Abstract
We report on ground-based studies in course of preparation of the experiment DCMIX2 (Diffusion coefficient in mixtures) to be performed on the International Space Station (ISS). In microgravity experiment the diffusion and thermodiffusion coefficients will be measured at six points with different compositions of the ternary mixture Toluene-Methanol-Cyclohexane. This mixture attracts attention of the researchers, as it has a miscibility gap and presumably large region with negative Soret coefficients. By using Optical Digital Interferometry we have measured the variations of refractive index Δn in the full parameter space of concentrations. The regions of stable and unstable behavior of system were determined from the time-dependent behavior of Δn. The system is hydrodynamically unstable in a wide region of compositions where Soret measurement should be done in microgravity. We present the results of the study of the compatibility of this mixture with often used sealing materials:Viton (R), Chemraz (R) and different types of Kalrez. To facilitate the expected theoretical studies we have measured density and thermal expansion in the points of the interest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many efforts are currently aimed to study thermodiffusion (Soret effect) in multicomponent mixtures. Recent advances in finding some intrinsic rules governing behaviour of mixtures on the basis of properties of single components, e.g. thermophobicity suggested by Hartmann et al. (2012), excite even more interest in such studies with the ambitious goal of expanding this regularity from binary equimolar compositions to the whole concentration coverage for certain classes of ternary mixtures.
Experiments in mixtures with more than two components impose severe practical difficulties with respect to binaries. The first steps on the way from binary to ternary systems have already been done, see Blanco et al. (2010) and Königer et al. (2010). Nevertheless, the results obtained by various methods are quite different and a method for their comparison was recently suggested by Mialdun and Shevtsova (2013).
The most often used ternary mixture used by oil industry for their numerical modeling is composed by 1,2,3,4-tetrahydronaphthalene (THN), isobutylbenzene (IBB) and dodecane (nC12) as it includes molecules of different families (polycyclic, alkane, aromatic). The different pairs of the components of the mixture were used by scientific community as binary benchmark liquids (see Platten et al. 2003; Mialdun and Shevtsova 2011a; Croccolo et al. 2012;Gebhardt et al. 2013) and it seems that this mixture also acts as a benchmark mixture among the ternary ones.
Nowadays along with revisited old techniques for measuring the thermodiffusion new ones appear. The modern optical techniques provide very good accuracy and reliability for measurement transport coefficients in binary mixtures since no perturbation is introduced into the diffusive process. The situation with ternary mixtures is more complicated as the sign of the Soret coefficients of the various components could be different and it destabilizes the system as discussed by Ryzhkov and Shevtsova (2009), Shevtsova et al. (2006). In this respect, orbital laboratories provide an ideal environment for the measurements due to the absence of buoyancy driven convection.
In the framework of a cooperative international project supported by ESA, European scientists expect to obtain reliable benchmark results on the ISS for different types of mixtures and validate their ground-based techniques. Instrument SODI (Selectable Optical Diagnostic) is equipped with two wavelength diagnostic which enables to measure Soret and diffusion coefficients in ternary mixtures. Accordingly, in the first experiment DCMIX1 on the ISS the benchmark mixture THN–IBB–nC12 was investigated.
This article is organized as follows. First, the experimental design and procedure are briefly outlined. Then, the analysis of benefits and disadvantages of the test mixture for the second experiment DCMIX2, which is going to be implemented in 2013, is presented. Fundamental theoretical and experimental results of ground activities on the subject are summarized in the next paragraphs. The conclusions are drawn at the end of article.
SODI Instrument on the ISS
The SODI instrument (Selectable Optical DIagnostic), launched to ISS in 2009 and operated inside the Microgravity Science Glovebox, is a multifunctional instrument. The basic feature of the instrument is the use of different exchangeable methods for optical probing of objects under investigation. The SODI instrument enables the possibility to perform thermal diffusion experiments. It includes an optical interferometer, and its real time results (images) can be sent to researchers via telemetry. The first experiment inside SODI facility, IVIDIL (Influence of VIbrations on DIffusion in Liquids) has examined diffusion controlled phenomena in binary mixtures with and without imposed vibrations, see Mazzoni et al. (2010), Shevtsova et al. (2011b).
