Abstract
Root-knot nematodes (RNKs) are injurious plant pests that have been managed mainly by synthetic nematicides. Regardless of their effectiveness, chemical nematicides can be deleterious to the environment and human health. The objective of this study was to assess the ecofriendly nematicidal properties of different crude protein extracts precipitated from Moringa oleifera seeds against the RNK, Meloidogyne incognita, on banana cv. Grande-Naine plants, in comparison with a biological control mean (Azotobacter chroococcum), a marine algae (Ulva lactuca), and a synthetic nematicide (Nemacur 10% G). Various ammonium sulfate concentrations were employed in the fractionation of proteins from M. oleifera. Different ammonium sulfate saturations (50, 60 and 70%) were used to obtain the first, second and third precipitate fractions (PFs), respectively. The in vivo test showed that the first PF gave the greatest reduction in number of nematode juveniles in soil (63.51%) and galls on roots (73.24%). Furthermore, these treatments and the marine algae increased plant growth more than the other tested materials. However, the effects on plant growth did not seem to be related with nematode control. PFs were tested for lectin hemagglutination using a microscopy. Hence, the first PF was the best fraction for the control of the nematode.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plant-parasitic nematodes cause severe yield losses of a wide range of agricultural crops, especially in tropical and subtropical regions (Sikora and Fernandez 2005). Also, nematodes can be one of the major constraints to sustainable banana and plantain. The use of certain chemical nematicides to control root-knot nematodes (RNKs) can result in high costs and often upset ecological equilibriums of treated environments; it pollutes and presents human health hazards (Javed et al. 2008; Kosma et al. 2011). Hence, investigations on novel approaches for pest control, such as the use of natural plant substances, have been carried out in various countries (Ioannina et al. 2004; Gahukar 2012; Zhao et al. 2017). As a result, a broad variety of plant species, representing several families, have shown to posses nematicidal compounds, including various secondary metabolites such as the alkaloid, oils, terpenoides, fatty acids, saponine and glycosides (Pavaraj et al. 2012; Pretali et al. 2016). For example, Moringa oleifera is the most widely cultivated species of the genus Moringa in the family Moringaceae (Fakayode and Ajav 2016); also, it is an exceptionally nutritious vegetable tree with a variety of prospective uses (Paliwal et al. 2011). The potential benefits of M. oleifera as a biological control of RKN in maize were demonstrated as moringa leaves and seeds have been found to possess pesticidal properties (Fahey 2005; Govardhan Singh et al. 2013; Arora and Onsare 2014; Ratshilivha et al. 2014; Ammer et al. 2016). The use of moringa extracts may provide an efficient and cheap method of nematode control that is environment friendly and safe to farmers and end users of the product.
Therefore, the use of indigenous plant extracts should be considered in integrated disease management strategies (Claudius-Cole et al. 2010; Akinsanya et al. 2016). Phytochemical analysis showed that M. oleifera contains saponin, tannins, alkaloids, steroids and reducing sugars and, also, enhances plant growth (Izuogu et al. 2013; Guil-Guerrero et al. 2016). The presence of active substances is essential to prevent the formation of reactive oxygen species that lead to cellular damage through oxidation of lipids and proteins (Hassan et al. 2015). Seed proteins are believed to function as protectant against pathogens, nematodes and insects, because of their content in lectins and protease inhibitors (Carlini and Grossi-de-Sá 2002; Salles et al. 2014; Dang and Van Damme 2015). The presence of active substances is essential to prevent the formation of reactive oxygen species that lead to cellular damage through oxidation of lipids and proteins (Hassan et al. 2015).
The purpose of this study was to evaluate the nematicidal properties of various crude proteins precipitated from M. oleifera seeds towards the RNK, M. incognita, in comparison with a marine algae (Ulva lactuca), a bacterium (Azotobacter chroococcum) and a synthetic chemical nematicide (Nemacur 10% G), in plastic pots.
