Abstract
Lactobionic acid (LBA) is a newly identified natural polyhydroxy acid that is widely used in the food industry. In this study, the antibacterial effects and underlying mechanism of action of LBA against Staphylococcus aureus were investigated. LBA exhibited significant antibacterial activity against S. aureus with a determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of 15 mg/mL and 50 mg/mL, respectively. The Growth curves indicated that LBA directly inhibited the growth of S. aureus. Moreover, LBA induced the leakage of alkaline phosphatase and nucleotides in the culture medium, indicating damage to the integrity of the S. aureus cell wall membrane, which was confirmed by transmission electron microscopy observations. The relative electric conductivity measurements indicated that LBA changed the cell membrane permeability. The preservation effect of LBA was evaluated by quantifying the total number of colonies, total volatile base nitrogen (TVB-N), and thiobarbituric acid reactive substances (TBARS). Overall, these results revealed that LBA exerts its antibacterial activity by breaking down the structure of the bacterial cell wall and membrane, thereby releasing the cellular contents as well as inhibiting protein synthesis, which ultimately lead to cell death. The total number of colonies, the TVB-N value, and the TBARS of cold fresh meat treated with preservatives were significantly lower than those of the control group (P < 0.05). With these antibacterial characteristics, LBA has potential to be used as a safe food additive in the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the rapid development in the field of food science, it has become increasingly evident that food spoilage and contamination are the most common causes of foodborne diseases. As a serious public health issue, protecting against such contamination represents one of the most urgent challenges currently facing the food industry (Sokmen et al. 2004; Aziman et al. 2014).
Several studies have focused on the causes of foodborne illness and disease worldwide, demonstrating that infection from foodborne pathogens and spoilage microorganisms is the most critical factor to target (Patra and Baek 2016; Diao et al. 2013). Thirty-one major pathogens were identified to be responsible for 9.4 million episodes of foodborne illness, resulting in 55,961 hospitalizations and 1351 deaths annually in the USA (Scallan et al. 2011; Xu et al. 2017). Preservatives are additives that can not only extend the shelf life of food but also effectively inhibit the colonization and growth of harmful microorganisms in food, thereby securing the health of consumers (Zhou et al. 2017). Despite the fact that, due to their low cost and good effects, the chemical preservatives are the preferred and most common type of food preservatives used in the market, they can also pose great harm to the human body (Derde et al. 2013). Therefore, the development of naturally based preservatives to avoid ingesting such potentially harmful chemicals is an important topic in food sciences (Goñi et al. 2013). In general, a natural preservative is defined as a natural substance that is safe and effective, including some already existing food components. In addition to not being harmful to health, such preservatives should also help to enhance the food product with regard to improving or maintaining flavor and quality. Natural antimicrobial substances are currently used as food preservatives; however, before their widespread application, it is important to understand the detailed antibacterial characteristics and underlying mechanism.
One such potential natural preservative with antibacterial effects is lactobionic acid (LBA), which is a versatile polyhydroxy acid comprising one galactose molecule attached to another molecule of gluconic acid via an ether-like linkage that is featured by the presence of eight hydroxyl groups (Alonso et al. 2013). LBA has been verified to be a safe and non-toxic substance (Van Dokkum et al. 1994) and currently plays an essential role in several industrial applications, including in the food, chemical, pharmaceutical, medical (Olivieri et al. 2018), and cosmetic industries. The role of LBA as a food preservative is highly anticipated, and its use in the form of calcium lactobionate has already been supported by the US Food and Drug Administration. Indeed, this versatile polyhydroxy acid can serve as an antioxidant, stabilizer, or gelling agent (Jéssica et al. 2016), acidifier agent (Faergemand et al. 2012), aging inhibitor (Oe and Kimura 2011), and food flavor enhancer and can also enhance mineral absorptions (Oe et al. 2008). LBA may also exert potential prebiotic effects as a bioactive ingredient in functional foods (Schaafsma 2008). However, its antibacterial activity and mechanism remain to be verified.
