Abstract
During 2010–14 surveys in the major sesame growing areas of Fars, Yazd and Isfahan provinces (Iran), genetic diversity and vector transmission of phytoplasmas associated with sesame phyllody were studied. Virtual RFLP, phylogenetic, and DNA homology analyses of partial 16S ribosomal sequences of phytoplasma strains associated with symptomatic plants revealed the presence of phytoplasmas referable to three ribosomal subgroups, 16SrII-D, 16SrVI-A, and 16SrIX-C. The same analyses using 16S rDNA sequences from sesame phyllody-associated phytoplasmas retrieved from GenBank database showed the presence of phytoplasmas clustering with strains in the same subgroups in other Iranian provinces including Bushehr and Khorasan Razavi. Circulifer haematoceps and Orosius albicinctus, known vectors of the disease in Iran, were tested for transmission of the strains identified in this study. C. haematoceps transmitted 16SrII-D, 16SrVI-A, and 16SrIX-C phytoplasmas, while O. albicinctus only transmitted 16SrII-D strains. Based on the results of the present study and considering the reported presence of phytoplasmas belonging to the same ribosomal subgroups in other crops, sesame fields probably play an important role in the epidemiology of other diseases associated with these phytoplasmas in Iran.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytoplasmas are members of the class Mollicutes, intracellular wall-less plant pathogens transmitted mainly by different leafhopper species, and inducing typical symptoms of yellowing, discoloration, witches’ broom, dwarfing, virescence, and phyllody in both wild and cultivated plants (Seemüller et al. 1998; Bertaccini et al. 2014). Phytoplasma diseases cause significant yield losses worldwide in more than 1000 plant species from different plant families (Lee et al. 2000; Bertaccini and Duduk 2009).
Sesame (Sesamum indicum L.) is an oilseed plant belonging to family Pedaliaceae that is one of the most economically important hosts of phytoplasmas. It has been described for the first time in ancient Assyria and Babylon about four thousand years ago. Due to high nutritional value, rich antioxidant compounds, tolerance to high temperatures, and short length of plant growth, sesame is considered very important by farmers compared to the other oilseed plants. Its short growing cycle enables farmers to use it as an intermediate crop between harvesting and sowing of winter fall crops (Weiss 2000; Ashri 2007). Sesame phyllody is a very important disease especially in tropical and subtropical areas of the world where it causes significant economic losses (Sertkaya et al. 2007; Rao et al. 2015).
In recent years, phyllody has been produced great losses to the sesame farmers and it is one of the main reasons for the reported reduction of its cultivated areas (Esmailzadeh Hosseini et al. 2015a). The disease was reported for the first time in Burma (Myanmar) (McGibbon 1924), and today, it has been reported in many parts of the world. Phytoplasmas infecting sesame plants were classified in 16SrI group subgroup -B, 16SrII subgroups -A, -C, and -D, 16SrVI subgroup -A and 16SrIX subgroup -C, and different leafhopper species were reported as vectors of the disease (Table 1).
Sesame is one of the oldest plants grown in Iran, where its cultivation started around 2000 BC; currently, it is used as food and for medical purposes (Hue 1996). In Iran, total reported yield of sesame seeds is 28,000.00 t with 0.7 ton/ha (FAOSTAT 2013). Sesame phyllody (SP) disease was firstly observed in 1965 in Varamin (Mostafavi 1970), and subsequently in most parts of the country, especially in tropical and subtropical areas (Ibrahimi and Minasian 1975; Salehi and Izadpanah 1992; Dehgham et al. 2009; Esmailzadeh Hosseini et al. 2015b). Although the wide distribution of SP in Iran, little is known about the genetic diversity of the associated phytoplasmas; therefore, the present work reports a study about genetic diversity and vector transmission of SP-associated phytoplasmas in Iran.
Materials and methods
Plant sampling
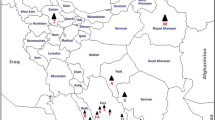
Sesame sampling areas were Fasa, Jahrom, Khafr, Mobarak Abad, Neyriz, Sarvestan and Zarghan (Fars province), Herat, Yazd, Ashkezar (Yazd province), Ardestan, and Mahabad (Isfahan province). In each area, five sesame fields were randomly selected for sampling both symptomatic plants and insects. During September, 300 sesame plants (5 plants per field) showing typical symptoms were selected for molecular analyses and vector transmission studies. Plants used in the latter study were grown from seed in an insect-proof greenhouse, every 2 weeks sprayed with Metasystox insecticide. Plastic cages were used for maintenance of leafhoppers on potted plants during the transmission trials.