2 Materials and methods
2.1 Collection of plant material
Mature Moringa oleifera seeds (Fig. 1) were obtained from the Moringa Production Unit, National Research Centre, Giza, Egypt. The seeds were air dried, de-shelled and ground to powder. A sample of 1 kg was defatted in a Soxhlet apparatus using n-hexane and the resulting powder was left to dry at room temperature.
2.2 Saline extraction and fractionation
One hundred grams of the defatted powder (moringa cake) was extracted with one litre of 0.2 M phosphate buffered saline (PBS), pH 7.2, containing 0.15 M sodium chloride. The mixture was stirred for 2–3 h and was left over night at 4 °C. The clear supernatant was collected by centrifugation at 6000 rpm for 20 min using the centrifuge Centurion Scientific LTD Model 1020 series.
The following procedure for fractionation was carried out according to the method of Hebert et al. (1973). Therefore, 850 ml of crude PBS extract was lightly stirred, while ammonium sulfate (NH4)2SO4 was slowly added and mixed well up to final amount of 270.02 g, with constant stirring for 1 h at 4 °C, until reaching a semi saturation state. The reaction mixture was set over night at 4 °C and then centrifuged to compact the precipitated protein. The supernatant fluid was removed and stored. The first precipitate in 50% saturated (NH4)2SO4 was dissolved in PBS to a final volume of 60 ml (fraction A). For the second precipitation in 60% saturated (NH4)2SO4, the supernatant fluid, which was obtained from the first step, was gently stirred while an additional quantity of (NH4)2SO4 was slowly added and mixed well up to final amount of 65.59 g. The mixture was centrifuged to compact the formed precipitate which was re-suspended in a 60 ml of PBS (fraction B). A third precipitation in 70% saturated (NH4)2SO4 was handled in the same manner using (NH4)2SO4 up to final amount of 70.46 g (fraction C). To purify proteins, all fractions were dialyzed against recurrent changes for 24–48 h at 4 °C against a continuous flow of the PBS.
2.3 Protein determination
The protein concentrations in all fractions were determined according to the method described by Bradford (1976) using a spectrophotometer (UV-200-RS LWScientific) at the absorbance of 280 nm. Bovine serum albumin (BSA) was used as a standard for comparison.
2.4 Preparation of bacterial inocula
An Azotobacter chroococcum isolate was grown on Jensen’s medium (g/L): 20.0 sucrose, 1.0 K2HPO4, 0.5 MgSO4·7H2O, 0.5 NaCl, 0.1 FeSO4, 0.005 Na2MoO4·2H2O, 2.0 CaCO3, 15.0 agar; for 3 days. A loop of bacterial culture was inoculated into 100 ml Jensen’s medium for 48 h at 28 °C ± 1. The bacterial inoculum was applied as a soil treatment at the rate of 5 ml of the bacterial suspension (1 × 108 cfu/ml) per plant.
2.5 Nematode isolation and inocula
Eggs of the RNK, Meloidogyne incognita, were extracted from tomato (Lycopersicon esculentum cv. Castle Rock) roots infected with the nematode using a 1% sodium hypochlorite solution (Hussey and Barker 1973). The egg suspension was incubated at 28 ± 2 °C and emerging second-stage juveniles (J2 s) were collected daily from eggs and were stored at 15 °C. The juveniles used in the experiments were less than 5 days old.
2.6 Nematode infection assay
Two months old banana plantlets cv. Grande-Naine were obtained from the Tissue Culture Lab of the Genetic Engineering and Biotechnology Research Institute (GEBRI). Bananas were planted in 30 cm diameter plastic pots containing about 4.5 kg of sterilized soil (1:3 mixture of clay: sand) with pH 7. The temperature of the soil was about 28 ± 2 °C for most of the experiment time. Thirty-two pots were inoculated with 3000 J2 s per pot at planting time. Seven days later, 12 pots were treated with 15 ml of each moringa protein fraction after precipitations, with fraction No. A (50%), fraction No. B (60%) and fraction No. C (70%) saturated (NH4)2SO4 in concentrations of 70, 25.5 and 4.5 mg/ml, respectively.