Therefore, the aim of the present study was to comprehensively evaluate the mode of action and promote the further application of LBA in the food industry by testing its antibacterial activity against Staphylococcus aureus, which is a significant pathogen and major cause of foodborne diseases worldwide.
Material and methods
Bacterial strain and growth conditions
The strain S. aureus ATCC 25923 obtained from the American Type Culture Collection was inoculated in nutrition broth (NB) containing 1% tryptone, 0.3% beef extract, and 0.5% NaCl, and on nutrient agar (NA) with the same components along with 1.7% agar powder. S. aureus was cultured by streaking onto NA petri dishes and incubating for 24 h at 37 °C. The turbidity of the cell suspensions was adjusted to the required concentration of 106 colony-forming units (CFU)/mL.
Determination of the minimum inhibitory concentration and minimum bactericidal concentration
LBA (≥ 97%; Sigma-Aldrich, St. Louis, USA) was diluted to 20% (v/v) in sterile water and then filtered through a 0.22-μm filter membrane. The antibacterial assay of LBA was conducted against S. aureus ATCC 25923 using the agar well diffusion method as reported previously (Zhang et al. 2017). In brief, the suspensions containing 106 CFU/mL of S. aureus were spread onto separate NA plates. Oxford cups (8 mm in diameter) were filled with 250 μL of the LBA solution so that the concentration of LBA ranged from 0.75 to 50 mg/mL. The inhibitory zones formed were measured after incubation of the plates at 37 °C for 24 h to calculate the minimum inhibitory concentration (MIC).
The minimum bactericidal concentration (MBC) was determined according to a previously reported procedure (Raja et al. 2011) with minor modifications. In brief, the bacterial suspensions were incubated at 37 °C for 24 h and then diluted in the NB to obtain a final inoculum of 106 CFU/mL. Bacterial suspensions (100 μL) were added to each well of a 96-well plate so that the final concentration of LBA ranged from 10 to 100 mg/mL, and the plates were incubated at 37 °C for 18 h. The MBC was determined by spreading the cultures from each well on NA plates and determining the absence of growth after 24-h incubation at 37 °C.
Growth curves
To evaluate the direct inhibitory effect of LBA on the growth of S. aureus, 106 CFU/mL of the bacterial suspension was poured into a 100-mL NB medium containing 1/2× MIC and 1× MIC of LBA. The bacterial suspension was adjusted to an inoculum amount of 1%. NB medium with the inoculum, but without LBA, was used as the control. All samples were incubated at 37 °C, and the absorption values at 600 nm were determined at 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, and 24 h. The growth curves were generated by plotting the optical density values at 600 nm (OD600) versus time. The experiment was performed in triplicate.
Leakage of alkaline phosphatase
The logarithmic-phase S. aureus (107 CFU/mL) was treated with LBA at a final concentration of 1× MIC and 2× MIC. The bacterial suspension without LBA treatment served as a control. The mixtures were incubated at 37 °C for 5 h and then centrifuged at 5000×g for 5 min. The amount of extracellular alkaline phosphatase (AKP) in the supernatant was determined using an AKP kit (Nanjing Jiancheng Institute of Bioengineering, Nanjing, Jiangsu, China) and evaluated on a microplate reader (Eon, Biotek, USA).
Leakage of nucleic acids
The leakage of nucleotides was determined according to a previous report (Diao et al. 2018) with some modifications. In brief, logarithmic-phase S. aureus cells were obtained by centrifugation (5000×g for 5 min). The cells were washed twice and resuspended at a concentration of 1 × 106 CFU/mL in 10 mM phosphate-buffered saline (PBS, pH 7.4). S. aureus was incubated with LBA at three concentrations (1/2× MIC, 1× MIC, 2× MIC) at 37 °C, and samples were collected at 0, 30, 60, 90, and 120 min and then sterilized through a 0.22-μm filter membrane. The amount of nucleotides in the filtrates was determined at 260 nm on a microplate reader (Eon, Biotek, USA).