Insect transmission trials
From each sesame field, insects were collected two times (June and September) during the growing season by using a D-vac aspirator. Among collected leafhoppers, Circulifer haematoceps (Mulsant and Rey 1855) and Orosious albicinctus Distant, 1918 Orosius x Orosius, two known SP vectors in Iran, were separated to test their ability to transmit SP phytoplasma strains. These specimens were identified by comparison to the voucher specimen identified previously by the British Museum (UK). Specimens of C. haematoceps and O. albicinctus randomly selected from each area (10 specimens of each species per area) were separately tested for phytoplasma presence using nested PCR assay. For transmission trials, non-inoculative and inoculative colonies of C. haematoceps and O. albicinctus leafhoppers were allowed to grow in an insect-proof greenhouse (no. 1), while healthy test plants (sesame, sugar beet, and periwinkle) were grown in a separate insect-proof greenhouse (no. 2). Non-inoculative colonies of C. haematoceps and O. albicinctus were separately established by transferring single-fertilized females on healthy sugar beet plants for egg deposition and subsequent hatching. Non-inoculative colonies and sugar beet plants were frequently monitored by nested PCR for phytoplasma presence. Inoculative colonies were developed by transferring adult C. haematoceps and O. albicinctus from non-inoculative colonies to sesame plants (infected by different SP phytoplasma strains and maintained in greenhouse no.2). In the time of inoculation, caged healthy sesame and periwinkle plants (free of insects) were brought to greenhouse no. 1 and inoculative leafhoppers (200 insects per species) were transferred on healthy plants (10 insects per plant) under the cages. The inoculation feeding time on each plant species was 10 days. After the inoculation access period (IAP), the insects under the cages were killed using Metasystox insecticide, cages were removed, and test plants (in pots) were transferred to greenhouse no. 2 (which was insect-free and insect-proof) for monitoring disease symptoms development and sampling for PCR assays. Fifty non-inoculative specimens of each leafhopper species fed on five plants of each species were used as negative controls. Three months post-inoculation, plants were molecularly tested for phytoplasma presence.
DNA extraction
Total DNA was extracted from 0.2-g midrib tissue of naturally SP-infected sesame and symptomatic experimentally insect-inoculated sesame and periwinkle plants using Zhang et al. (1998) procedure. Total nucleic acids were extracted from the field-collected leafhoppers by the Doyle and Doyle (1990) method. Total DNA extracted from symptomless sesame and periwinkle plants and insect samples from healthy colonies were used as negative controls. Positive control was a symptomatic periwinkle plant infected with Fars (Iran) alfalfa witches’ broom phytoplasmas (Salehi et al. 2005).
PCR amplification of 16S rRNA gene
Total DNA samples were tested for phytoplasma presence using primer pair P1/P7 (Deng and Hiruki 1991; Schneider et al. 1995) followed by R16F2n/R16R2 (Gundersen and Lee 1996). Primer pair P1/P7 amplifies a 1.8 kbp fragment of the ribosomal operon which encloses the 16S rRNA gene, the 16S–23S intergenic spacer region (SR) and a portion of the 5′ region of the 23S rRNA gene and R16F2n/R16R2 primer pair that amplifies 1.25 kbp of 16S rRNA gene. PCR conditions were as described previously (Salehi et al. 2011). The amplifications were carried out in a programmable thermocycler (Bio-Rad, USA); PCR products were electrophoresed in 1 % agarose gels in TAE buffer and visualized with a UV transilluminator following ethidium bromide staining. The molecular weight of the PCR products was estimated by comparison with 100-bp DNA ladder (Fermentas, Vilnius, Lithuania).
Nucleotide sequencing and analysis
Five nested PCR products of samples from each surveyed areas in Fars province were ligated onto pTZ57R⁄T vector and cloned into Escherichia coli DH5a cells using InsT ⁄ A cloneM PCR Product Cloning Kit (Fermentas, Vilnius, Lithuania) according to manufacturer instructions. The presence of the correct insert was confirmed by restriction endonuclease analysis using EcoR1 and Pst1 enzymes. Plasmid DNA from cultures of recombinant colonies was purified using GF-1 PCR Clean-Up Kit (Vivantis, Malysia, HQ). Sequencing was performed by Macrogen (South Korea) on both strands by using M13 (−21) forward (5′-TGTAAAACGACGGCCAGT-3′) and M13 (−29) reverse (5′-CAGGAAACAGCTATGACC-3′) primers (BioNeer, DNA sequencing service, S. Korea). The Yazd and Isfahan SP strains were directly sequenced using nested PCR products obtained with P1/P7 and R16mF2/R16mR2, and after assembling, the resulting sequences were trimmed to the R16F2n/R16R2 fragment (1250 bp). A database search of homologous sequences was then performed by Blast analyses at the National Center for Biotechnology Information (NCBI).
