatible with 16S rRNA sequence analysis findings and was a powerful identification tool with classical microbiological tests and 16S rRNA sequence data, especially in pure cultures. Schwieger and Tebbe (1998) reported that isolates from different species gave different band positions on SSCP gel. In the same studies, the researchers reported that some isolates gave more than one band. In parallel with this study, Schmalenberger et al. (2001) studied the SSCP band profiles of 13 different bacterial species, V2–V3, V4–V5, V6, V7, and V8 variable regions of the 16S rRNA, and selected the most suitable band-giving region as the V4–V5 gene region. In addition, Schmalenberger et al. suggested that the most suitable site was V4–V5 by referring only to the SSCP profile of 13 bacteria, indicating the lack of sample, but the validity of this hypothesis was questioned. In our study, by analyzing the same region, we determined that 47 of 49 pure cultures as single bands and determining their compatibility with sequence data contributed to the accuracy of this hypothesis. When we compare 16S rRNA sequence analysis and SSCP technique, SSCP technique provides the advantage of rapid decision making in bacterial diversity with similar band profile for most bacteria of the same species. Furthermore, it allows to compare the known reference strains of the non-sequenced pure cultures with the band profiles and reveal their possible affinities. However, it is a disadvantage that it rarely gives different band profiles among isolates of the same species and requires verification by 16S rRNA sequence analysis.
In our study, 1F-1R, 2F-2R, and F-1622R primer sets of nosZ gene regions were used as molecular markers. In our study, Table 2 shows that 1F-1R and 2F-2R primer sets are similar to environmental clones, and the findings are not compatible with 16S rRNA data in Table 1. On the other hand, although the sample number was low, 4 of the 5 isolates with nosZ F-1622R primer set were compatible with 16S rRNA results; in this case, it supported the hypothesis that this primer set could be used as a molecular marker for the studies of detection of denitrifies in environmental samples. However, the small number of samples questioned this situation and led to more extensive studies with the primer set F-1622R or more effective primer designs expressing the nosZ gene region. From previous studies on this topic, Scala and Kerkhof (1999) performed sequence analysis of 37 nosZ gene copies obtained from environmental samples and pure bacterial cultures and formed phylogenetic trees. Three clusters obtained in phylogenetic trees have reported very little compatibility with environmental and pure culture clones. Delorme et al. (2003) reported that there was no similarity between the 16S rRNA and nosZ phylogeny they formed for fluorescent Pseudomonas. Horn et al. (2006), in their study comparing nosZ and 16S rRNA, reported that the cultured denitrifiers give < 48% similarity rate for nosZ and showed < 97% similarity level for the 16S rRNA, and the 16S rRNA gene region was much more conserved. He also reported that there was horizontal gene transfer for nosZ, so it would be difficult to predict new species due to nosZ. Mills et al. (2008), in parallel with our study, reported that the 42 nosZ clones generally show similarities to non-cultured clones. Bowles et al. (2012) compared nosZ and 16S rRNA gene regions and reported that nosZ clones do not show similarity to pure culture denitrifiers but rather resemble to non-cultured environmental clones. Henry et al. (2006) based on the suggestion that the nosZ 2F-2R primer attracted greater interest than the nosZ 1F-1R primer based on the intuition that the more diverse primer set was more appropriate, and our findings were found to be consistent with the study. Heylen et al. (2006) proposed that denitrification genes support the hypothesis that horizontal gene transfer events occur and that denitrification genes should not be related to the diversity of organisms of denitrifers in culture-independent studies. On the other hand, from Dandie et al. (2007), 16S rRNA and nosZ in their study in the comparison of both gene regions reported that the results are compatible. Ishii et al. (2011) stated that the preferred nirS, nirK, and nosZ environmental clone sequences as molecular markers reach a wide population in the databases, but most denitrifers carrying these gene regions are still unknown and are represented by much less records in the databases than 16S rRNA.