Transmission electron microscopy
Logarithmic-phase S. aureus cells were treated with LBA at 2× MIC and incubated at 37 °C for 6 h. The bacterial pellets were processed for transmission electron microscopy (TEM) as reported previously (Liu et al. 2016), and the ultrathin sections were observed on an H-600IV microscope (Hitachi, Tokyo, Japan).
Relative electric conductivity
The permeability of the bacterial membrane was assessed according to a previously reported method (Diao et al. 2014) with slight modifications. Briefly, the bacterial suspension was centrifuged at 5000×g for 5 min after incubation with the MIC of LBA for 0, 1, 2, 3, 4, 5, 6, and 7 h at 26 °C. Untreated bacterial cells were used as the control. The changes in permeability of the supernatant were determined by measuring the relative electric conductivity with a conductivity meter.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of whole-cell proteinase
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of S. aureus proteins was performed according to a previously reported method with few modifications (Li et al. 2014; Zhao et al. 2015). Logarithmic-phase cells of S. aureus (1 × 107 CFU/mL) in NB were added to an equal volume of LBA solution with different concentrations (1/2× MIC, 1× MIC, 2× MIC). The treated bacterial suspensions were incubated at 37 °C with shaking at 150 rpm for 4 h and then centrifuged at 5000×g for 10 min at 4 °C. The pellets were washed twice with PBS (10 mM, pH 7.4) and resuspended to make 1 × 107 CFU/mL solution in PBS. Ten microliters of proteins was combined with 10 μL 2× SDS sample dilution buffer. After boiling for 10 min, the samples were centrifuged at 5000×g for 10 min, and then 15 μL of the supernatant of each sample was loaded onto the gel. SDS-PAGE was performed on a 5% stacking gel and 12% separating gel, and the bands were visualized after silver staining.
Application of LBA in meat preservation
The meat (Sheng Rong Xiang Sunshine Pork Co. Ltd) was cut into pieces of about 500 g and chilled in a refrigerator at 4 °C. The meat pieces were separately coated with 3 mL of LBA solution (0, 1, 2, 3, 4, 5 g/100 mL) and placed in a 14.5 × 11.5 × 5.5-cm transparent crisper, marked, and placed in a refrigerator at 4 °C. GB 4789.2-2016 (“Determination of the total number of colonies in food microbiology test”) and GB5009.228-2016 (“Determination of volatile base nitrogen in food”) were used to determine the total number of colonies and volatile base nitrogen values after 6 days of storage at 4 °C. The method by Oussalah et al. (2004) was used with slight modifications. Ten grams of meat was crushed with a pulverizer and mixed with 50 mL of 7.5% trichloroacetic acid (containing 0.1% EDTA.Na2). This mixture was shaken for 30 min, filtered twice with double-layered filter paper, and 5 mL of the supernatant was added to 5 mL of 0.02 mol/L thiobarbituric acid solution and incubated at 90 °C for 40 min in a water bath. This mixture was then cooled to room temperature (cooling process was completed within 1 h) and centrifuged at 1100×g for 5 min, and the supernatant was transferred to a clean tube, shaken with 5 mL of chloroform, and left to stand for formation of layers. The supernatant from the tube was analyzed colorimetrically at 532 nm and 600 nm, and the light absorption value was recorded.
Data analysis
Each assay was performed at least three times in duplicate, and the data was analyzed by SPSS 17.0 software. Data are expressed as mean ± SD. The significance level was set to P < 0.05 and P < 0.01.
Results and discussion
Antibacterial activity of LBA to S. aureus
The MIC and MBC values of LBA against S. aureus ATCC 25923, obtained by using the agar well diffusion method, were 15 mg/mL and 50 mg/mL, respectively.
Effect of LBA on S. aureus growth
As shown in Fig. 1, the growth curves of LBA-treated and LBA-untreated S. aureus exhibited significant differences. Bacterial growth in the untreated control group entered the logarithmic phase after 4 h and showed rapid growth by 10 h with an increasing trend (Serpa et al. 2012; Ferro et al. 2015). However, S. aureus treated with LBA at 1/2× MIC grew much more slowly, demonstrating clearly inhibited growth. Upon treatment with 1× MIC LBA, the absorbance value of the bacterial suspensions did not show a clear change over time, remaining essentially the same as the initial concentration. Therefore, LBA negatively affected the growth of S. aureus in a dose-dependent manner. LBA not only significantly inhibited the normal growth of S. aureus, but also inhibited the growth activity and rates.