Virtual RFLP analysis
Virtual RFLP analysis using iPhyClassifier (Zhao et al. 2009) was used to determine subgroup affiliation of SP phytoplasmas. Each aligned DNA fragment was digested in silico with 17 distinct restriction enzymes: AluI, BamHI, BfaI, BstUI (ThaI), DraI, EcoRI, HaeIII, HhaI, HinfI, HpaI, HpaII, KpnI, MboI (Sau3AI), MseI, RsaI, SspI, and TaqI that have been used for the phytoplasma 16S rDNA RFLP analysis (Lee et al. 1998).
Sequence homology and phylogenetic analysis
Partial 16S rDNA sequences of 12 SP phytoplasma strains from Fasa, Jahrom, Khafr, Mobarak Abad, Neyriz, Sarvestan, Zarghan (Fars province), Herat, Yazd, Ashkezar (Yazd province), Ardestan and Mahabad (Isfahan province) (present study) and seven SP phytoplasma sequences from Iranian Fars, Bushehr and Khorasan Razavi provinces, retrieved from GenBank (Table 2) were separately aligned and related phylogenetic trees and sequence homologies generated using MEGA6 software (Tamura et al. 2013). Acholeplasma laidlawii was used as an outgroup to root the tree. Bootstrapping was performed 1000 times to estimate the stability and support for the tree branches.
Results
Symptomatology
Occurrence of SP disease was observed in the all surveyed areas including Fasa, Jahrom, Khafr, Mobarak Abad, Neyriz, Sarvestan, Zarghan (Fars province), Herat, Yazd, Ashkezar (Yazd province), Ardestan and Mahabad (Isfahan province). Disease incidence rate in some sesame fields in Zarghan and Ashkezar approached 100 %. The prevalent symptoms of disease were severe little leaf, internode shortening, witches’ broom (Fig. 1a), flower virescence, phyllody, proliferation and sterility (Fig. 1c), cracking of seed capsules (Fig. 1d), germination of seeds in capsules, dwarfing, and yellowing.
DNA amplification
After 35 cycles, DNA fragments of approximately 1.8 and 1.2 kbp were amplified in direct and nested PCR, respectively, from 300 naturally SP-affected sesame plants (25 samples per area) from 12 areas in Fars, Yazd, and Isfahan provinces and from all the experimentally inoculated symptomatic sesame and periwinkle plants. Neither control plants nor leafhopper samples tested positive in direct PCR. Forty-three out of 120 samples of C. haematoceps from all surveyed areas and 29 out of 120 samples of O. albicinctus from Mobarak Abad, Fasa, Jahrom, Khafr, Yazd, Ashkezar, Mahabad, and Ardestan were positive in nested PCR assay.
Nucleotide sequence analyses
Sixty R16F2n/R16R2 amplified fragments of SP phytoplasmas from 12 areas in three provinces were sequenced. In each area, one example of identical sequences was submitted to the GenBank; totally, 12 sequences from nine areas including Fasa (two sequences), Jahrom (three sequences), Khafr, Mobarak Abad, Neyriz, Sarvestan, Zarghan, Yazd, and Mahabad (one sequence per area) were submitted under accession numbers (Acc. nos.): KT265703, KT265697, KT265698, KT265699, KT265700, KT265706, KT265705, KT265701, KT265704, KT265702, KT923671, and KU297203, respectively. BLAST search using these sequences and similar sequences (1.2 kbp of ribosomal RNA operon) retrieved from GenBank showed that three SP phytoplasma strains from Jahrom (Jahrom1, Jahrom2, Jahrom4) in addition to one strain from each of Fasa (Fasa3), Neyriz, Bushehr1, and Khorasan Rasavi1 blast with 16SrVI group phytoplasmas, while Darab, Fasa1, Sarvestan, Zarghan, Khorasan Razavi2, and Sabzevar blast with 16SrIX group phytoplasmas, Bushehr2, Jahrom, Mobarak Abad, Khafr, Yazd, and Mahabad strains with 16SrII group phytoplasmas.
Virtual RFLP analysis
Results of virtual RFLP analyses of the 1.2 kbp 16S rDNA of sequences from strains Jahrom1, Jahrom2, Jahrom4, Neyriz, Khorasan Razavi1, Bushehr1, and Fasa3 were referable to those of ‘Candidatus Phytoplasma trifolii’ (Genbank Acc. no. AY390261), representative of 16SrVI-A subgroup; however, while the sequences of strains Khorasan Razavi1 and Bushehr1 were identical to the one of ‘Ca. P. trifolii,’ sequences of Jahrom1, Jahrom2, Jahrom4, Neyriz, and Fasa3 were differentiable from it in DraI, EcoRI, MseI, and RsaI restriction profiles. Based on the same enzymes, the sequence of Fasa 3 also was differentiable from the others and from ‘Ca. P. trifolii’. Zarghan, Fasa1, Darab, Sarvestan, Khorasan Razavi2, and Sabzevar strains produced profiles identical to the one of Knautia arvensis phyllody phytoplasma (GenBank Acc. no. EF186823), classified in 16SrIX-C subgroup (Lee et al. 2012). Sequences of strains Khafr, Yazd, Mahabad, Bushehr2, and Jahrom showed identical patterns to the one of ‘Ca. P. australasia’ (GenBank Acc. no. Y10096), a 16SrII-D phytoplasma (Lee et al. 2010), while the sequence of Mobarakabad strain was distinguishable from the one of ‘Ca. P. austaliense’ by HinfI virtual restriction profile (Fig. 2).