Our data related to the structure of the community were in parallel with the studies which successfully applied SSCP analysis to the dynamics and definition of microbial community (Schwieger and Tebbe 1998; Stach et al. 2001; Backman et al. 2003; Hori et al. 2006; Rossmann et al. 2012). Smalla et al. (2007) tested the Denaturing gradient gel electrophoresis (DGGE), T-RFLP, and SSCP techniques to determine the bacterial community structure in soil samples and reported similar results in all three techniques. Hori et al. (2006) compared the SSCP and DGGE techniques to determine the structure of the bacterial community and reported that the SSCP was superior to DGGE in determining the dynamics of microbial community. Backman et al. (2003) compared the SSCP and DGGE techniques in determining the structure of the community and reported that the solubility of DNA fragments at the time of separation was better at SSCP. Uncultured bacterium clones were found to be dominant in our study. In parallel with our study, Tsai et al. (2009) reported 350 clones obtained from 3 different soil samples, Wolińska et al. (2017) reported 40 clones obtained from 16 different soil samples, and Bunge and Lechner (2004) reported that 15 clones of 16S rRNA were similar to non-cultured bacterial clones.
As a result, this study can be expressed in three different stages; in the first stage, it has been observed the successful isolation of denitrifers which can be cultured by conventional microbiological methods from different cultivated land soils. In addition to the morphological tests, the results obtained with API test kits were insufficient for the identification of the samples; therefore, it was determined that a successful identification was made with the combination of classical microbiological tests, SSCP fingerprint, and 16S rRNA sequence analysis. In the second stage, because of the large variety of denitrifier bacterial diversity and the difficulty of detecting this group with only 16S rRNA sequence analysis, nosZ was extracted from some isolated bacteria and compared to being a molecular marker with 16S rRNA. However, the nosZ gene region was found to be inadequate as a molecular marker for denitrification. In the third stage, the culture-independent bacterial community structure using the SSCP technique was successfully identified. In order to determine the diversity of the bacterial community, SSCP technique was combined with clone library analysis and it was found that uncultured bacterium clones in soil samples were dominant. In addition, in the first stage, cultivated denitrifers isolated from soil samples were not found to be dominant in the community.
References
Avşar C (2018) Quantification of denitrifier population size in soil, bacteria community structures and comparison of nosZ and 16S rRNA genes from culturable denitrifying. PhD Thesis, Ankara University, Ankara, Turkey, 188p.
Awong-Taylor J, Craven KS, Griffiths L, Bass C, Muscarella M (2008) Comparison of biochemical and molecular methods for the identification of bacterial isolates associated with failed loggerhead sea turtle eggs. J Appl Microbiol 104(5):1244–1251. https://doi.org/10.1111/j.1365-2672.2007.03650.x
Backman JS, Hermansson A, Tebbe CC, Lindgren PE (2003) Liming induces growth of a diverse flora of ammonia-oxidising bacteria in acid spruce forest soil as determined by SSCP and DGGE. Soil Bio Biochem 35(10):1337–1347. https://doi.org/10.1016/s0038-0717(03)00213-x
