Abstract
Phylogenetic analysis was conducted to examine ruminal bacteria in two ruminal fractions (adherent fraction vs. liquid fraction) collected from cattle fed with two different diets: forage alone vs. forage plus concentrate. One hundred forty-four 16S rRNA gene (rrs) sequences were obtained from clone libraries constructed from the four samples. These rrs sequences were assigned to 116 different operational taxonomic units (OTUs) defined at 0.03 phylogenetic distance. Most of these OTUs could not be assigned to any known genus. The phylum Firmicutes was represented by approximately 70% of all the sequences. By comparing to the OTUs already documented in the rumen, 52 new OTUs were identified. UniFrac, SONS, and denaturing gradient gel electrophoresis analyses revealed difference in diversity between the two fractions and between the two diets. This study showed that rrs sequences recovered from small clone libraries can still help identify novel species-level OTUs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A complex microbiome consisting of bacteria, archaea, fungi, and protozoa has evolved to efficiently degrade various types of forages in the rumen (Flint 1997), and a biofilm formed on the surface of the ruminal forages is especially important to feed digestion (Cheng et al. 1977). Defining the phylogenetic diversity of ruminal microbiome, particularly the bacterial community, is intriguingly interesting to many microbiologists because it is essential to functional analysis of this microbiome. As for other microbiomes, 16S rRNA gene (rrs) sequence-based analysis is primarily used in identifying the phylogenetic diversity of ruminal bacterial community (Stahl et al. 1998). Most studies focused on the microbes recovered from rumen fluid (e.g., Tajima et al. 2000, 2007; Ozutsumi et al. 2005), though some studies examined the microbes present in separated liquid and solid fractions (Larue et al. 2005; Yu et al. 2006; Brulc et al. 2009) and on the rumen wall (Cho et al. 2006; Lukas et al. 2010). Based on a recent meta-analysis (Kim et al. 2011), the rrs sequences of rumen origin that have been archived in the RDP database represent more than 3,500 species-level operational taxonomic units (OTUs). These OTUs represent approximately 70% of the global bacterial diversity estimated to be present in the rumen. Therefore, more studies are needed to further discovery of phylogenetic diversity of ruminal bacterial communities. However, it is uncertain if typical clone library-based analysis can still identify new OTUs. In this study, we analyzed four rrs clone libraries constructed from liquid and adherent fractions collected from two cows fed with different diets (forage alone vs. forage plus concentrate), defined species-level OTUs, and compared the defined OTUs with existing ruminal OTUs reported recently (Kim et al. 2011). This study enabled us to identify many novel ruminal OTUs. The difference in phylogenetic diversity between the two fractions and the two diets was also assessed using UniFrac, shared OTUs and similarity (SONS), and DGGE analyses.
Materials and methods
Sample collection, fractionation, and DNA extraction
Whole rumen content was collected from four cannulated cows: two Jersey cattle fed with only forage composed predominantly of Timothy grass and two Holstein cattle fed with a typical dairy diet consisting of 14% alfalfa forage, 42% corn silage, 6% cottonseed, and 38% grains. The two groups of cows were fed twice daily (early morning and late afternoon) and adapted to their respective diets for more than 3 weeks before rumen sampling, which took place approximately 6 h after the morning feeding. Both the liquid fraction and the adherent fraction of each rumen digesta were separated as reported previously (Larue et al. 2005). Bacteria present in the liquid fraction (Lq) were recovered by centrifugation, while bacteria adherent to the solid digesta were recovered by using a detaching buffer (Dehority and Grubb 1980) and centrifugation. Metagenomic DNA was extracted from each of the fractionated samples as described previously (Yu and Morrison 2004a). To recover bacteria that might fail to detach, the remaining solid digesta was also subjected to DNA extraction. The resultant metagenomic DNA extracts from the adhering fraction and the solid particles were combined and designated as the adherent fraction (Ad).
DGGE analysis
Denaturing gradient gel electrophoresis (DGGE) analysis was conducted to profile and compare the bacterial communities among the four samples using the GC-357f and 519r primer set, and band patterns were analyzed using the BioNumerics program as described previously (Yu and Morrison 2004b).
Construction of rrs clone libraries
The DNA extracts were pooled based on diets and fractions, resulting in four composite samples representing the liquid fraction and the adhering fraction collected from the two cattle fed with forage alone (Lq-H and Ad-H, respectively) and from the two cattle fed with forage plus concentrate (Lq-C and Ad-C, respectively). The nearly full-length rrs gene was amplified by PCR from each composite DNA sample using universal bacterial primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1525R (5′-AAGGAGGTGWTCCARCC-3′) and cloned using a TOPO-TA cloning kit (Invitrogen, Carlsbad, CA). Three hundred and eighty-four random clones from the four libraries (96 clones per library) were subjected to screening for the presence of the insert using PCR (Yu and Mohn 2001).