Virtual RFLP profiles generated with program iPhyClassifier from in silico digestion of the R16F2n/R16R2 DNA fragments of the sesame phyllody phytoplasmas and of selected representatives of subgroups of 16SrVI-A, -B, and -C, 16SrIX-D and -E and 16SrII-F and -G. Profiles are in a, ‘Ca. P. trifolii’ (Acc. no. AY39026) (16SrVI-A); in b, Jahrom1, Jahrom2, Jahrom4, and Neyriz1, Khorasan Razavi1; in c, Fasa 3; in d, Knautia arvensis phyllody phytoplasma strain KAP (Acc. no. EF186823) (16SrIX-C); in e, profiles of Darab, Fasa1, Sarvestan, Zarghan, Khorasan Razavi2, Sabzevar; in f; ‘Ca. P. austaliense’ (Acc. no. Y1009) (16SrII-D) and profiles of Bushehr, Fasa1, Jahrom5, Esfahan, Yazd strains; in g, Mobarak Abad strain. Boxes show the patterns of differentiating enzymes; MW, virtual ØX174 HaeIII digested size marker
Phylogenetic analyses
The phylogenetic trees generated by the analysis of 1.2 kbp 16S rRNA genes belonging to representatives or subgroups of 16SrI, 16SrII, 16SrIII, 16SrIV, 16SrVI, 16SrIX, and 16SrXII phytoplasma groups, and SP phytoplasma strains from Iran showed that Jahrom1, Jahrom2, Jahrom4, Neyrizi, and Fasa3 (Fars province), Bushehr1 (Bushehr province), and Khorasan Razavi1 (Khorasan Razavi province) clustered with 16SrVI; Zarghan, Fasa1, Sarvestan, Darab (Fars province), Khorasan Razavi2, and Sabzevar (Khorasan Rasavi province) with 16SrIX while Mobarak Abad, Khafr, Jahrom (Fars province), Yazd (Yazd province), Mahabad (Isfahan province), and Bushehr2 (Bushehr province) clustered with16SrII phytoplasma group (Fig. 3). Phylogenetic trees and percent sequence homology (Table 3) confirmed the results of virtual RFLP analyses. In these analyses, Jahrom1, Jahrom2, Jahrom4, Neyrizi, Fasa3, Bushehr, and Khorasan Razavi1 strains were closer to vinca virescence phytoplasma (Genbank Acc. no. AY500817), enclosed in the 16SrVI-A subgroup (Lee et al. 2010) than other subgroups of 16SrVI group. These strains had maximum homology (99.2–99.8 %) with “Ca. P. trifolii’ (GenBank Acc. no. AY390261), representative of 16SrVI-A subgroup. Zarghan, Fasa1, Sarvestan, Darab, Khorasan Razavi2, and Sabzevar SP strains clustered with Knautia arvensis phyllody phytoplasma (Genbank Acc. no. EF18682) representative of 16SrIX-C subgroup (Lee et al. 2012) showing maximum homology (99.8–99.9 %) with this strain. Mobarak Abad, Khafr, Jahrom, Bushehr2, Yazd, and Mahabad clustered with ‘Ca. P. australiense’, a representative of 16SrII-D subgroup with sequence homology of 99.9–100 %. Based on these results, 16SrVI-A strains were identified in Fars, Bushehr, and Korasan Razavi provinces, extending from south to north Iran; 16SrII-D strains were present in Fars, Bushehr, Isfahan, and Yazd provinces, central and southern regions, and 16SrIX in Fars, and Khorasane Razavi, central and northern regions. It appears that 16SrII-D is present only in the central regions of Iran.