Belak Á, Kovács M, Hermann Z, Holczman Á, Márta D, Stojakovič S, Maraz A (2011) Molecular analysis of poultry meat spoiling microbiota and heterogeneity of their proteolytic and lipolytic enzyme activities. Acta Aliment 40(1):3–22. https://doi.org/10.1556/aalim.40.2011.suppl.2
Benga L, Benten WPM, Engelhardt E, Köhrer K, Gougoula C, Sager M (2014) 16S ribosomal DNA sequence-based identification of bacteria in laboratory rodents: a practical approach in laboratory animal bacteriology diagnostics. Lab Anim 48(4):305–312. https://doi.org/10.1177/0023677214538240
Bosshard PP, Abels S, Altwegg M, Böttger EC, Zbinden R (2004) Comparison of conventional and molecular methods for identification of aerobic catalase-negative Gram-positive cocci in the clinical laboratory. J Clin Microbiol 42(5):2065–2073. https://doi.org/10.1128/jcm.42.5.2065-2073.2004
Bowles MW, Nigro LM, Teske AP, Joye SB (2012) Denitrification and environmental factors influencing nitrate removal in Guaymas Basin hydrothermally altered sediments. Front Microbiol 3:1–11. https://doi.org/10.3389/fmicb.2012.00377
Bunge M, Lechner U (2004) Studying the microbial dynamics of a trichlorobenzene-dechlorinating community by single-strand conformation polymorphism. Kapitel 6:127–140. https://doi.org/10.1002/0471684228.egp11609
Byun SO, Fang Q, Zhou H, Hickford JGH (2009) An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Analytic Biochem 385(1):174–175. https://doi.org/10.1016/j.ab.2008.10.024
Carlson JM, Leonard AB, Hyde ER, Petrosino JF, Primm TP (2017) Microbiome disruption and recovery in the fish Gambusia affinis following exposure to broad-spectrum antibiotic. Infect Drug Res 10:143. https://doi.org/10.2147/idr.s129055
Čuhel J, Šimek M, Laughlin RJ, Bru D, Chèneby D, Watson CJ, Philippot L (2010) Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl Environ Microbiol 76(6):1870–1878. https://doi.org/10.1128/aem.02484-09
Cui P, Fan F, Yin C, Song A, Huang P, Tang Y, Liang Y (2016) Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Bio Biochem 93:131–141. https://doi.org/10.1016/j.soilbio.2015.11.005
Dandie CE, Burton DL, Zebarth BJ, Trevors JT, Goyer C (2007) Analysis of denitrification genes and comparison of nosZ, cnorB, and 16S rDNA from culturable denitrifying bacteria in potato cropping systems. Syst Appl Microbiol 30:128–138. https://doi.org/10.1016/j.syapm.2006.05.002
Delorme S, Philippot L, Edel-Hermann V, Deulvot C, Mougel C, Lemanceau P (2003) Comparative genetic diversity of the narG, nosZ, and 16S rRNA genes in fluorescent pseudomonads. Appl Environ Microbiol 69(2):1004–1012. https://doi.org/10.1128/aem.69.2.1004-1012.2003
Gaimster H, Alston M, Richardson D, Gates A, Rowley G (2017) Transcriptional and environmental control of bacterial denitrification and N2O emissions. FEMS Microbiol Let fnx277. https://doi.org/10.1093/femsle/fnx277
Graf DRH, Jones CM, Hallin S (2014) Intergenomic comparisons highlight modularity of the denitrification pathway and underpin the importance of community structure for N2O emissions. PLoS ONE 9:e114118. https://doi.org/10.1371/journal.pone.0114118
Hayatsu M, Tago K, Saito M (2008) Various players in the nitrogen cycle: diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci Plant Nut 54:33–45. https://doi.org/10.1111/j.1747-0765.2007.00195.x
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soil. Appl Environ Microbiol 72:5181–5189. https://doi.org/10.1128/aem.00231-06