Restriction fragment length polymorphism analysis, DNA sequencing, and phylogenetic analysis
To reduce sequencing redundancy of similar clones, the PCR products of the aforementioned screening were digested using both HaeIII and AluI as described previously (Larue et al. 2005). The restriction fragment length polymorphism (RFLP) patterns were compared using BioNumerics (BioSystematica, Tavistock, Devon, UK), and the clones with a unique RFLP pattern were sequenced using the 27F primer at High-Throughput Genomics Unit (University of Washington, Seattle, WA).
Low-quality sequence regions at both ends of each sequence read were trimmed off using the FinchTV program V1.4 (Geospiza, Inc., Seattle, WA). All the sequences were then subjected to chimera check using the Pintail program (Ashelford et al. 2005), and suspected chimeric sequences were excluded from further analysis. Classification, alignment, and construction of a taxonomic tree at genus level were performed using the bioinformatic programs as described previously (Kim et al. 2011). Using the Mothur program (Schloss et al. 2009), the species-level OTUs (defined at 0.03 phylogenetic distance) identified in this study were compared to all the OTUs recognized in the recent meta-analysis of global diversity of ruminal bacteria (Kim et al. 2011).
Comparison of ruminal bacterial communities among the four composite samples
Principal coordinate analysis (PCA) was conducted to examine relationship among the four composite samples using the UniFrac program (Lozupone and Knight 2005). The SONS program (Schloss and Handelsman 2006) was used to determine the number of shared OTUs across the four samples.
Nucleotide sequence accession numbers
The rrs sequences obtained in this study have been deposited in the GenBank database (JF319298 - JF319441).
Results and discussion
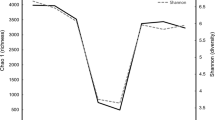
The four composite samples were first compared using DGGE to visualize the impact on the ruminal bacterial communities from the diets and the fractions. As shown in Fig. 1, the four samples shared a number of DGGE bands, but bands distinct to each sample were also evident. Clustering analysis showed that both the diets and the microenvironments (liquid vs. solid fractions) had affected the ruminal bacterial communities, with the diets having a greater impact than the fractions. These results are consistent with a previous study where both corn supplementation and fractions were found to affect the ruminal bacterial communities in sheep (Larue et al. 2005).
Clustering analysis of DGGE banding profiles based on the V3 region of 16S rRNA genes. Lq-C and Ad-C represent the liquid fraction and the adhering fraction recovered from cattle fed with forage plus concentrate, while Lq-H and Ad-H represent the liquid fraction and the adhering fraction recovered from cattle fed with forage alone. The dendrogram was constructed using the BioNumerics program
The bacterial communities in the composite samples were further examined and compared using the rrs clone libraries. From the 384 clones screened for the presence of insert, 288 clones were found to produce a unique RFLP pattern, which were then sequenced. After removing the sequences of low quality and suspected chimeric sequences, 144 high-quality rrs sequences were obtained and analyzed phylogenetically. The phyla Firmicutes, Bacteroidetes, Proteobacteria, Spirochaetes, and Verrucomicrobia were represented by 140 sequences, and the remaining four sequences could not be classified into any existing phylum (Fig. 2). Firmicutes was the most predominant phylum and accounted for 69.4% of all the 144 sequences. Approximately 60.2% of the Firmicutes sequences could not be classified into any known family or genus, with “Unclassified_Lachnospiraceae” being the most abundant (27 sequences) group. Genera Butyrivibrio, Ruminococcus, and Succiniclasticum each were represented by more than nine sequences, while the remaining genera identified were represented by no more than five sequences each. The 144 sequences were assigned to 116 species-level OTUs, of which 52 appeared to be novel when compared to the existing ruminal OTUs reported previously (Kim et al. 2011), indicating new ruminal species. This is the first study that compared OTUs identified in individual studies to those already documented. The results of this study also demonstrated that small numbers of rrs sequences could still contribute towards identifying the full phylogenetic diversity of ruminal bacteria. Future individual studies using different PCR primers, sampling methods, and DNA extraction techniques would help improve discovery of novel OTUs and eventually lead to full coverage of phylogenetic diversity (Edwards et al. 2004; Hong et al. 2009).