The following project, DCMIX (Diffusion Coefficients in MIXtures), is aimed at measuring the diffusion and Soret coefficients in ternary mixtures. Presently the project includes three experiments with mixtures of different origins. The experiment DCMIX1 has been recently performed with nC12-THN-IBB mixture, DCMIX2 will operate with Toluene-Methanol-Cyclohexane (further Tol-Meth-Ch) mixture and DCMIX3 with Water-Ethanol-TEG mixture. The authors of this paper are working on the preparation of DCMIX2 experiment.
Both experiments, IVIDIL and DCMIX analyze diffusive phenomena in liquids using the same two-step procedure: separation by thermodiffusion and back isothermal diffusion. However, they have different optical design. Both use the concept of Mach-Zehnder interferometer but with different ways to obtain the optical phase data from raw interferometric images. The IVIDIL approach is based on 2D Fourier Transform, e.g. see Mialdun and Shevtsova (2008, 2011b), while DCMIX uses phase shift technique, e.g. Hariharan et al. (1987), Mialdun et al. (2013). In the general case of N-component mixture, (N − 1) independent diagnostics are required, for example (N − 1) laser beams of different wavelengths.
Experimental Design and Procedure
The five transparent rectangular cells 10 mm × 10 mm × 5 mm are filled with different compositions of the same ternary system. Since two independent concentrations should be measured in a ternary mixture, two laser diodes emitting light with different wavelengths that ensure different optical behavior of the components are required for a complete composition analysis. The SODI/DCMIX is based on a two-wavelength (670 nm and 935 nm) Mach-Zehnder interferometer with the possibility of changing alignment and magnification (see Fig. 1). It is complex, but very sensitive and precise. The instrument includes two optical bridges, a moving and a fixed one.
Movable optical parts, consisting of illumination block and imaging block (CCD camera plus optics), successively inspects all five cells to follow the evolution of separation, as it is shown in Fig. 2. The interferometric patterns for two wavelengths are successively recorded with the small delay in time. The fixed optical part monitors only the cell with binary mixture using laser λ = 670 nm. The temperature difference ΔT is applied across the cell to maintain thermodiffusion process.
In multicomponent fluid mixtures, subjected to a temperature gradient at a constant pressure, the diffusive transport is caused not only by gradients of concentrations but also by gradients of temperature; when the latter tend to separate species, the former always tend to homogenize them back. All the coefficients characterizing both effects enter simultaneously into the mathematical model of the process. If we denote the mass fraction of component i by c i , \(\sum _{i=1}^{N} c_i=1,\) then the diffusive flux of this component can be written as
where D ik are the mass diffusion coefficients, and D′ Ti are the thermal diffusion coefficients. Since the diffusive flux must vanish in the two dilute limits c i = 0 and c i = 1, the thermal diffusion coefficients are usually represented as D′ Ti = c i0 (1− c i0)DTi where c i0 is the mean mass fraction of the i-component. In a binary mixture there are two independent coefficients (D T and D), while in case of ternary it grows to six (two thermal- and four mass diffusion coefficients). The appearance of cross-diagonal diffusion (D ij , i ≠ j) significantly complicates the measurement of the thermodiffusion coefficients in ternary in comparison to binary mixtures. The Soret coefficients S Ti in ternary mixtures depends on four mass diffusion coefficients D ij and on thermal diffusion coefficients D′ Ti , see Shevtsova et al. (2011a)
All experimental runs will last a long time with the purpose to reach steady state. Experiment in each sample includes 15h for thermodiffusion step, and then 15h for isothermal diffusion. The Soret coefficients S′ Ti can be extracted from observations in the transient regime or at steady state, when diffusion fluxes vanished, J i = 0. The mass diffusion coefficients D ij can be determined from the transient behavior in both regimes. After finishing the experiment in one cell, the optical system is displaced to another cell. To avoid non complete homogeneity at the end of the diffusion step, an experiment in the first cell will be conducted again only after the scanning of all five cells. The cell with binary mixture can be monitored continuously, but again, to avoid residual concentration, the experiments in binary mixture will be conducted each second run. As result, it is assumed to conduct 20 experimental runs with ternary mixtures (applying different ΔT or mean temperature) and 10 experimental runs with binary mixture. Experimental runs are conducted in automatic regime according to the script established before flight.