Effect of LBA on the cell wall
AKP is located between the cell wall and the cell membrane and therefore cannot be detected extracellularly through the cell wall. Nevertheless, when the cell wall is destroyed or compromised, AKP will leak out of the cell (Tang et al. 2017; Hsouna et al. 2011). Therefore, AKP activity in the culture medium can be an indicator of cell wall integrity. As shown in Fig. 2, compared with the control group, the OD520 value of AKP in the cell suspension obviously increased within 5 h. After treatment with LBA, the OD520 value increased from 0.137 to 0.174 for 1 × MIC and from 0.137 to 0.252 for 2 × MIC. Thus, LBA was more effective at increasing the extracellular AKP level at greater concentrations. We speculate that LBA was able to increase permeability of the cell wall, suggesting that damage to the integrity of the cell wall might be the main cause of the release of AKP into the supernatant.
Effect of LBA on the cell membrane
Nucleic acids are essential life substances in microorganisms, playing important roles in the vital activities of the cell. The maximum absorbance value of nucleic acids is 260 nm (Wu et al. 2016; Yang et al. 2016). Therefore, a microplate reader can be used to detect the presence of nucleic acid substances outside the cell. The degree of leakage of nucleic acids from S. aureus cells treated with 1/2× MIC, 1× MIC, and 2× MIC of LBA is displayed in Fig. 3. There was a clear increase in the amounts of nucleotides that leaked out of S. aureus cells with greater LBA concentrations and longer exposure time. These results suggest that LBA might induce damage to the outer membrane of S. aureus, resulting in leakage of nucleic acids from the bacterial cells.
TEM
To further examine the effect of LBA on S. aureus cells and cell membrane integrity, the cell morphology and ultrastructural changes were visualized using TEM. As shown in Fig. 4, the untreated S. aureus showed intact cell walls with smooth membranes, and the cytoplasm was uniformly distributed and full. In contrast, the bacteria treated with 2× MIC of LBA for 6 h showed an irregular shape and a loss of the clear boundary of the cell walls. Lysis of the membrane was evident, and the intracellular contents were clearly leaking out of the cells, leading to electron density heterogeneity. Therefore, LBA damaged the S. aureus cell walls and membranes, contributing to the leakage of cellular cytoplasmic contents (Ahamed et al. 2017; Wang et al. 2017). This damage is likely responsible for the observed growth inhibition and bacterial killing effect.
Permeability of the cell membrane
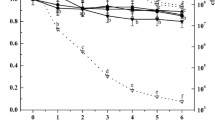
The permeability changes in the cell membrane were determined by the relative electric conductivity of bacterial suspension. Figure 5 shows that the values of the control group were slightly increased, which may be partly due to the normal apoptosis of cells (Diao et al. 2014). Compared with the control, S. aureus exposed to LBA at MIC showed a significant increase in conductivity with increased time, and the value for the treatment group was obviously higher than that of the control group after 2 h. Moreover, with the increase in LBA concentration and treatment time, the relative electric conductivity increased correspondingly. These results demonstrated that LBA could affect the permeability and integrity of S. aureus cell membranes, causing leakage of electrolytes (K+, Na+, and so on) and damage to metabolic pathways.
Protein analysis of S. aureus cells treated with LBA
The SDS-PAGE profiles of bacterial proteins from LBA-treated and control S. aureus were vastly distinct (Fig. 6). The protein bands of the control group were strong and clear, whereas those of the LBA-treated bacteria were weak, or even disappeared altogether in some cases. In addition, there was a marked LBA concentration-dependent effect, with less protein amounts and types expressed at higher LBA concentrations. These results indicated that LBA could cause the infiltration of S. aureus proteins into the culture medium by disrupting the bacterial cell membrane to release intracellular proteins. Similar results were reported for bacterial cells treated with other types of natural antibacterial substances, including lactic acid (Wang et al. 2015) and sugar fatty acid esters (Zhao et al. 2015).
