Abstract
The yeast Saccharomyces cerevisiae accumulates the high levels of inorganic polyphosphates (polyPs) performing in the cells numerous functions, including phosphate and energy storage. The effects of vacuolar membrane ATPase (V-ATPase) dysfunction were studied on polyP accumulation under short-term cultivation in the Pi–excess media after Pi starvation. The addition of bafilomycin A1, a specific inhibitor of V-ATPase, to the medium with glucose resulted in strong inhibition of the synthesis of long-chain polyP and in substantial suppression of short-chain polyP. The addition of bafilomycin to the medium with ethanol resulted in decreased accumulation of high-molecular polyP, while the accumulation of low-molecular polyP was not affected. The levels of polyP synthesis in the mutant strain with a deletion in the vma2 gene encoding a V-ATPase subunit were significantly lower than in the parent strain in the media with glucose and with ethanol. The synthesis of the longest chain polyP was not observed in the mutant cells. The synthesis of only the low-polymer acid-soluble polyP fraction occurred in the cells of the mutant strain. However, the level of polyP1 was nearly tenfold lower than compared to the cells of the parent strain. Both bafilomycin A1 and the mutation in vacuolar ATPase subunit vma2 lead to a considerable decrease of cellular polyP accumulation. Thus, the defects in ΔμH+ formation on the vacuolar membrane resulted in the decrease of polyP biosynthesis in S. cerevisiae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-polymeric inorganic polyphosphates (polyPs) are linear polymers containing a few to several hundred orthophosphate residues and performing numerous functions in the cells, including phosphate and energy storage, sequestration of cations, formation of membrane channels, involvement in cell envelope formation and function, gene activity control, regulation of enzyme activities, stress response, and stationary phase adaptation (Kulaev et al. 2004; Rao et al. 2009; Achbergerová and Nahálka 2011; Orell et al. 2012). The yeast Saccharomyces cerevisiae is a good model for investigation of the metabolism of these biologically active polyanions. However, polyP biosynthesis in these microorganisms has been little studied. S. cerevisiae lacks polyphosphate kinase, the enzyme responsible for the synthesis of the most part of polyP in bacteria (Rao et al. 2009). Only two enzymes of polyP synthesis in S. cerevisiae are known at present: dolychylpyrophosphate: polyphosphate transferase responsible for the synthesis of a minor part of yeast cell polyP localized mainly in the cell wall (Kulaev et al. 1987) and Vtc4 protein, a transport chaperone localized in the vacuolar membrane (Hothorn et al. 2009).
The important role of vacuoles in polyP metabolism was postulated rather long ago (Durr et al. 1979; Ogawa et al. 2000). However, it is still little understood. The vacuolar membrane ATPase (V-ATPase) is important for many cellular processes in yeasts (Bouillet et al. 2012; Zhang and Rao 2012; Marshall et al. 2012). The mutation in the vma2 gene encoding the protein B of subunit V1 of V-ATPase (Milgrom et al. 2007) decreases the content of polyP in S. cerevisiae during cultivation on glucose (Tomaschevsky et al. 2010). This result suggests an important role of vacuolar membrane energization in polyP biosynthesis in S. cerevisiae. However, the processes of polyP accumulation in yeast cells under cultivation on different carbon sources are characterized by distinctive features. Glucose and ethanol are indicative carbon sources which differ in main mechanism of energy supply in S. cerevisiae cells: under glucose consumption, it is glycolysis, while under ethanol consumption, it is oxidative phosphorylation. Previously, we have revealed that the accumulation of long-chain and short-chain polyPs increases and decreases, respectively, under cultivation on ethanol (Vagabov et al. 2008). The question arises whether this effect is associated with the changes in polyP biosynthetic pathways under cultivation on ethanol compared to cultivation on glucose.
The goal of this work was to find out the effects of V-ATPase dysfunction on polyP biosynthesis in S. cerevisiae under cultivation in media with glucose and ethanol.
Materials and methods
Research objects and culture conditions
The objects of research were the wild strain of S. cerevisiae VKM Y-1173 (All-Russian Collection of Microorganisms, Russian Academy of Sciences), the mutant strain BY 4741 vma2Δ (a deletion in the vma2 gene), and its parent strain BY 4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0). The strains BY 4741 and BY 4741 vma2Δ were kindly provided by Dr. P. Kane, SUNY Upstate Medical University, USA (Milgrom et al. 2007). The BY 4741 and BY 4741 vma2Δ yeast cultures were maintained on YPD agar slants; the strain VKM Y-1173 was maintained on Wort agar slants.