Four inoculated pots were treated with 3 g/kg soil of the fine dried powder of the marine algae, Ulva lactuca (El-Ansary and Hamouda, 2014). Also, four inoculated pots were treated with 5 ml bacterial suspension of Azotobacter chroococcum. The same number of inoculated pots was treated with 3 g of Nemacur 10% G, (Fenamiphos), per pot. The remaining four inoculated pots served as inoculated and untreated control. Moreover, four more pots served as untreated and non-inoculated control. The tested materials were added to the soil in 3 holes followed by the addition of 50 ml of water. The pots were arranged in a completely randomized design in a greenhouse of the Genetic Engineering Biotechnology Research Institute (GEBRI). Plants were evaluated after 60 days of inoculation. The roots were washed carefully to remove the soil and stained with Phloxine B (3.5 g in 750 ml distilled water + 250 ml acetic acid 5%) solution for 5 min to facilitate counting of all nematode stages in the root. Nematode variables observed per root system were galls, egg masses and females. Also, juveniles per 250 g soil were extracted according to Cobb’s sieving and decanting method, using sieves (60 meshes and 325 meshes). Eggs in soil and in egg masses were not counted. The banana growth variables shoot length (cm), shoot weight (g), root length (cm), root weight (g), corm weight (g) and number of leaves were also recorded.
2.7 Testing for agglutinating activity
The precipitate from the (NH4)2SO4 fractionation was examined microscopically for recognition of phytolectin by a qualitative agglutination method. Therefore, series of twofold dilutions were made and were added to a solution of 20% trypsinized cattle erythrocytes in PBS. The mixture was incubated for 20 min and then observed under microscope (Leica DM300). In the blank experiment, the PBS was mixed with erythrocytes instead of the sample solution (Adamova et al. 2014). Hemagglutination activity was expressed as the inverse of the minimum amount of sample in mg/ml in the last dilution giving affirmative agglutination.
2.8 Statistical analysis
All data were subjected to analysis of variance (ANOVA) (Sokal and Rohlf 1995). Significance of the variable mean differences was determined using Duncan’s multiple range tests (p ≤ 0.05). All analyses were carried out using SPSS 16 software.
3 Results and discussion
Table 1 shows the protein contents (mg/ml) in saline and ammonium sulfate extracts obtained from M. oleifera seeds. The results indicated that the saline extract (0.15 M NaCl) has a protein content of 8.88 mg/ml. Furthermore, the increase of (NH4)2SO4 saturation reduced the protein precipitation gradually. The first precipitate in 50% saturated (NH4)2SO4 (fraction No. A), the second precipitate in 60% saturated (NH4)2SO4 (fraction No. B) and the third precipitate in 70% saturated (NH4)2SO4 (fraction No. C) had protein contents of 4.18, 1.53 and 0.27 mg/ml, respectively.