Effect of LBA on meat preservation
The results of the effect of LBA solution (0, 1, 2, 3, 4, 5 g/100 mL) on the 6th day are shown in Table 1. The logarithm of the total number of colonies in the control group was 6.75 ± 0.167 CFU/g, which exceeded the national standard, and except for one result of 1 g/100 mL LBA, the concentrations of bacteria in the treatment group were significantly lower than those in the control group (P < 0.05). This indicated that LBA could inhibit the growth of spoilage microorganisms. On the 6th day, the total volatile base nitrogen (TVB-N) value of the control group was 18.30 ± 0.098 mg/100 g, greater than 15 mg/100 g, which exceeded the range for cold meat. In contrast, the values of the 2, 3, 4, and 5 g/100 mL groups were significantly lower than those of the control group (P < 0.05) and still in the range of fresh meat. This result indicated that LBA could inhibit the proliferation of some aerobic microorganisms and slow down the increase of TVB-N value. The value of thiobarbituric acid reactive substances (TBARS) of the control group was 1.30 ± 0.069 mg/kg, which was higher than that in groups treated with different concentrations of lactobionic acid (P < 0.05). The logarithm of the total number of colonies, the TVB-N value, and the TBARS value of the 4-g/100 m and 5-g/100 m groups were not significantly different, indicating that 4 g/100 mL lactobionic acid could provide optimal preservation effect.
Conclusion
We have confirmed that LBA has good antibacterial activity against S. aureus. LBA changed the growth curve of S. aureus. In addition, LBA induced damage to the integrity of the cell wall and cell membrane, resulting in ultrastructural alterations and membrane dysfunction leading to leakage of macromolecules (electrolytes, proteins, and nucleic acids). The change in protein patterns indicated that LBA might inhibit the synthesis of proteins or reduce degradation of bacterial cell proteins. The logarithm of the total number of colonies, the TVB-N value, and the TBARS value of chilled fresh meat treated with LBA were significantly lower than those of the control group on the 6th day (P < 0.05). Thus, our study provides a strong basis for further investigation of the antibacterial properties of LBA toward its development as a natural food preservative and antibacterial agent for application in the food industry and other fields.
Abbreviations
- AKP:
-
Alkaline phosphatase
- CFU:
-
Colony-forming units
- LBA:
-
Lactobionic acid
- MBC:
-
Minimum bactericidal concentration
- MIC:
-
Minimum inhibitory concentration
- PBS:
-
Phosphate-buffered saline
References
Ahamed AAP, Rasheed MU, Peer MNK, Reehana N, Santhoshkumar S et al (2017) In vitro antibacterial activity of MGDG-palmitoyl from Oscillatoria acuminata NTAPC05 against extended spectrum β-lactamase producers. J Antibiot 70:754–762
Alonso S, Rendueles M, Diaz M (2013) Bio-production of lactobionic acid: current status, applications and future prospects. Biotechnol Adv 31:1275–1291. https://doi.org/10.1016/j.biotechadv.2013.04.010
Aziman N, Abdullah N, Noor ZM, Kamarudin WS, Zulkifli KS (2014) Phytochemical profiles and antimicrobial activity of aromatic Malaysian herb extracts against food-borne pathogenic and food spoilage microorganisms. J Food Sci 79:583–592. https://doi.org/10.1111/1750-3841.12419
Derde M, Lechevalier V, Guérin-Dubiard C, Cochet MF, Jan S, Baron F, Nau F (2013) Hen egg white lysozyme permeabilizes Escherichia coli outer and inner membranes. J Agric Food Chem 61:9922–9929. https://doi.org/10.1021/jf4029199