Choice of the DCMIX2 Test Mixture
The ternary liquid mixture Toluene-Methanol-Cyclohexane was selected for experiments in ISS due to some of its specific features.
First, it exhibits a miscibility gap and for a long time attracts the attention of scientists from different areas of research. The system with demixing contains critical point, so-called consolute point. On the basis of the statistical physics it was derived that the diffusion coefficient D should drastically diminish approaching to the critical point, while thermodiffusion coefficients D T remains almost constant. In binary mixture the Soret coefficient S T = D T /D grows as ∼ 1/D. Experimentally an increase of Soret coefficient by four orders of magnitude has been observed in polymer solutions by Voit et al. (2007). A similar behavior is expected in the ternary systems. The map of the mixture in mass fraction is shown in Fig. 3 where green points outline the demixing zone.
Second, the mass at 298.15K diffusion coefficients in the wide range of compositions have recently been measured at 298.15 K by Grossmann and Winkelmann (2009a, b) using the Taylor dispersion technique. The points, at which diffusion coefficients were measured are shown by blue dots in Fig. 3. Independent data on diffusion are very important to cross-check the future results, especially, at the step of fitting procedure. The values of the diffusion coefficients are influential because the sign of the Soret coefficients in ternary mixture depends on the mass diffusion coefficients, see Eq. 2.
Third, this mixture possess a region of compositions where the Soret effect is negative, i.e. denser components segregate to the hot regions due to thermal diffusion. The negative Soret sign has a destabilizing effect on the system in the gravity field and these ternary mixtures cannot be treated quantitatively in terrestrial conditions, at least, by now. The reported data by Bou-Ali et al. (2000) in the constituent binary mixture Meth-Tol showed a sign change at mass fraction of methanol c = 0.23. To cross-check, we conducted experiments with three compositions of this mixture and the results of all measurements are summarized in Fig. 4. The comparison shows an excellent agreement. For binary Meth-Ch the measurements were conducted in the small range of mass fractions 0.13 < c Ch < 0.28 by Story and Turner (1969) and the authors reported about the positive Soret coefficient in this region with respect to less denser component (Ch). A negative sign was reported in a single point with concentration of cyclohexane close to 1 with respect to Ch.
Measured Soret coefficients of Tol-Meth as a function of mass fraction of Toluene. Rhombus corresponds to the results by Bou-Ali et al. (2000) and triangles show our measurements using ODI
Fourth, the mixture satisfied to the safety condition on the ISS and its use was approved by toxical level assessment.
Along with the attractive features, this system has the negative one: non compatible with a variety of sealing materials, namely due to high solvent permeation and sorption in the sealing material. This point will be addressed in section “Analysis of Sealing Materials”.
Results and Discussion
Choice of the Compositions of the Mixture
There are two equivalent ways of choosing optimal experimental conditions: by choosing a concentration set or by choosing a proper wavelengths. In the case of interest for some specific liquids one should search for proper light sources because for the prescribed wavelengths not all compositions are suitable. To conclude, the available laser diodes inside SODI (670 nm and 935 nm) are not suitable for arbitrary liquids and concentrations. Below one may find short explanation.
Optical measurements on the ISS (or at the ground laboratory) provide variation of the refractive index in space and in time. Let us denote this Δn 1 and Δn 2 the change of refractive index due to variation of c and T for the measurements at wavelength λ 1 and λ 2. To obtain concentration profiles one needs to solve the system of equations
Equations 3–4 can be written in a vector form where the matrix A should be determined from preparatory laboratory experiments,
By inversion of the matrix A, we find the variations of the concentration fields,
and then the Soret coefficients can be defined.