Phylogenetic tree constructed by the Neighbor-Joining method using partial 16S rRNA gene sequences (1250 bp) of 48 phytoplasmas and A. laidlawii as the outgroup; ‘Ca. P.’: “Candidatus phytoplasma”; CWB cactus witches’ broom; numbers at the nodes are bootstrap (confidence) values are based on 1000 repetitions; only bootstrap values higher than 70 are reported; GenBank accession numbers for sequences are given in parentheses after the phytoplasma names, Iranian sesame phyllody phytoplasmas are in bold; bar 1 nucleotide substitution per 100 nucleotides
Vector transmission
C. haematoceps and O. albicinctus (Fig. 4) were the most abundant leafhoppers collected in the sesame fields and the only species reared in caged sesame plants. Out of 120 DNA samples of C. haematoceps 42 specimens from Fasa, Jahrom, Khafr, Mobar Ababad, Neyriz, Sarvestan, Zarghan, Yazd and Isfahan were positive in nested PCR assay. In the case of O. albicinctus, 32 samples from Jahrom, Fasa, Kafr, Mobarak, Darab, Yazd, and Isfahan were PCR positive.
In the trials for the transmission of SP strains identified in this study (Table 4), C. haematoceps leafhopper transmitted 16SrII-D, 16SrVI-A, and 16SrIX-C phytoplasmas from affected sesame plants to healthy sesame and periwinkle plants. Twenty-one out of 30 sesame seedlings and 19 out of 30 periwinkle plants inoculated with each of these phytoplasma strains showed symptoms and were positive in PCR assay. O. albicinctus only transmitted 16SrII-D phytoplasma and seven of 10 inoculated sesame and nine of 10 periwinkle plants were symptomatic and PCR positive. Neither C. haematoceps nor O. albicinctus from non-inoculative colonies were able to transmit SP phytoplasma strains.
Disease symptoms in vector inoculated sesame plants were similar to those of naturally diseased plants, while same age periwinkle plants inoculated at the same time with different SP phytoplasma strains show differentiable symptomatology. Little leaf, internode shortening, and stunting in periwinkle plants inoculated with 16SrIX-C, 16SrVI-A, and 16SrII-D strains were severe, moderate, and almost absent, respectively. More yellowing was seen in 16SrVI-A strain inoculated periwinkles than in those inoculated with 16SrIX-C and 16SrII-D strains (Fig. 5).
Reaction of periwinkle plants 2 months old to the insect vector inoculation carried out in the same time with different SP phytoplasma strains, 130 days after the beginning of transmission trials. a Severe little leaf, internode shortening and stunting in a periwinkle plant inoculated with a 16SrIX phytoplasmas. b Yellowing moderate little leaf, internode shortening and stunting in a periwinkle plant inoculated with a 16SrVI-A phytoplasmas. c Virescence, phyllody, and nearly normal leaves and internodes in a periwinkle plant inoculated with 16SrII-D phytoplasmas. d Two healthy seed grown periwinkle plants
Discussion
Occurrence of sesame phyllody phytoplasma disease was reported from different sesame growing areas in Iran (Salehi and Izadpanah 1992; Dehghan et al. 2009; Esmailzadeh Hosseini et al. 2015b), but a comprehensive studies on diversity and classification of sesame phyllody strains was never performed. In the present work, molecular identification of phytoplasmas associated with sesame phyllody disease and ability of C. haematoceps and O. albicinctus to vector different sesame phyllody phytoplasma strains is reported. Based on Blast search using partial 16S rRNA gene, SP phytoplasma strains from 21 areas in five provinces are shown to belong to three 16Sr phytoplasma groups including 16SrII, 16SrVI, and 16SrIX. Collectively, virtual RFLP, phylogenetic analyses, and percent sequence homology showed that all 16SrIX phytoplasma strains were related to -C subgroup, while those classified in the 16SrVI and 16SrII groups were identical or very similar to phytoplasmas in 16SrVI-A and 16SrII-D subgroups, respectively. Different reaction of periwinkle plants to SP phytoplasma strains confirmed the genetic diversity of SP disease agents in Iran.
The 16SrIX-C subgroup phytoplasmas have been previously detected in almond (Salehi et al. 2006) and eggplant (Tohidi et al. 2015) in Fars and Hormozgan provinces of Iran; however, other subgroups of 16SrIX group including -B in almond and GF-677 trees (Motamedi et al. 2016), -D in lettuce (Salehi et al. 2007a; Lee et al. 2012) and -E in tomato (Jamshidi et al. 2014) were also reported. In Iran, in addition to sesame, tomato (Jamshidi et al. 2014) was reported as host of 16SrVI-A phytoplasmas. Phytoplasmas associated with safflower phyllody (Salehi et al. 2009), cabbage yellows (Salehi et al. 2007b), greenhouse cucumber phyllody (Esmailzadeh Hosseini et al. 2015c), and alfalfa witches’ broom (Esmailzadeh Hosseini et al. 2015b) are other three 16SrVI group phytoplasma reported in the country, but their subgroup affiliation was not determined. Other reported hosts of 16SrII-D phytoplasmas in Iran are squash (Salehi et al. 2015b), tomato (Salehi et al. 2014), sunflower (Salehi et al. 2015a), and alfalfa (Esmailzadeh Hosseini et al. 2015a).