Diao WR, Hu QP, Feng SS, Li WQ, Xu JG (2013) Chemical composition and antibacterial activity of the essential oil from green huajiao (Zanthoxylum schinifolium) against selected foodborne pathogens. J Agric Food Chem 61:6044–6049. https://doi.org/10.1021/jf4007856
Diao WR, Hu QP, Zhang H, Xu JG (2014) Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 35:109–116. https://doi.org/10.1016/j.foodcont.2013.06.056
Diao M, Qi D, Xu M, Lu Z, Lv F, Bie X, Zhang C, Zhao H (2018) Antibacterial activity and mechanism of monolauroyl-galactosylglycerol against Bacillus cereus. Food Control 85:339–344. https://doi.org/10.1016/j.foodcont.2017.10.019
Faergemand M, Gilleladen C, Qvist KB (2012) Method for producing an acidified milk product. United States Patent Application Pub. No.: US 20120045546 A1
Ferro BE, Ingen J, Wattenberg M et al (2015) Time–kill kinetics of antibiotics active against rapidly growing mycobacteria. J Antimicrob Chemother 70:811–817. https://doi.org/10.1093/jac/dku431
Goñi MG, Tomadoni B, Moreira MR, Roura SI (2013) Application of tea tree and clove essential oil on late development stages of butterhead lettuce: impact on microbiological quality. LWT- Food Sci Technol 54:107–113
Hsouna AB, Trigui M, Mansour RB, Jarraya RM, Damak M, Jaoua S (2011) Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int J Food Microbiol 148:66–72. https://doi.org/10.1016/j.ijfoodmicro
Jéssica C, Ribeiro B, Granato D, Masson ML, Andriot I, Mosca A, Salles C, Guichard E (2016) Effect of lactobionic acid on the acidification, rheological properties and aroma release of dairy gels. Food Chem 207:101–106. https://doi.org/10.1016/j.foodchem
Li YQ, Han Q, Feng JL, Tian WL, Mo HZ (2014) Antibacterial characteristics and mechanisms of ɛ-poly-lysine against Escherichia coli and Staphylococcus aureus. Food Control 43:22–27. https://doi.org/10.1016/j.foodcont.2014.02.023
Liu G, Ren G, Zhao L, Cheng L, Wang C, Sun B (2016) Antibacterial activity and mechanism of bifidocin A against Listeria monocytogenes. Food Control 73:854–861. https://doi.org/10.1016/j.foodcont.2016.09.036
Oe K, Kimura T (2011) Aging inhibitor for bread. Japan Patent Application Pub. No.: JP2011177121
Oe K, Nishikawa Y, Kimura T, Kiryu T, Kiso T, Murakami H et al (2008) Oxidation of lactose to lactobionic acid by acetic acid bacteria. Presentation at the 2nd International Conference on Acetic Acid Bacteria
Olivieri M, Cristaldi M, Pezzino S, Lupo G, Anfuso CD, Gagliano C, Genovese C, Rusciano D (2018) Experimental evidence of the healing properties of lactobionic acid for ocular surface disease. Cornea 37:1058–1063. https://doi.org/10.1097/ICO.0000000000001594
Oussalah M, Salmieri S, Lacroix M (2004) Antimicrobial and antioxidant effects of milk protein-based film containing essential oils for the preservation of whole beef muscle. J Agric Food Chem 52:5598–5605. https://doi.org/10.1021/jf049389q
Patra JK, Baek KH (2016) Antibacterial activity and action mechanism of the essential oil from enteromorpha linza L. against foodborne pathogenic bacteria. Molecules 21:388. https://doi.org/10.3390/molecules21030388
Raja AF, Ali F, Khan IA, Shawl AS, Arora DS, Shah BA, Taneja SC (2011) Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-beta-boswellic acid from Boswellia serrata. BMC Microbiol 11:54. https://doi.org/10.1186/1471-2180-11-54
Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM (2011) Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis 17:7–15. https://doi.org/10.3201/eid1701.P11101
Schaafsma G (2008) Lactose and lactose derivatives as bioactive ingredients in human nutrition. Int Dairy J 18:458–465. https://doi.org/10.1016/j.idairyj.2007.11.013