The method described above is simple and straightforward, but there is a serious obstacle to its practical implementation because the matrix A can be ill-conditioned. For each matrix one can calculate the control parameter, so-called condition number. Obviously, before carrying out the Soret measurements it is necessary to estimate the condition number of the matrix A. To understand the essence of the problem the appendices of the paper by Shevtsova et al. (2011a) are recommended.
The matrix A for Tol-Meth-Ch mixture was determined by the measurements of refractive indices for the full concentration space in two wavelengths. The refractive indices were determined at 66 points for both wavelengths λ = 670 nm and λ = 925 nm. Continuous 3D surfaces in the form n 1f = n f (c 1,c 2), n 2f = n f (c 2, c 3), n 3f = n f (c 1, c 3) were constructed on the basis of the experimental points with the aim to fit the set of data points as closely as possible for a specified function n f . The smoothness of the surface is important as the final target is the calculation of the derivatives with a good accuracy. The polynomial fit for n 1f (c 1, c 2) was chosen as
where the coefficients α ij depend on the concentrations set under consideration and they were sought by the non-linear regression method, see Sechenyh et al. (2012). The polynomials for other functions were constructed in a similar way. The concentration derivatives ∂ nλ j / ∂ c i , i, j = 1, 2, vital for the determination of S T and D ij were calculated according to Eq. 6. The values on triangle sides, i.e. for binary mixtures, were compared with literature data. The correctness of the fitting functions was also controlled by calculating the sum of the three derivatives obtained for each wavelength, which must be equal to zero
Although it is only internal consistency check, taking into account that we present experimental results, it provides us confidence to the obtained derivatives.
Then, the condition numbers were calculated for matrices A. The smaller is condition number, the better is precision of the results. The variation of the condition number over composition space is shown in Fig. 5 for mixture Tol-Meth-Ch. The tabulated values of refractive indices, fitting coefficients as well as contrast factors are given in recent paper by Sechenyh et al. (2012).
Isolines of condition numbers of matrix A when λ 1 = 670 nm and λ 2 = 925 nm and compositions for DCMIX2 experiment (Sechenyh et al. 2012). Shaded region outlines demixing zone. The selected points for DCMIX2 experiment are shown by circles with numbers
The compositions of ternary mixtures are selected along the two lines in the direction of consolute point, as shown in Fig. 6. In the vicinity of the critical point exists a turbidity region where mixture is non-transparent. To avoid it, the experimental points are selected at some distance from demixing zone. The numbered experimental cells with selected composition are listed in Table 1.
Physical Properties of the Mixture
Along with refractive index we have measured density and thermal expansion in the points of the interest to facilitate the expected theoretical studies. For these measurements each mixture was prepared in a narrow-distributed concentration around the values of interest. An electronic balance of Sartorius (model 1712, ser. no. 35070147) with an accuracy of 10−2 mg/30 g was used. The weights of all components were measured with an accuracy ±5· 10−5 g. The density was measured at different temperatures using Anton Paar densimeter DMA 5000M. The densities of all the mixtures are linear function of temperature and for two mixtures they are shown in Fig. 7. The values of density and thermal expansion coefficients β T are given in the last two columns of Table 1. The difference in density between the mixtures as well as in thermal expansion coefficients is about 5 %.
Analysis of Sealing Materials
The process of permeation of chemicals through polymers is a combination of two processes, absorption of the chemical in the polymer and diffusion through the polymer. There is a limit to the amount of the chemicals that can be absorbed under particular set of conditions. The often used seal materials do not provide high resistance to permeation of Tol-Meth-Ch mixture. Experiment with Tol-Meth-Ch system was supposed to be uploaded to the ISS in 2011 together with DCMIX1 mixture. However, two weeks after the cell filling bubbles appeared in the cells with well degassed mixtures. The conclusion drawn after the detailed analysis was that seals made of VITON were not compatible with the mixture.