3.1 Effects of crude seed protein fractions of moringa on Meloidogyne incognita
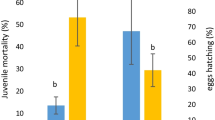
The effect of the treatments on the RNK, Meloidogyne incognita, infecting banana plants resulted in a marked suppression of the nematode population (Table 2). Different crude proteins of Moringa oleifera reduced numbers of M. incognita in soil and roots. This agrees with the results obtained by earlier investigations (Fahey 2005; Claudius-Cole et al. 2010; Izuogu et al. 2013; Salles et al. 2014). Numbers of galls, females and egg masses in the roots and nematodes in the soil were greatly reduced when compared with those of the untreated plants (with nematode). M. oleifera was the best treatment to suppress number of J2 s in soil. Likewise, the soluble plant lectins may function as an active defense mechanism. Lectins detected in precipitates of M. olifera proteins, using saturated ammonium sulfate (0–60%), are important and responsible for the anti-nematode effect (Etzler 1986). Generally, all the tested treatments with different protein contents of moringa significantly (p ≤ 0.05) reduced the number of root galls, juveniles in soil, females, and egg-masses per root (Table 2). The most effective precipitate treatment in reducing nematode infestation in soil was fraction A, (63.51% reduction) compared to the chemical control, Nemacur 10% G (67.59% reduction), followed by marine algae, Ulva lactuca (56.09% reduction), and then by the bacterium, Azotobacter chroococcum (48.77% reduction). Lectins exhibit a variety of effects including antimicrobial, antitumoral, mitogenic and insecticide activities, because lectins recognize and bind to specific carbohydrates present on the surface cells (Napoleão et al. 2011; Paiva et al. 2012). Phytochemical screening showed that M. oleifera contains saponin, tannins, alkaloids, steroids and reducing sugars. These basic phytochemicals have nematicidal activity and had been reported to confer pesticidal effects in plants (Adeniyi et al. 2010). Also, the phytochemicals tannins and saponins disrupt membranes in organisms, thereby facilitating penetration of toxic principles and thus suppressing infecting organism (Agrios 2005).
3.2 Effect of moringa protein fractions on the growth of banana plants infected with Meloidogyne incognita
There was no difference in plant growth between inoculated and non-inoculated control (Table 2). Only treatments with moringa protein fractions and marine algae improved plant growth of banana and this effect was greater on shoots and corms than on roots and leaves. The greatest effect on shoot height and corm was given by marine algae, while this treatment was as good as moringa fractions in increasing shoot weight and number of leaves. El-Ansary and Hamouda (2014) also reported that shoot weight of banana infected by Meloidogyne sp. was increased more by extracts of U. lactuca than by extracts from other plants (Jania rubens, Laurencia obtuse and Sargassum vulgare).
According to Foidl et al. (2001), M. oleifera leaf extracts stimulated plant growth and increased yield (20–35%) of different vegetables when applied as a seed treatment at diluted rates.
3.3 Detection of phytolectins in crude proteins of moringa seeds
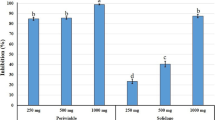
At room temperature, both 50 and 60% ammonium sulfate crude proteins were selected to detect lectin activity in the fractions using the agglutination technique of trypsinized cattle erythrocytes on microscope glass slide, after dialysis against phosphate buffered saline (PBS) directly instead of a purified lectin. As shown in Fig. 2a, control trials in the absence of crude protein fractions did not show any visible agglutination of erythrocytes. Agglutination of the second precipitate in fraction B, 60% saturated (NH4)2SO4, was more visible than fraction A, 50% saturated (NH4)2SO4 (Fig. 2b), and the best results (the biggest clusters) were obtained using a concentration of crude protein of 1 mg/ml. Lectins are a class of glycoproteins that are abundantly found in plant seeds, agglutinate blood erythrocytes, and play a role in the defense mechanism of plants against attack by different microorganisms including pests and insects. Phytolectins are known to control RNKs (Marban-Mendoza et al.1987; Al-Saman et al. 2015). Moreover, Santos et al. (2009) revealed the presence of two lectins in M. oleifera seed extracts based on the affinity support used and extraction solvent; water-soluble M. oleifera lectin (WSMoL) with the carbohydrate binding site of lectin recognized D(+)-fructose and N acetylglucosamine (Coelho et al. 2009) and coagulant M. oleifera lectin (cMoL) from saline solvent with the several carbohydrates binding site of lectin recognized and with the exception of D(+)-fructose. Salles et al. (2014) found that the nematicidal activity of M. oleifera seeds involves different bio-molecules, particularly molecules with low molecular weight.
4 Conclusion
In our study, the level of the nematode inoculum used was not sufficient to affect plant growth as no significant difference was observed between inoculated and non-inoculated controls (Table 3). Therefore, the improvement of the plant growth following the treatments is not the result of their nematicidal effect, but rather of their stimulant activity. Nevertheless, it could be concluded that the use of the first precipitate of M. oleifera proteins in 50% saturated (NH4)2SO4 (fraction A), showed the highest antagonistic effect on M. incognita on banana plants.