C. haematoceps was previously reported in Iran as vector of sesame phyllody disease (Salehi, and Izadpanah 1992); however, at that time, these sesame phyllody-associated phytoplasmas were not characterized while in the present study, it is shown that the three phytoplasmas associated with sesame phyllody disease detected in Iran were all transmitted by this leafhopper. While the transmission of phytoplasmas related to 16SrI and 16SrVI groups with C. haematoceps was reported from Iran (Salehi et al. 2007b, 2011), to our knowledge, this is the first report of transmission of 16SrII and 16SrIX group phytoplasmas by this insect vector.
In all surveyed areas also O. albicinctus was collected but only 16SrII-D phytoplasmas associated with sesame phyllody were transmitted by this species. This is in agreement with reports from Turkey demonstrating the transmission of only 16SrII-D phytoplasma by this insect vector (Ikten et al. 2014). It seems that in sesame fields, O. albicinctus is vector of 16SrII phytoplasmas and this and the other sesame phyllody-associated phytoplasmas are vectored by C. haematoceps or other, still not known, insect vectors.
In the surveyed sesame fields Hishimonus phycitis, vector of sesame phyllody in India (Nabi et al. 2015b) was not collected.
Based on of the present study, it seems that sesame fields are important sources of 16SrVI-A, 16SrIX-C, and 16SrII-D phytoplasmas and are also hosting their insect vectors able to transmit these phytoplasmas to other different crops. However, the transmission of sesame phyllody phytoplasmas to other crops still is under investigation.
References
Akhtar KP, Dickinson M, Sarwar G, Jamil FF, Haq MA (2008) First report on the association of a 16SrII phytoplasma with sesame phyllody in Pakistan. Plant Pathol 57:771
Akhtar KP, Sarwar G, Dickinson M, Ahmad M, Haq MA, Hameed S, Iqbal J (2009) Sesame phyllody disease: symptomatology, etiology and transmission in Pakistan. Turk J Agric For 5:477–486
Al-Sakeiti MA, Al-Subhi AM, Al-Saady NA, Deadman ML (2005) First report of witches’ broom disease of sesame (Sesamum indicum) in Oman. Plant Dis 89:530
Ashri A (2007) Sesame (Sesamum indicum L.). In: Singh RJ (ed) Genetics resources, chromosome engineering, and crop improvement, Vol.4, oilseed crops. CRC Press, Boca Raton
Bertaccini A, Duduk B (2009) Phytoplasma and phytoplasma diseases: a review of recent research. Phytopath Medit 48:355–378
Bertaccini A, Duduk B, Paltrinieri S, Contaldo N (2014) Phytoplasmas and phytoplasma diseases: a severe threat to agriculture. Am J Pl Sci 5:1763–1788
Catal M, Ikten C, Yol E, Ustun R, Uzun B (2013) First report of 16SrIX group phytoplasma associated with sesame phyllody in Turkey. Plant Dis 97:835
Choopanya D (1973) Mycoplasma like bodies associated with sesamum phyllody in Thailand. Phytopathology 63:1536–1537
Dehghan A, Salehi M, Khajazadeh Y, Taghizadeh M (2009) Studies on sesame phyllody and effect of sowing date and insecticide spray on disease control in Khuzestan. Appl Entomol Phytopath 77:23–36
Deng SJ, Hiruki C (1991) Amplification of 16S ribosomal- RNA genes from culturable and non culturable mollicutes. J Microbiol Meth 14:53–61
Desmits M, Laboucheix J (1974) Relationship between cotton phyllody and a similar disease of sesame. F.A.O. Plant Prot Bull 22:19–20
Doyle JJ, Doyle JI (1990) Isolation of DNA from fresh plant tissue. Focus 12:13–15
Esmailzadeh Hosseini SA, Khodakaramian G, Salehi M, Fani SR, Mirchenari SR, Elham S (2015a) Incidence, distribution and economic importance of alfalfa witches’ broom disease in Sistan-Baluchestan (Iran) and characterization of associated phytoplasma. Phytopath Moll 5:1–7
Esmailzadeh Hosseini SA, Salehi M, Khodakaramian G, Bolok Yazdi HR, Salehi M, Jafari Nodooshan A, Jadidi O, Bertaccini A (2015b) Status of sesame phyllody and its control methods in Yazd, Iran. Phytopath Moll 5(1-Suppl):S119–S120
Esmailzadeh Hosseini SA, Salehi M, Mirchenari SM, Tarizeh D, Gholampoor H (2015c) First report of a 16SrVI group related phytoplasma associated with cucumber phyllody in a greenhouse in Iran. New Dis Rep 32:5
Esmailzadeh Hosseini SA, Mirzaie A, Jafari-Nodooshan A, Rahimian H (2007) The first report of transmission of a phytoplasma associated with sesame phyllody by Orosius albicinctus in Iran. Austral Plant Dis Notes 2:33–34
Faostat (2013) Food and agriculture organization corporate statistical database. [online] URL: http://faostat3.fao.org
Gundersen DE, Lee I-M (1996) Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopath Medit 35:144–151
Hue, YH (1996) Bailey’s industrial oil and products. A Wiley Interscience Publication. 457 pp.