We have performed comprehensive study on the permeation of Tol-Meth-Ch through different sealing materials which are made from polymers (elastomers). A piece of elastomer under the test was immersed into studied liquid mixture of a particular concentration. Each sample had been immersed into approximately 10 ml liquid volume in tightly closed glass flask. Samples were stored at atmospheric pressure and room temperature. Time to time samples had been picked up from the flasks, dried externally by filter paper and weighted by the same balance as described in section “Physical Properties of the Mixture”. The study was continued during 25 days, the samples were weighted a few times per day in the first week and then once per day.Figure 8 shows the relative increase of elastomer mass with time due to permeation of the mixture Tol/Meth/Ch with the concentration in mass fractions 0.25/0.15/0.60 (cell #1) by three different materials: Viton, Chemraz and Kalrez. A second attempt to fill the cells for the experiment on the ISS with the mixture was in 2012, using sealing material Chemraz 505 (75 shore) as the first line of O-rings and VITON as second line of O-rings. The compatibility tests were performed in company Qinetiq ( http://www.QinetiQ.be), which designed and developed SODI instrument. The bubbles appear in cell #1 and cell #3 two months after filling the cells with mixtures of Tol-Meth-Ch. The used sealing materials are listed in Table 2.
Among the studied 12 seals here we discuss only those, which have already been used in SODI (Viton 75, Chemraz 505) and the best one, Kalrez 6375. Usually, the compatibility of seal is assumed as acceptable when saturation is less than 1 %. Because the bubbles appear on the shortest time scale in the cell #1, we present results in Fig. 8 for this particular composition. Among the studied 12 seals the less resistant is the Viton which weight was increased by 5 % in less than 15 days and it is still far from saturation. Chemraz seal attains saturation in about 2 weeks and the relative mass increase is about 1.4 %. Our study demonstrated that for all the six mixtures in Table 1 the best seal is that made of Kalrez 6375, for which the change of mass is less than 1 % after 25 days. After the completing of weighing, the samples of elastomers remains in the mixtures for more than 7 months and no modification of the elastomer was visible, chemical compatibility was excellent.
Despite this results the following attempt to upload experiment on the ISS in 2013 will be done with the seals made of Chemraz expecting the late load access. Because the change of the seal at this step would further delay experiment, all measurements will be conducted on the ISS as fast as possible and, preferably, during first two months after the filling.
Hydrodynamic Instability
It is common knowledge that light fluids rise while heavy fluids sink in the gravity field. The most obvious case is the isothermal Rayleigh-Taylor instability when a more dense fluid is placed a on top of the less dense one. Unstable density stratification might be established in a binary mixture with a negative Soret effect in the case of heating from above: the denser liquid is accumulated on the less denser one. Situation is more complicated in ternary mixtures where the final scenario depends on the sum of Soret coefficients.
Using Optical Digital Interferometry (ODI) with one wave length λ = 670 nm we have performed Soret experiments in ternary mixture Tol-Meth-Ch in wide region of compositions. The experimental technique and picture processing are described in details by Mialdun and Shevtsova (2008, 2009, 2011a). One wave length experiments allow to determine the total variation of the optical phase in whole field of view or, by other words, net variation of the refractive index, \(\Delta \tilde n~=~\Delta n_{1}(x,z,t)\) defined in Eq. 3, although it doesn’t allow to determine both Soret coefficients.
All experiments were conducted in the rectangular cell of height L = 6.1 mm (in the direction of temperature gradient) imposing ΔT = 6 K, resulting in gradient ≈ 1 K/mm. The duration of the experiments was about 12h. These experiments allow us to determine the time dependent behavior of the refractive index difference near the hot and cold walls, \(\Delta n~=~\Delta \tilde n(z=L)-\Delta \tilde n(z=0)\). To illustrate stability of the flow in the gravity field, the evolution of \(\Delta n(t)\) with time is shown Fig. 9 for the three representative cases: stable, semi-stable and unstable. These three cases correspond to different initial compositions. Figure 9 clearly shows that even in the case of unstable regime Δn attains stationary value after 7h. ODI technique enables to observe the concentration field in the entire cross-section of the cell and this has allowed us to identify, that this stationary behavior of Δn simply means that there is no change near the walls, but convection continues to evolve in the interior region.