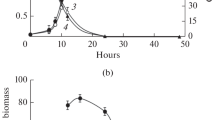
For the experiments, the yeast was cultivated in liquid phosphate-containing (+P) and phosphate-free (−P) media. The growth of the strains BY 4741 and BY 4741 vma2Δ is shown in Fig. 1. The growth of the strain VKM Y-1173 was similar to BY 4741 (data not shown). The cultures were grown in shakers at 29 °С in the flasks containing 200 ml of the liquid medium supplemented with 2 % glucose as a carbon source at 120 rpm or 50 ml of the medium with 1 % ethanol at 200 rpm.
The growth of the strains BY 4741 and BY 4741 vma2Δ of S. cerevisiae. The strains were grown in (+P) media to a culture density of 0.4–0.6 (point A). Then, the cells were precipitated at 3,000×g and washed with the (−P) medium. The cells were re-inoculated in the (−P) medium and cultivated for 9 h (strains BY 4741 and BY 4741 vma2Δ) or for 7 h (strain VKM Y-1173) (point B). After that, the cells were precipitated at 3000×g, re-inoculated in the (+P) medium, and cultivated for 1 h (strains BY 4741 and BY 4741 vma2Δ) or for 0.5 h (strain VKM Y-1173) (point C). White circle strain BY 4741, the medium with glucose; black circle BY 4741 vma2Δ, the medium with glucose; triangle strain BY 4741, the medium with ethanol; black up-pointing triangle BY 4741 vma2Δ, the medium with ethanol
The (+P) medium contained (in gram per liter): (NH4)2SO4, 5; MgSO4·7H2O, 1.025; NaCl, 0.1; CaCl2, 0.1; KH2PO4, 0.85; K2HPO4, 0.15; (NH4)2SO4·FeSO4·6H2O, 0.25 × 10−3; yeast extract, 2; and trace elements (Vagabov et al. 2008) for cultivation of the strain VKM Y-1173. For cultivation of the strains BY 4741 and BY 4741 vma2Δ, the same medium was supplemented with 60 mg/L of histidine, methionine, and uracyl and with 90 mg/L of leucine. For the cultivation of BY 4741 vma2Δ strain, the pH values of all media used were adjusted to 5.0 by adding 50 mM succinate–NaOH buffer.
The (−P) medium contained (in gram per liter): (NH4)2SO4, 5; MgSO4·7H2O, 1.025; NaCl, 0.1; CaCl2, 0.1; KCl, 0.6; (NH4)2SO4·FeSO4·6H2O, 0.25 × 10−3; phosphate-free yeast extract, 2; and trace elements. The phosphate-free yeast extract was obtained as described (Rubin 1973).
The yeast strains were grown in (+P) media to a culture concentration corresponding to 0.4–0.6 (A 530, measured in a 3.07-mm cuvette, point A in Fig. 1). Then, the cells were precipitated at 3,000×g and washed with the (−P) medium. The cells were re-inoculated in the (−P) medium and cultivated for 9 h (strains BY 4741 and BY 4741 vma2Δ) or for 7 h (strain VKM Y-1173) (point B, Fig 1). The times of cultivation in the (−P) medium were selected in preliminary experiments to obtain minimal polyP levels in the cells.
After that, the cells were precipitated at 3,000×g, re-inoculated in the (+P) medium, and cultivated for 1 h (strains BY 4741 and BY 4741 vma2Δ) or for 0.5 h (strain VKM Y-1173) (point C, Fig 1).
The strain VKM Y-1173 was cultivated for 0.5 h in the absence (control) or presence of 0.375 μmol/L bafilomycin A1. Biomass was harvested by centrifugation at 3,000×g, washed twice with cold distilled water, and used for the assay.
Polyphosphate extraction and assay
PolyP fractions were obtained and the polyP content was quantified as described earlier (Vagabov et al. 2008). The following polyP fractions were obtained: acid-soluble fractions (polyP1), salt-soluble fractions (polyP2), two alkaline-soluble fractions (polyP3 and polyP4), and a hot chlorine extract fraction (polyP5). Pi was assayed by the method of Heinonen and Lahti (1981).