Ibrahimi, A, Minasian, V (1975) A list of diseases of cultivated and wild plants of Khusestan. Gondishapoor University 28 pp.
Ikten C, Catal M, Yol E, Ustun R, Furat S, Toker C, Uzun B (2014) Molecular identification, characterization and transmission of phytoplasmas associated with sesame phyllody in Turkey. Eur J Plant Pathol 139:217–229
Jamshidi E, Jafarpour B, Rouhani H, Salehi M (2014) Association of members of clover proliferation (16SrVI) and pigeon pea witches’ broom (16SrIX) phytoplasma groups with tomato big bud disease in Iran. Iranian. J Plant Pathol 50:77–89
Kersting U (1993) Symptomatology, etiology and transmission of sesame phyllody in Turkey. J Turk Phytopathol 22:47–54
Khan AJ, Bottner K, Al-Saadi N, Al-Subhi AM, Lee I-M (2007a) Identification of phytoplasma associated with witches’ broom and virescence diseases of sesame in Oman. Bull Insectol 60:133–134
Khan MS, Raj SK, Snehi SK (2007b) First report of molecular identification of ‘Candidatus Phytoplasma asteris’ affecting sesame (Sesamum indicum) cultivation in India. J Pl Pathol 89:301–305
Klein M (1977) Sesame phyllody in Israel. Phytopathology 88:165–171
Kolte, SJ 1985. Diseases of annual edible oil seed crops. Vol. II. CRS Press 135 pp
Lee I-M, Gundersen-Rindal DE, Davis RE, Bartoszyk IM (1998) Revised classification scheme of phytoplasmas based on RFLP analyses of 16SrRNA and ribosomal protein gene sequences. Int J Syst Bacteriol 48:1153–1169
Lee I-M, Davis RE, Gundersen-Rindal DE (2000) Phytoplasma: phytopathogenic mollicutes. Annu Rev Microbiol 54:221–255
Lee I-M, Gundersen-Rindal D, Davis RE, Bottner KD, Marcone C, Seemüller E (2004) Candidatus Phytoplasma asteris’, a novel taxon associated with aster yellows and related diseases. Int J Syst Bacteriol 54:1037–1048
Lee I-M, Bottner KD, Zhao Y, Davis RE, Harrison NA (2010) Phylogenetic analysis and delineation of phytoplasmas based on SecY gene sequences. Int J Syst Evol Microbiol 60:2887–2897
Lee I-M, Bottner-Parker KD, Zhao Y, Bertaccini A, Davis RE (2012) Differentiation and classification of phytoplasmas in the pigeon pea witches’-broom group (16SrIX): an update based on multiple gene sequence analysis. Int J Syst Evol Microbiol 62:2279–2285
Madhupriya, Rao GP, Kumar A, Baranwal VK (2015) Classification of sesame phytoplasma strain in India at 16Sr subgroup level. J Plant Pathol 3:523–528
Manjunatha N, Prameela HA, Rangaswamy KT, Palanna KB, Wickramaaracgchi WART (2012) Phyllody phytoplasma infecting sesame in South India. Phytopath Moll 2:29–32
McGibbon, TD (1924) Annual report of the Economic Botanist, Burma for the year ending 30th June, 5 pp.