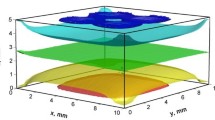
Conducting such experiments at different compositions, we have determined the areas where the concentration field either is non perturbed or convective flow occurs in the mixture. Figure 10 presents stability map in the full parameter space of concentrations. The concentrations, at which experiments were carried out, are shown by small dots. The major part of the parameter space of concentrations display unstable behavior and Soret measurement should be done in microgravity. The only region, rich by methanol and poor in cyclohexane is gravitationally stable and can be measured in laboratory experiments. Notice, that the demixing zone is entirely inside unstable region. In the experiments on the ISS usually the temperature gradient is ΔT / L = 10 K/5 mm = 2 K/mm.
The stability of a stratified system very much depends on the temperature difference and on the cell height. The stability map shown in Fig. 10 correspond to ΔT / L ≈ 1 K/mm and it will be shifted applying another ΔT (≠ 6 K) or choosing different thickness L (≠ 6.1 mm) of the cell.
Conclusions
In the frame of DCMIX project the measurements of diffusion and thermodiffusion coefficients will be conducted in the mixtures of different origins using SODI instrument on the ISS. We have reported motivations for the selection of Tol-Meth-Ch mixture for DCMIX2 experiment and ground-based activities in preparation of microgravity experiment.
The refractive indices (RI) have been measured in this mixture in the whole concentration space at two wave lengths, available in the SODI instrument. Knowledge of RI allowed calculation of the contrast factors and conditions numbers of the corresponding matrices. The mixture concentrations have been selected for microgravity experiment in the region of low condition numbers at different distances from demixing zone.
Tol-Meth-Ch mixture is chemically active and not compatible with often used seals (elastomers). We presented the results of the studying the compatibility of this mixture with Viton, Chemraz and Kalrez. The density and thermal expansion have been measured in the points of interest.
The previous measurements of Soret coefficients in binary mixture Tol-Meth are confirmed. We have performed comprehensive study of the hydrodynamic stability of the mixture in the gravity field. The ternary system is hydrodynamically stable only in a small region of concentrations where the mixture is rich by methanol and poor in cyclohexane. The most interesting region around the demixing zone is unstable and, presently, the themodiffusion coefficients can only be measured in microgravity.
References
Blanco, P., Bou-Ali, M.M., Platten, J.K., de Mezquia, D.A., Madariaga, J.A., Santamaria, C.M.: Thermodiffusion coefficients of binary and ternary hydrocarbon mixtures. J. Chem. Phys. 132, 114506 (2010)
Bou-Ali, M.M., Ecenarro, O., Madariaga, J.A., Santamaria, C.M., Valencia, J.J.: Measurement of negative Soret coefficients in a vertical fluid layer with an adverse density gradient. Phys. Rev. 62, 1420 (2000)
Croccolo, F., Bataller, H., Scheffold, F.: A light scattering study of non equilibrium fluctuations in liquid mixtures to measure the Soret and mass diffusion coefficient. J. Chem. Phys. 137, 234202 (2012)
Gebhardt, M., Köhler, W., Mialdun, A., Yasnou, V., Shevtsova, V.: Diffusion, thermal diffusion, Soret coefficients, and optical contrast factors of the binary mixtures of dodecane, isobutylbenzene and 1,2,3,4-tetrahydronaphthalene. J. Chem. Phys. 138, 114503 (2013)
Grossmann, T., Winkelmann, J.: Part 1. Toluene-rich area. J.Chem. Eng. Data 54, 405 (2009a)