Polyphosphate chain lengths were determined by electrophoresis in 20 % polyacrylamide gel prepared in 200 mmol/L Tris–borate buffer, pH 8.3, with 7 mol/L urea (Kumble and Kornberg 1996). Commercial polyphosphates with different average chain lengths (Sigma and Monsanto, USA) were used as markers.
ΔpH formation on membrane of isolated vacuoles
Vacuoles were obtained as described earlier (Lichko and Okorokov 1984). Formation of the ∆pH on membrane of isolated vacuoles was registered by 9-amino-6-chloro-2-methoxyacridine (ACMA) fluorescence quenching in a Hitachi MPF-4 microfluorimeter (Japan) as described earlier (Lichko and Okorokov, 1984). The incubation medium contained (in millimole per liter): glucitol, 100; MES, 10; ACMA, 0.125; and MgSO4, 50; and pH was adjusted to 7.2 with NaOH. The reaction was started by adding 2 mmol/L ATP and MgSO4.
ATP assay
ATP was extracted from the cells by adding dimethyl sulfoxide (0.2 ml per 25–50 mg of wet biomass). ATP was assayed with luciferin–luciferase assay kit (Sigma, USA) using a luminometer 1250 (LKB, Sweden).
All experiments were performed three times and the average values are presented. The χ 2 values were no more than 10 % in all experiments presented.
Results and discussion
Bafilomycin A1, the specific inhibitor of V-ATPase, suppresses ΔμH+ formation on the vacuolar membrane (Bowman et al. 1988). We have studied the effect of bafilomycin on polyP synthesis in the cells of S. cerevisiae VKM Y-1173. Phosphate-starved cells (Fig. 1, point B) contained low levels of polyP (Table 1). These cells were re-inoculated in a fresh medium with 9 mmol/L Pi supplemented with glucose or ethanol. The cells accumulated polyP after 0.5-h cultivation (Fig. 1, point C). Table 2 shows the levels of polyP in the fractions different in chain length. The average chain lengths of polyP were 15, 25, 65, 75, and more than 200 phosphate residues for polyP1, polyP2, polyP3, polyP4, and polyP5, respectively. These average chain lengths did not depend on the carbon source, which is in agreement with the data obtained previously for this strain (Vagabov et al. 2008), and did not vary in the presence of bafilomycin. More short-chain polyP (polyP1 and polyP2) and less long-chain polyP (polyP3, polyP4, and polyP5) were synthesized in the medium with glucose, while more long-chain polyP and less short-chain polyP were synthesized in the medium with ethanol (Table 2). The addition of bafilomycin to the medium with glucose resulted in strong inhibition of the synthesis of long-chain polyP3, polyP4, and polyP5 and substantial suppression of short-chain polyP1 and polyP2 (Table 2). The addition of bafilomycin to the medium with ethanol resulted in the decrease of the quantity of high-molecular fractions polyP3, polyP4, and polyP5, while the accumulation of low-molecular fractions polyP1 and polyP2 was not inhibited (Table 2).
The ATP level increased in the cells grown on glucose in the presence of bafilomycin (Table 3). It may be caused by the decrease of ATP consumption by V-ATPase. The enhancement of Pi level correlated with the decrease in polyP biosynthesis (Table 3). In the cells grown on ethanol, bafilomycin had no effect on Pi and ATP levels and the suppression of polyP synthesis was less pronounced. The less pronounced effect of bafilomycin on the cells cultivated in the medium with ethanol may be explained by the lower penetration of the inhibitor across the cytoplasmic membrane. The cultivation on ethanol changes the lipid composition and decreases the cytoplasmic membrane permeability (Herve et al. 1994).
Another approach to revealing the role of the ΔμH+ on the vacuolar membrane in polyP biosynthesis is to use mutants in the genes encoding V-ATPase subunits. Taking into account possible differences in polyP levels in different yeast strains, we used the parent strain BY-4741 as a control. We have observed the ATP-dependent formation of ΔpH on the vacuolar membrane of the parent strain. The ΔpH was not formed on the vacuolar membrane of the mutant strain vma2Δ (Fig. 2).