A taxonomic tree showing the bacterial genera represented by the 144 sequences. The lineage at phylum level is labeled: V phylum Verrucomicrobia, B phylum Bacteroidetes, S phylum Spirochaetes, P phylum Proteobacteria, F phylum Firmicutes. In total, 14 known genera were represented by 49 sequences, while the remaining 95 sequences could not be assigned to any existing genus. Numbers in parenthesis indicate the number of sequences from respective fraction
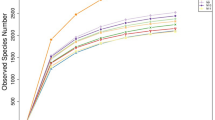
A similar number of species-level OTUs were identified from all the four samples, with Ad-H, Ad-C, Lq-H, and Lq-C fractions having 31, 29, 34, and 31 OTUs, respectively. However, based on comparison using the SONS program, three or fewer OTUs were shared between any two of the four composite samples, and no OTU was shared by all the four samples (Fig. 3). Ten of the 31 Ad-H OTUs were classified into known genera, with Ruminococcus (four OTUs) being abundant. The Ad-C fraction also included ten OTUs classified into existing genera, with each genus being represented by one OTU except Ruminococcus (two OTUs) and Succiniclasticum (two OTUs). As expected, Ruminococcus was predominant in the adherent fraction recovered from the rumen of cattle fed with only forage. However, most of the OTUs from both the Ad-H and the Ad-C fractions could not be assigned to any known genus (21 and 19 OTUs, respectively). Eight OTUs could only be classified to the order Clostridiales in the Ad-H fraction, while another seven OTUs were only assigned to the family Lachnospiraceae in the Ad-C fraction. These OTUs might represent new families or genera. The distinct distribution of these two groups of bacteria is probably attributed to dietary effect. Future studies using quantitative analysis are needed to confirm and help explain this finding.
The Lq-H and the Lq-C fractions contained eight and ten OTUs (of the 34 and 31 OTUs) assigned to known genera, respectively. The most predominant genus in the Lq-H sample was Butyrivibrio (4 OTUs), while each genus in the Lq-C sample was represented by only one of the ten OTUs except Butyrivibrio (two OTUs) and Treponema (two OTUs). It appeared that versatile Butyrivibrio species that can degrade several types of polysaccharides (hemicellulose, starch, and pectin) are abundant in the liquid fraction irrespective of the diets. The two OTUs assigned to Treponema are thought to be associated with diets rich in concentrate as described previously (Bekele et al. 2011). “Unclassified_Bacteroidetes” (seven OTUs) and “Unclassified_Clostridiales” (six OTUs) were the first and second most abundant unclassified groups in the Lq-H sample, while “Unclassified_Lachnospiraceae” (eight OTUs) was the most abundant unclassified group in the Lq-C sample. Again, “Unclassified_Clostridiales” was predominant in the adhering fraction of the forage-fed cattle, while “Unclassified_Lachnospiraceae” was predominant in both fractions of cattle fed with both forage and concentrate. As noted previously (Kim et al. 2011), “Unclassified_Clostridiales” rather than “Unclassified_Lachnospiraceae” seems to play an important role in fiber digestion.
Based on the PCA analysis, the P1 separated the bacterial communities based on the diets, while the P2 separated the ruminal bacterial communities based on the fractions (Fig. 4). This result, in general, agrees with the DGGE data and the sequence-based comparison by SONS. However, the DGGE data showed a greater similarity among the samples than the Venn diagram. This discrepancy is probably due to the limited resolution of DGGE. These results suggest significant impact on the ruminal bacterial community from diets and microenvironments (liquid vs. solid surface), although difference between the two breeds may also have some effect on the ruminal bacterial community. The effects of diets on the ruminal bacterial community have been investigated in several studies (e.g., Tajima et al. 2000; Larue et al. 2005). The partition of the bacterial populations between the solid and the liquid fractions has also been examined (Michalet-Doreau et al. 2001; Larue et al. 2005). In all the studies reported (including this present study), the same OTUs were not commonly found between diets or fractions. More studies are needed to define the core and variable bacterial communities in the rumen. As demonstrated recently (Pitta et al. 2010), studies using comprehensive analysis may help achieve such a goal.