Grossmann, T., Winkelmann, J.: Part 2. Toluene-rich area near the Binodal curve. J.Chem. Eng. Data 54, 485 (2009b)
Hariharan, P., Oreb, B.F., Eiju, T.: Digital phase-shifting interferometry: a simple error-compensating phase calculation algorithm. Appl. Opt. 26, 2504 (1987)
Hartmann, S., Wittko, G., Köhler, W., Morozov, K.I., Albers, K., Sadowski, G.: Thermophobicity of liquids: heats of transport in mixtures as pure component properties. Phys. Rev. Lett. 109, 065901 (2012)
Königer, A., Wunderlich, H., Köhler, W.: Measurement of diffusion and thermal diffusion in ternary fluid mixtures using a two-color optical beam deflection technique. J. Chem. Phys. 132, 174506 (2010)
Mazzoni, S., Shevtsova, V., Mialdun, A., Melnikov, D., Gaponenko, Y., Lyubimova, T., Saghir, Z.: Vibrating liquids in space. Europhys. News 41, 14 (2010)
Mialdun, A., Shevtsova, V.: Development of optical digital interferometry technique for measurement of thermodiffusion coefficients. Int. J. Heat Mass Transfer 51, 3164 (2008)
Mialdun, A., Shevtsova, V.: Measurement of Soret coefficients: open questions. Microgravity Sci. Technol. 21, 31 (2009)
Mialdun, A., Shevtsova, V.: Measurement of the Soret and diffusion coefficients for benchmark binary mixtures by means of digital interferometry. J. Chem. Phys. 134, 044524 (2011a)
Mialdun, A., Shevtsova, V.: Digital interferometry as a powerful tool to study the thermodiffusion effect. C.R. Mecanique 339, 362 (2011b)
Mialdun, A., Shevtsova, V.: Communication: new approach for analysis of thermodiffusion coefficients in ternary mixtures. J. Chem. Phys. 138, 161102 (2013)
Mialdun, A., Minetti, C., Gaponenko, Y., Shevtsova, V., Dunois, F.: Towards SODIDCMIX experiments on the ISS: analysis of the thermal performance. Microgravity Sci. Technol. 25, 83 (2013)
Platten, J.K., Bou-Ali, M.M., Costeseque, P., Dutrieux, J.F., Köhler, W., Leppla, C., Wiegand, S., Wittko, G.: Benchmark values for the Soret, thermal diffusion and diffusion coefficients of three binary organic liquid mixtures. Phil. Mag. 83, 1965 (2003)
Ryzhkov, I.I., Shevtsova, V.M.: Long-wave instability of a multicomponent fluid layer with the Soret effect. Phys. Fluids 21, 014102 (2009)
Shevtsova, V., Melnikov, D.E., Legros, J.C.: Onset of convection in Soret-driven instability. Phys. Rev. E 73, 047302 (2006)
Shevtsova, V., Sechenyh, V., Nepomnyashchy, A., Legros, J.C.: Analysis of the application of optical two-wavelength techniques to measurement of the Soret coefficients in ternary mixtures. Philos. Mag. 91, 3498 (2011a)
Shevtsova, V., Lyubimova, T., Saghir, Z., Melnikov, D., Gaponenko, Y., Sechenyh, V., Legros, J.C., Mialdun, A.: IVIDIL: on-board g-jitters and diffusion controlled phenomena. J. Phys. Conf. Ser. 327, 012031 (2011b)
Sechenyh, V., Legros, J.C., Shevtsova, V.: Measurements of optical properties in binary and ternary mixtures containing cyclohexane, toluene, and methanol. J. Chem. Eng. Data 57, 1036 (2012)
Story, M.J., Turner, J.C.R.: Flow cell studies of thermal diffusion in liquids. Part 5.Binary mixtures of CH3OH with CCl4, benzene and cyclohexane at 25 °C. Trans. Faraday Soc. 65, 1523 (1969)
Voit, A., Krekov, A., Köhler, W.: Laser-induced structures in a polymer blend in the vicinity of the phase boundary. Phys. Rev. E 76, 011808 (2007)
Acknowledgments
This work is supported by the PRODEX programme of the Belgian Federal Science Policy Office, ESA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shevtsova, V., Santos, C., Sechenyh, V. et al. Diffusion and Soret in Ternary Mixtures. Preparation of the DCMIX2 Experiment on the ISS. Microgravity Sci. Technol. 25, 275–283 (2014). https://doi.org/10.1007/s12217-013-9349-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12217-013-9349-6