Therefore, crude proteins of M. oleifera seeds could be proposed as an effective, safe and efficient nematicide that is environmentally friendly and safe to farmers and users of the product. However, trials under field conditions are necessary to confirm the most appropriate rates and timing of application and the economics of such treatment.
References
Adamova L, Malinovska L, Wimmerova M (2014) New sensitive detection method for lectin hemagglutination using microscopy. Microsc Res Tech 77:841–849
Adeniyi SA, Orjiekwe CL, Ehiagboare JE, Arimah BD (2010) Preliminary phytochemical analysis and insecticidal activity of ethanolic extracts of four tropical plants (Vernonia amygdalina, Sidaacuta, Ocimum gratissimum and Telfaria occidentalis) against bean weevil (Acanthscelides obtectus). Int J Phys Sci 5(6):753–762
Agrios GN (2005) Plant pathology, 5th edn. Academic press, San Diego
Akinsanya B, Utoh OU, Ukwa UD (2016) Toxicological, phytochemical and anthelminthic properties of rich plant extracts on Clarias gariepinus. J Basic Appl Zool 74:75–86
Al-Saman MA, Farfour SA, Tayel AA, Rizk NM (2015) Bioactivity of lectin from Egyptian Jatropha curcas seeds and its potentiality as antifungal agent. Glob Adv Res J Microbiol 4(7):87–97
Ammer MR, Zaman S, Khalid M, Bilal M, Erum S, Huang D, Che S (2016) Optimization of antibacterial activity of Eucalyptus tereticornis leaf extracts against Escherichia coli through response surface methodology. J Radiat Res Appl Sci 9(4):376–385
Arora DS, Onsare JG (2014) In vitro antimicrobial evaluation and phytoconstituents of Moringa oleifera pod husks. Ind Crops Prod 52:125–135
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carlini CR, Grossi-de-Sá MF (2002) Plant toxic proteins with insecticidal properties: a review on their potentialities as bioinsecticides. Toxicon 40(11):1515–1539
Claudius-Cole AO, Aminu AE, Fawole B (2010) Evaluation of plant extracts in the management of root-knot nematode Meloidogyne incognita on cowpea [Vigna unguiculata]. Mycopath 8(2):53–60
Coelho JS, Santos ND, Napoleão TH, Gomes FS, Ferreira RS, Zingali RB, Coelho LC, Leite SP, Navarro DM, Paiva PM (2009) Effect of Moringa oleifera lectin on development and mortality of Aedes aegypti larvae. Chemosphere 77(7):934–938
Dang L, Van Damme EJ (2015) Toxic proteins in plants. Phytochemistry 117:51–64
El-Ansary MSM, Hamouda RA (2014) Biocontrol of root knot nematode infected banana plants by some marine algae. Russ J Mar Biol 40(2):140–146
Etzler ME (1986) Distribution and function of plant lectins. In: Liener IE, Sharon N, Goldstein IJ (eds) The lectins. Academic Press, San Diego, pp 371–435
Fahey JW (2005) Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Tree Life J 1:5
Fakayode OA, Ajav EA (2016) Process optimization of mechanical oil expression from Moringa (Moringa oleifera) seeds. Ind Crops Prod 90:142–151
Foidl N, Makkar HPS, Becker K (2001) The potential of Moringa oleifera for agricultural and industrial uses. What development potential for Moringa products? October 20th–November 2nd 2001. Dar Es Salaam, Tanzania
Gahukar RT (2012) Evaluation of plant-derived products against pests and diseases of medicinal plants: a review. Crop Protect 42:202–209
Govardhan Singh RS, Negi PS, Radha C (2013) Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J Funct Food 5:1883–1891