References
Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ (2005) At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Appl Environ Microbiol 71:7724–7736
Bekele AZ, Koike S, Kobayashi Y (2011) Phylogenetic diversity and dietary association of rumen Treponema revealed using group-specific16S rRNA gene-based analysis. FEMS Microbiol Lett 316:51–60
Brulc JM, Antonopoulos DA, Miller ME, Wilson MK, Yannarell AC, Dinsdale EA, Edwards RE, Frank ED, Emerson JB, Wacklin P, Coutinho PM, Henrissat B, Nelson KE, White BA (2009) Gene-centric metagenomics of the fiber-adherent bovine rumen microbiome reveals forage specific glycoside hydrolases. Proc Natl Acad Sci U S A 106:1948–1953
Cheng KJ, Akin DE, Costerton JW (1977) Rumen bacteria: interaction with particulate dietary components and response to dietary variation. Fed Proc 36:193–197
Cho SJ, Cho KM, Shin EC, Lim WJ, Hong SY, Choi BR, Kang JM, Lee SM, Kim YH, Kim H, Yun HD (2006) 16S rDNA analysis of bacterial diversity in three fractions of cow rumen. J Microbiol Biotechn 16:92–101
Dehority BA, Grubb JA (1980) Effect of short-term chilling of rumen contents on viable bacterial numbers. Appl Environ Microbiol 39:376–381
Edwards JE, McEwan NR, Travis AJ, Wallace RJ (2004) 16S rDNA library-based analysis of ruminal bacterial diversity. Antonie Van Leeuwenhoek 86:263–281
Flint HJ (1997) The rumen microbial ecosystem—some recent developments. Trends Microbiol 5:483–488
Hong S, Bunge J, Leslin C, Jeon S, Epstein SS (2009) Polymerase chain reaction primers miss half of rRNA microbial diversity. ISME J 3:1365–1373
Kim M, Morrison M, Yu Z (2011) Status of the phylogenetic diversity census of ruminal microbiomes. FEMS Microbiol Ecol 76:49–63
Larue R, Yu Z, Parisi VA, Egan AR, Morrison M (2005) Novel microbial diversity adherent to plant biomass in the herbivore gastrointestinal tract, as revealed by ribosomal intergenic spacer analysis and rrs gene sequencing. Environ Microbiol 7:530–543
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235
Lukas F, Simunek J, Mrazek J, Kopecny J (2010) PCR-DGGE analysis of bacterial population attached to the bovine rumen wall. Folia Microbiol 55:345–348
Michalet-Doreau B, Fernandez I, Peyron C, Millet L, Fonty G (2001) Fibrolytic activities and cellulolytic bacterial community structure in the solid and liquid phases of rumen contents. Reprod Nutr Dev 41:187–194
Ozutsumi Y, Tajima K, Takenaka A, Itabashi H (2005) The effect of protozoa on the composition of rumen bacteria in cattle using 16S rRNA gene clone libraries. Biosci Biotechnol Biochem 69:499–506
Pitta DW, Pinchak E, Dowd SE, Osterstock J, Gontcharova V, Youn E, Dorton K, Yoon I, Min BR, Fulford JD, Wickersham TA, Malinowski DP (2010) Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb Ecol 59:511–522
Schloss PD, Handelsman J (2006) Introducing SONS, a tool for operational taxonomic unit-based comparisons of microbial community memberships and structures. Appl Environ Microbiol 72:6773–6779
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Stahl DA, Flesher B, Mansfield HR, Montgomery L (1998) Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol 54:1079–1084
Tajima K, Arai S, Ogata K, Nagamine T, Matsui H, Nakamura M, Aminov RI, Benno Y (2000) Rumen bacterial community transition during adaptation to high-grain diet. Anaerobe 6:273–284
Tajima K, Nonaka I, Higuchi K, Takusari N, Kurihara M, Takenaka A, Mitsumori M, Kailkawa H, Aminov RI (2007) Influence of high temperature and humidity on rumen bacterial diversity in Holstein heifers. Anaerobe 13:57–64
Yu Z, Mohn WW (2001) Bacterial diversity and community structure in an aerated lagoon revealed by ribosomal intergenic spacer analyses and 16S ribosomal DNA sequencing. Appl Environ Microbiol 67:1565–1574
Yu Z, Morrison M (2004a) Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36:808–812
Yu Z, Morrison M (2004b) Comparisons of different hypervariable regions of rrs genes for use in fingerprinting of microbial communities by PCR-denaturing gradient gel electrophoresis. Appl Environ Microbiol 70:4800–4806
Yu Z, Yu M, Morrison M (2006) Improved serial analysis of V1 ribosomal sequence tags (SARST-V1) provides a rapid, comprehensive, sequence-based characterization of bacterial diversity and community composition. Environ Microbiol 8:603–611
Acknowledgment
This study was supported by an OARDC ward (2010–007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M., Morrison, M. & Yu, Z. Phylogenetic diversity of bacterial communities in bovine rumen as affected by diets and microenvironments. Folia Microbiol 56, 453–458 (2011). https://doi.org/10.1007/s12223-011-0066-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-011-0066-5