Formation of the ∆pH on membrane of isolated vacuoles (ΔF/F—the relative fluorescence quenching). The incubation medium contained (in millimole per liter): glucitol, 100; MES, 10; ACMA, 0.125; and MgSO4, 50; pH was adjusted to 7.2 with NaOH. The reaction was started by adding 2 mmol/L ATP and MgSO4. Lower curve–vacuoles from the cells of parent strain BY 4741, upper curve–vacuoles from the cells of strain BY 4741 vma2Δ
Phosphate-starved cells of the parent and mutant strains (Fig. 1, point B) contained similarly low levels of polyP (Table 1). We have compared the levels of polyP synthesis in the parent strain BY-4741 and the mutant strain BY-4741 vma2Δ during 1-h cultivation (Fig. 1 point C) in the media with glucose or ethanol. The cells of parent strain accumulated polyP in both media (Table 4). The level of polyP synthesis in the mutant strain was significantly lower than in the parent strain in the media with glucose and with ethanol (Table 4). The synthesis of the most long-chain polyP (fractions polyP4 and polsyP5) was not observed, and the synthesis of polyP3 and polyP2 was insignificant. The synthesis of only the low-polymer fraction polyP1 was observed in the cells of the mutant strain (Table 4). However, the level of polyP1 was yet nearly tenfold lower than that in the cells of the parent strain.
The Vtc4 protein, a transport chaperone localized in the vacuolar membrane, is known to be a polyphosphate synthetase (Hothorn et al. 2009). Up to now, no experimental model yielding the high level of polyP biosynthesis in cell-free extracts or preparations of vacuoles has been described. We have failed to obtain polyP synthesis in the preparation of isolated vacuoles (data not shown). So, we have used the native cells of S. cerevisiae, for which a model for the study of polyP synthesis has been developed (Vagabov et al. 2008).
Two approaches are commonly used in such studies: the specific inhibitors in a valid concentration and mutations in genes important for the process. The effect bafilomycin A1 on polyP synthesis in the cells of S. cerevisiae under glucose consumption was analyzed earlier (Trilisenko et al. 2003). Bafilomycin at concentration of 0.05 μmol/L according to reference (Bowman et al. 1988) exhibited a little effect on polyP biosynthesis. Only the level of polyP3 decreased for ∼30 % (Trilisenko et al. 2003). In our preliminary experiments, an effective concentration of bafilomycin A1 (0.375 μmol/L) was selected. It had no effect on cell growth and exopolyphosphatase activity (not shown). A pronounced inhibitory effect of bafilomycin A1 on polyP accumulation was revealed at first in this study (Table 2). The effects of bafilomycin were firstly compared under consumption of two different carbon sources.
The decrease of polyP content in mutant in the vacuolar ATPase subunit vma2 was observed earlier under cultivation on glucose (Tomaschevsky et al. 2010). This effect was confirmed under cultivation on ethanol in this work. Besides, a more adequate model for polyP biosynthesis assay was used. It should be noted that exopolyphosphatase activities in cell homogenates of the parent strain and vma2Δ strain were similar (∼10 mU/mg protein).
The disturbance of ΔμH+ on the vacuolar membrane resulted in the decrease of polyP biosynthesis in S. cerevisiae independent of the prevalence of glycolysis (cultivation in the medium with glucose) or oxidative phosphorylation (cultivation in the medium with ethanol). Probably, polyP biosynthesis by polyphosphate synthetase Vtc4 localized in the vacuolar membrane (Hothorn et al. 2009) needs ΔμH+ at this membrane.
The data obtained suggest the importance of vacuolar systems for polyP biosynthesis in S. cerevisiae. There are many indirect data suggesting the difference in metabolic pathways and cellular localization of long-chained and short-chained polyPs in yeasts (Kulaev et al. 2004). For example, short-chained polyPs are localized presumably in cytoplasm, while the long-chained polymers are localized in other organelles and cellular compartments (Lichko et al. 2006). In this study, we demonstrated that the biosynthesis of the shortest chain fraction polyP1 is less dependent on the vacuolar membrane energization. It seems that the biosynthesis of these polyPs involves not only Vtc4, but also other, yet unidentified enzymes.