Mostafavi M (1970) Green flowering of sesamum. Iran J Plant Path 5:36–37
Motamedi E, Salehi M, Sabbagh SK, Salari M (2016) Differentiation and classification of phytoplasmas associated with almond and GF-677 witches’ broom diseases using rRNA and rp genes. J Phytopathol 164:185–192
Nabi S, Madhupriya, Dubey DK, Rao GP, Baranwal VK, Sharma P (2015a) Characterization of phytoplasmas associated with sesame (Sesamum indicum) phyllody disease in North India utilizing multilocus genes and RFLP analysis. Indian Phytopathol 68:112–119
Nabi S, Madhupriya, Kumar Dubey D, Rao GP, Baranwal VK, Sharma P (2015b) Molecular characterization of ‘Candidatus Phytoplasma asteris’ subgroup I-B associated with sesame phyllody disease and identification of its natural vector and weed reservoir in India. Austral Plant Pathol 44:289–247
Nakashima K, Hayashi T, Chaleeprom W, Wongkaew P, Sirithorn P (1995) Detection of DNA of phytoplasmas associated with phyllody disease of sesame in Thailand. Ann Phytopathol Soc Jpn 61:519–528
Rao GP, Nabi SU, Madhupriya (2015) Overview on a century progress of research on sesame phyllody disease. Phytopath Moll 5:74–83
Salehi M, Izadpanah K (1992) Etiology and transmission of sesame phylloy in Iran. J Phytopathol 135:37–47
Salehi M, Heydarnejad J, Izadpanah K (2005) Molecular characterization and grouping of 35 phytoplasmas from central and southern provinces of Iran. Iran J Plant Pathol 41:62–64
Salehi M, Izadpanah K, Heydarnejad J (2006) Characterization of a new almond witches’ broom phytoplasma in Iran. J Phytopathol 154:386–391
Salehi M, Izadpanah K, Nejat N, Siampour M (2007a) Partial characterization of phytoplasmas associated with lettuce and wild lettuce phyllodies in Iran. Plant Pathol 65:669–670
Salehi M, Izadpanah K, Siampour M (2007b) Characterization of a phytoplasma associated with cabbage yellows in Iran. Plant Dis 91:625–630
Salehi M, Izadpanah K, Siampour M, Firuz R, Salehi E (2009) Molecular characterization and transmission of safflower phyllody phytoplasma in Iran. J Plant Pathol 91:453–458
Salehi M, Izadpanah K, Siampour M (2011) Occurrence, molecular characterization and vector transmission of a phytoplasma associated with rapeseed phyllody in Iran. J Phytopathol 159:100–105
Salehi E, Salehi M, Taghavi SM, Izadpanah K (2014) A 16SrII-D phytoplasma strain associated with tomato witches’ broom in Bushehr province, Iran. J Crop Protect 3:377–388
Salehi M, Esmailzadeh SA, Salehi E (2015a) Characterisation of a phytoplasma associated with sunflower phyllody in Fars, Isfahan and Yazd provinces of Iran. New Dis Repr 31:6
Salehi M, Siampour M, Esmailzadeh Hosseini SA, Bertaccini A (2015b) Characterization and vector identification of phytoplasmas associated with cucumber and squash phyllody in Iran. Bull Insectol 68:311–319
Schneider B, Seemueller E, Smart CD, Kirkpatrick BC (1995) Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasmas. In: Razin S, Tully JG (eds) In: molecular and diagnostic procedures in mycoplasmology, vol Vol. 1. Academic Press, San Diego, pp. 369–380
Seemüller E, Marcone C, Lauer U, Ragozzino A, Göschl M (1998) Current status of molecular classification of the phytoplasmas. J Plant Pathol 80:3–26
Sertkaya G, Martini M, Musetti R, Osler R (2007) Detection and molecular characterization of phytoplasmas infecting sesame and solanaceous crops in Turkey. Bull Insectol 60:141–142
Tamimi KM, Fattah FA, Al-Hamdany MA (1989) Shoot apex fasciation in Sesamum indicum associated with mycoplasma-like organisms. Plant Pathol 38:300–304
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tohidi Z, Salehi M, Ghasemi S, Khanchezar A, Shahamiri M (2015) Association of a 16SrIX-C phytoplasma with eggplant phyllody in Iran. J Crop protect 4:247–256
Tseng YW, Deng WL, Chang CJ, Huang JW, Jan FJ (2014) First report on the association of a 16SrII-A phytoplasma with sesame (Sesamum indicum) exhibiting abnormal stem curling and phyllody in Taiwan. Plant Dis 98:990
Weiss EA (2000) Oilseed crops, 2nd edn. Blackwell Science, Oxford 131-164 pp
Win NKK, Back C, Jung HY (2010) Phyllody phytoplasma infecting sesame in Myanmar belongs to group 16SrI and subgroup 16SrI-B. Trop Pl Pathol 35:310–313
Zhang YP, Uyemoto JK, Kirkpatrick BC (1998) A small-scale procedure for extracting nucleic acids from woody plants infected with various phytoplasmas for PCR assay. J Virol Methods 71:45–50
Zhao Y, Wei W, Lee I-M, Shao J, Suo X, Davis RE (2009) Construction of an interactive online phytoplasma classification tool, iPhyClassifier, and its application in analysis of the peach X-disease phytoplasma group (16SrIII. Int J Syst Evol Microbiol 59:2582–2593
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salehi, M., Esmailzadeh Hosseini, S.A., Salehi, E. et al. Genetic diversity and vector transmission of phytoplasmas associated with sesame phyllody in Iran. Folia Microbiol 62, 99–109 (2017). https://doi.org/10.1007/s12223-016-0476-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-016-0476-5