Heylen K, Gevers D, Vanparys B, Wittebolle L, Geets J, Boon N (2006) The incidence of nirS and nirK and their genetic heterogeneity in cultivated denitrifiers. Environ Microbiol 8:2012–2021. https://doi.org/10.1111/j.1462-2920.2006.01081.x
Hori T, Haruta S, Ueno Y, Ishii M, Igarashi Y (2006) Direct comparison of single-strand conformation polymorphism (SSCP) and denaturing gradient gel electrophoresis (DGGE) to characterize a microbial community on the basis of 16S rRNA gene fragments. J Microbiol Meth 66:165–169. https://doi.org/10.1016/j.mimet.2005.11.007
Horn MA, Drake HL, Schramm A (2006) Nitrous oxide reductase genes (nosZ) of denitrifying microbial populations in soil and the earthworm gut are phylogenetically similar. Appl Environ Microbiol 72(2):1019–1026. https://doi.org/10.1128/aem.72.2.1019-1026.2006
Ishii S, Ashida N, Otsuka S, Senoo K (2011) Isolation of oligotrophic denitrifiers carrying previously uncharacterized functional gene sequences. Appl Environ Microbiol 77(1):338–342. https://doi.org/10.1128/aem.02189-10
Jones CM, Graf DRH, Bru D, Philippot L, Hallin S (2013) The unaccounted yet abundant nitrous oxide-reducing microbial community: a potential nitrous oxide sink. The ISME J 7:417–426. https://doi.org/10.1038/ismej.2012.125
Mills HJ, Hunter E, Humphrys M, Kerkhof L, McGuinness L, Huettel M, Kostka JE (2008) Characterization of nitrifying, denitrifying, and overall bacterial communities in permeable marine sediments of the northeastern Gulf of Mexico. Appl Environ Microbiol 74(14):4440–4453. https://doi.org/10.1128/aem.02692-07
Morkved PT, Dörsch P, Bakken LR (2007) The N2O product ratio of nitrification and its dependence on long-term changes in soil pH. Soil Bio Biochemist 39(8):2048–2057. https://doi.org/10.1016/j.soilbio.2007.03.006
Morley N, Baggs EM, Dörsch P, Bakken L (2008) Production of NO, N2O and N2 by extracted soil bacteria, regulation by NO2− and O2 concentrations. FEMS Microbe Eco 65(1):102–112. https://doi.org/10.1111/j.1574-6941.2008.00495.x
Philippot L, Hallin S, Schloter M (2007) Ecology of denitrifying prokaryotes in agricultural soil. Adv Agro 96:249–305. https://doi.org/10.1016/s0065-2113(07)96003-4
Philippot L, Spor A, Henault C, Bru D, Bizouard F, Jones CM, Sarr A, Maron P (2013) Loss in microbial diversity affects nitrogen cycling in soil. The ISME J 7:1609–1619. https://doi.org/10.1038/ismej.2013.34
Pihlatie M, Syväsalo E, Simojoki A, Esala M, Regina K (2004) Contribution of nitrification and denitrification to N2O production in peat, clay and loamy sand soils under different soil moisture conditions. Nut Cycle Agro 70(2):135–141. https://doi.org/10.1023/b:fres.0000048475.81211.3c
Richardson D, Felgate H, Watmough N, Thomson A, Baggs E (2009) Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle could enzymic regulation hold the key. Trends Biotech 27:388–397. https://doi.org/10.1016/j.tibtech.2009.03.009
Rossmann B, Müller H, Smalla K, Mpiira S, Tumuhairwe JB, Staver C, Berg G (2012) Banana-associated microbial communities in Uganda are highly diverse but dominated by Enterobacteriaceae. Appl Environ Microbiol 78(14):4933–4941. https://doi.org/10.1128/aem.00772-12
Saggar S, Jha N, Deslippe J, Bolan NS, Luo J, Giltrap DL, Tillman RW (2013) Denitrification and N2O:N2 production in temperate grasslands: processes, measurements, modelling and mitigating negative impacts. Sci Total Environ 465:173–195. https://doi.org/10.1016/j.scitotenv.2012.11.050
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Clonning: A Laboratory Manual, 2nd Edition. Cold Spring Harbor Laboratory Press, New York, U.S.A., p 1102.