References
Achbergerová L, Nahálka J (2011) Polyphosphate—an ancient energy source and active metabolic regulator. Microb Cell Fact 10:63–70
Bouillet LE, Cardoso AS, Perovano E, Pereira RR, Ribeiro EM, Trópia MJ, Fietto LG, Tisi R, Martegani E, Castro IM, Brandão RL (2012) The involvement of calcium carriers and of the vacuole in the glucose-induced calcium signaling and activation of the plasma membrane H(+)-ATPase in Saccharomyces cerevisiae cells. Cell Calcium 51:72–81
Bowman EJ, Siebers A, Altendorf K (1988) Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA 85:7972–7976
Dürr M, Ürech K, Boller T, Wiemken A, Schwencke J, Nagy M (1979) Sequestration of arginine by polyphosphate in vacuoles of yeast Saccharomyces cerevisiae. Arch Microbiol 121:169–175
Heinonen YK, Lahti RY (1981) A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem 113:313–317
Herve A, Rousseaux I, Charpentier C (1994) Relationship between ethanol tolerance, lipid composition and plasma membrane fluidity in Saccharomyces cerevisiae and Kloeckera apiculata. FEMS Microbiol Lett 124:17–20
Hothorn M, Neumann H, Lenherr ED, Wehner M, Rybin V, Hassa PO, Uttenweiler A, Reinhardt M, Schmidt A, Seiler J, Ladurner AG, Herrmann C, Scheffzek K, Mayer A (2009) Catalytic core of a membrane-associated eucaryotic polyphosphate polymerase. Science 324:513–516
Kulaev IS, Vagabov VM, Shabalin YA (1987) New data on biosynthesis of polyphosphates in yeasts. In: Torriani-Gorini A et al (eds) Phosphate metabolism and cellular regulation in microorganisms. American Society for Microbiology, Washington, pp 233–238
Kulaev IS, Vagabov VM, Kulakovskaya TV (2004) The biochemistry of inorganic polyphosphates. Wiley, Chichester
Kumble KD, Kornberg A (1996) Endopolyphosphatases for long chain polyphosphate in yeast and mammals. J Biol Chem 271:27146–27151
Lichko LP, Okorokov LA (1984) Some properties of membrane-bound, solubilized and reconstituted into liposomes H+-ATPase of vacuoles of Saccharomyces carlsbergeisis. FEBS Lett 174:233–237
Lichko L, Kulakovskaya T, Pestov N, Kulaev I (2006) Inorganic polyphosphates and exopolyphosphatases in cell compartments of the yeast Saccharomyces cerevisiae under inactivation of PPX1 and PPN1 genes. Biosci Rep 26:45–54
Marshall PA, Netzel N, Guintchev JW (2012) Assessing compensation for loss of vacuolar function in Saccharomyces cerevisiae. Can J Microbiol 58:132–144
Milgrom E, Diab H, Middleton F, Kane PM (2007) Loss of vacuolar proton-translocating ATPase activity in yeast results in chronic oxidative stress. J Biol Chem 282:7125–7136
Ogawa N, DeRisi J, Brown PO (2000) New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell 11:4309–4321
Orell A, Navarro CA, Rivero M, Aguilar JS, Jerez CA (2012) Inorganic polyphosphates in extremophiles and their possible functions. Extremophiles 16:573–583
Rao NN, Gómez-García MR, Kornberg A (2009) Inorganic polyphosphate: essential for growth and survival. Ann Rev Biochem 78:605–647
Rubin GM (1973) The nucleotide sequence of Saccharomyces cerevisiae 5.8 S ribosomal ribonucleic acid. J Biol Chem 11:3860–3875
Tomaschevsky AA, Ryasanova LP, Kulakovskaya TV, Kulaev IS (2010) Inorganic polyphosphate in the yeast Saccharomyces cerevisiae with a mutation disturbing the function of vacuolar ATPase. Biochem Mosc 75:1052–1054
Trilisenko LV, Andreeva NA, Kulakovskaya TV, Vagabov VM, Kulaev IS (2003) Effect of inhibitors on polyphosphate metabolism in the yeast Saccharomyces cerevisiae under hypercompensation conditions. Biochem Mosc 68:577–581
Vagabov VM, Trilisenko LV, Kulakovskaya TV, Kulaev IS (2008) Effect of a carbon source on polyphosphate accumulation in Saccharomyces cerevisiae. FEMS Yeast Res 8:877–882
Zhang Y, Rao R (2012) The V-ATPase as a target for antifungal drugs. Curr Protein Pept Sci 13:134–140
Acknowledgments
The work was supported by the Russian Foundation for Basic Research (grant 11-04-01498). We are thankful to E. Makeeva for the help in preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trilisenko, L., Tomashevsky, A., Kulakovskaya, T. et al. V-ATPase dysfunction suppresses polyphosphate synthesis in Saccharomyces cerevisiae . Folia Microbiol 58, 437–441 (2013). https://doi.org/10.1007/s12223-013-0226-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-013-0226-x