Serpa R, Franca EJG, Furlaneto-Maia L, Andrade CGTJ, Diniz A, Furlaneto MC (2012) In vitro antifungal activity of the flavonoid baicalein against Candida species. J Med Microbiol 61:1704–1708. https://doi.org/10.1099/jmm.0.047852-0
Sokmen A, Gulluce M, Akpulat HA, Daferera D, Tepe B, Polissiou M et al (2004) The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 15:627–634. https://doi.org/10.1016/j.foodcont.2003.10.005
Tang H, Chen WX, Dou ZM, Chen RH, Hu YY, Chen WJ, Chen H (2017) Antimicrobial effect of black pepper petroleum ether extract for the morphology of Listeria monocytogenes and Salmonella typhimurium. J Food Sci Technol 54:2067–2076. https://doi.org/10.1007/s13197-017-2644-2
Van Dokkum W, Wezendonk LJW, Van ASP, Kistemaker C (1994) Tolerance of lactobionic acid in man. TNO Nutrition and Food Research V94, The Netherlands, p 115
Wang C, Chang T, Yang H, Cui M (2015) Antibacterial mechanism of lacticacid on physiological and morphological properties of Salmonella enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 47:231–236. https://doi.org/10.1016/j.foodcont.2014.06.034
Wang F, Wei F, Song C, Jiang B, Tian S, Yi J, Yu C, Song Z, Sun L, Bao Y, Wu Y, Huang Y, Li Y (2017) Dodartia orientalis L. essential oil exerts antibacterial activity by mechanisms of disrupting cell structure and resisting biofilm. Ind Crop Prod 109:358–366. https://doi.org/10.1016/j.indcrop.2017.08.058
Wu Y, Bai J, Zhong K, Huang Y, Qi H, Jiang Y, Gao H (2016) Antibacterial activity and membrane-disruptive mechanism of 3-p-trans-Coumaroyl-2-hydroxyquinic acid, a novel phenolic compound from pine needles of Cedrus deodara, against Staphylococcus aureus. Molecules 21:1–12. https://doi.org/10.3390/molecules21081084
Xu C, Li J, Yang L, Shi F, Yang L, Ye M (2017) Antibacterial activity and a membrane damage mechanism of Lachnum YM30 melanin against Vibrio parahaemolyticus and Staphylococcus aureus. Food Control 73:1445–1451. https://doi.org/10.1016/j.foodcont.2016.10.048
Yang S, Liu L, Li D, Xia H, Su X, Peng L, Pan S (2016) Use of active extracts of poplar buds against Penicillium italicum and possible modes of action. Food Chem 196:610–618. https://doi.org/10.1016/j.foodchem.2015.09.101
Zhang Y, Wu YT, Zheng W, Han XX, Jiang YH, Hu PL, Tang ZX, Shi LE (2017) The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae. J Funct Foods 38:273–279. https://doi.org/10.1016/j.jff.2017.09.047
Zhao L, Zhang H, Hao T, Li S (2015) In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem 187:370–377. https://doi.org/10.1016/j.foodchem.2015.04.108
Zhou H, Ren J, Li Z (2017) Antibacterial activity and mechansm of pinoresinol from Cinnamomum Camphora leaves against food-related bacteria. Food Control 79:192–199. https://doi.org/10.1016/j.foodcont.2017.03.041
Funding
This work was supported by the Scientific Study Project of Liaoning Province Education Department (grant no. LSNZD201607) and National Key Technology R&D Program of the Ministry of Science and Technology (grant no. 2015BAD16B0903).
Author information
Authors and Affiliations
Contributions
Jiarong Cao designed the study, interpreted the results, and drafted the manuscript. Hongjie Fu and Lihong Gao collected the test data. Yan Zheng provided guidance.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, J., Fu, H., Gao, L. et al. Antibacterial activity and mechanism of lactobionic acid against Staphylococcus aureus. Folia Microbiol 64, 899–906 (2019). https://doi.org/10.1007/s12223-019-00705-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-019-00705-3