Sanford RA, Wagner DD, Wu Q, Chee-Sanford JC, Thomas SH, Cruz-García C, Nissen S (2012) Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. PNAS 109(48):19709–19714. https://doi.org/10.1073/pnas.1211238109
Scala DJ, Kerkhof LJ (1999) Diversity of nitrous oxide reductase (nosZ) genes in continental shelf sediments. Appl Environ Microbiol 65(4):1681–1687. https://doi.org/10.1111/j.1574-6968.1998.tb12979.x
Schmalenberger A, Schwieger F, Tebbe CC (2001) Effect of primers hybridizing to different evolutionarily conserved regions of the small-subunit rRNA gene in PCR-based microbial community analyses and genetic profiling. Appl Environ Microbiol 67(8):3557–3563. https://doi.org/10.1128/aem.67.8.3557-3563.2001
Schwieger F, Tebbe CC (1998) A new approach to utilize PCR-single-strand-conformation polymorphism for 16S rRNA gene-based microbial community analysis. Appl Environ Microbiol 64:4870–4876. https://doi.org/10.1007/springerreference_76320
Schwieger F, Tebbe CC (2000) Effect of Field Inoculation with Sinorhizobium meliloti L33 on the composition of bacterial communities in rhizospheres of a target plant (Medicago sativa) and a non-target plant (Chenopodium album)-linking of 16S rRNA gene-based single-strand conformation polymorphism community profiles to the diversity of cultivated bacteria. Appl Environ Microbiol 66:3556–3565. https://doi.org/10.1128/aem.66.8.3556-3565.2000
Shcherbak I, Millar N, Robertson GP (2014) Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. PNAS 111(25):9199–9204. https://doi.org/10.1073/pnas.1322434111
Skiba UM, Sheppard LJ, MacDonald J, Fowler D (1998) Some key environmental variables controlling nitrous oxide emissions from agricultural and seminatural soils in Scotland. Atmospheric Environ 32(19):3311-3320. https://doi.org/10.1016/s1352-2310(97)00364-6
Smalla K, Oros-Sichler M, Milling A, Heuer H, Baumgarte S, Becker R, Neuber G, Kropf S, Ulrich A, Tebbe CC (2007) Bacterial diversity of soils assessed by DGGE, T-RFLP and SSCP fingerprints of PCR-amplified 16S rRNA gene fragments: do the different methods provide similar results. J Microbiol Met 69:470–479. https://doi.org/10.1016/j.mimet.2007.02.014
Song B, Leff LG (2005) Identification and characterization of bacterial isolates from the Mir space station. Microbiol Res 160(2):111–117. https://doi.org/10.1016/j.micres.2004.10.005
Stach JE, Bathe S, Clapp JP, Burns RG (2001) PCR-SSCP comparison of 16S rDNA sequence diversity in soil DNA obtained using different isolation and purification methods. FEMS Microbiol Eco 36(2-3):139–151. https://doi.org/10.1016/s0168-6496(01)00130-1
Throbäck IN, Enwall K, Jarvis Å, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Eco 49(3):401–417. https://doi.org/10.1016/s0168-6496(04)00147-3
Tsai S, Selvam A, Chang Y, Yang S (2009) Soil bacterial community composition across different topographic sites characterized by 16S rRNA gene clones in the Fushan Forest of Taiwan. Bot Stud 50(1):57–68
Wallenstein MD, Myrold DD, Firestone M, Voytek M (2006) Environmental controls on denitrifying communities and denitrification rates: insights from molecular methods. Ecological Appl 16(6):2143-2152. https://doi.org/10.1890/1051-0761(2006)016
White JD, Scott NA (2006) Specific leaf area and nitrogen distribution in New Zealand forests: species independently respond to intercepted light. Forest Ecol Manage 226:319–329. https://doi.org/10.1016/j.foreco.2006.02.001
Wittorf L, Bonilla-Rosso G, Jones CM, Bäckman O, Hulth S, Hallin S (2016) Habitat partitioning of marine benthic denitrifier communities in response to oxygen availability. Environ Microbiol Rep 8(4):486–492. https://doi.org/10.1111/1758-2229.12393
Wolińska A, Górniak D, Zielenkiewicz U, Goryluk-Salmonowicz A, Kuźniar A, Stępniewska Z, Błaszczyk M (2017) Microbial biodiversity in arable soils is affected by agricultural practices. Int Agrophys 31(2):259–271. https://doi.org/10.1515/intag-2016-0040
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Molec Bio Rev 61(4):533–536
Funding
This research has been supported by Ankara University Scientific Research Project Coordination Unit. Project Number: 17L0430004, 2017-2018.
Author information
Authors and Affiliations
Contributions
CA and ESA made a contribution to designing the study. CA was responsible for completing the experiments and data analysis. CA and ESA made a contribution to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 55 kb)
Rights and permissions
About this article
Cite this article
Avşar, C., Aras, E.S. Community structures and comparison of nosZ and 16S rRNA genes from culturable denitrifying bacteria. Folia Microbiol 65, 497–510 (2020). https://doi.org/10.1007/s12223-019-00754-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-019-00754-8