Guil-Guerrero JL, Ramos L, Moreno C, Zúñiga-Paredes JC, Carlosama-Yepez M, Ruales P (2016) Antimicrobial activity of plant-food by-products: a review focusing on the tropics. Livest Sci 189:32–49
Hassan N, El-bastawisy Z, Ebeed H, Nemat Alla M (2015) Role of defense enzymes, proteins, solutes and Δ1-pyrroline-5-carboxylate synthase in wheat tolerance to drought. Rend Fis Acc Lincei 26(3):281–291
Hebert GA, Pelham PL, Pittman B (1973) Determination of the optimal ammonium sulfate concentration for the fractionation of rabbit, sheep, horse, and goat antisera. Appl Microbiol 25(1):26–36
Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis Rep 57:1025–1028
Ioannina OG, Dimitrios GK, Demetra PA (2004) A novel non-chemical nematicide for the control of root-knot nematodes. Appl Soil Ecol 26:69–79
Izuogu NB, Badmos AA, Raji SO (2013) The potency of Moringa oleifera and Jatropha curcas leaf extracts as control for root-knot-nematode in maize (Zea mays). Int J Phytofuels Allied Sci 2(1):116–124
Javed N, Gowen SR, El-Hassan SA, Inam-ul-Haq M, Shahina F, Pembroke B (2008) Efficacy of neem (Azadirachta indica) formulations on biology of root-knot nematodes (Meloidogyne javanica) on tomato. Crop Prot 27:36–43
Kosma P, Ambang Z, Begoude BAD, Ten Hoopen GM, Kuaté J, Akoa A (2011) Assessment of nematicidal properties and phytochemical screening of neem seed formulations using Radopholus similis, parasitic nematode of plantain in Cameroon. Crop Prot 30(6):733–738
Marban-Mendoza N, Jeyaprakash A, Jansson H-B, Damon JRRA, Zuckerman BM (1987) Control of root-knot nematodes on tomato by lectins. J Nematol 19(3):331–335
Napoleão TH, Gomes FS, Lima TA, Santos NDL, Sá RA, Albuquerque AC, Coelho LCBB, Paiva PMG (2011) Termiticidal activity of lectins from Myracrodruon urundeuva against Nasutitermes corniger and its mechanisms. Int Biodeterior Biodegrad 65:52–59
Paiva PMG, Napoleão TH, Sá RA, Coelho LCBB (2012). Insecticide activity of lectins and secondary metabolites, insecticides—advances in integrated pest management. In: Farzana Perveen (ed), ISBN: 978-953-307-780-2, InTech, Available from: http://www.intechopen.com/books/insecticides-advances-in-integrated-pest-management/insecticide-activityof-lectins-and-secondary-metabolites
Paliwal R, Sharma V, Pracheta E (2011) A review on horse radish tree (Moringa oleifera): a multipurpose tree with high economic and commercial importance. Asian J Biotechnol 3(4):317–318
Pavaraj M, Bakavathiappan G, Baskaran S (2012) Evaluation of some plant extracts for their nematicidal properties against root-knot nematode, Meloidogyne incognita. J Biopestic 5 (Supplementary):106–110
Pretali L, Bernardo L, Butterfield TS, Trevisan M, Lucini L (2016) Botanical and biological pesticides elicit a similar induced systemic response in tomato (Solanum lycopersicum) secondary metabolism. Phytochemistry 130:56–63
Ratshilivha N, Awouafack MD, du Toit ES, Eloff JN (2014) The variation in antimicrobial and antioxidant activities of acetone leaf extracts of 12 Moringa oleifera (Moringaceae) trees enables the selection of trees with additional uses. S Afr J Bot 92:59–64
Salles HO, Braga ACL, Nascimento MTC, Sousa AMP, Lima AR, Vieira LS, Cavalcante ACR, Egito AS, Andrade LBS (2014) Lectin, hemolysin and protease inhibitors in seed fractions with ovicidal activity against Haemonchus contortus. Revista Brasileira de ParasitologiaVeterinária 23(2):136–143
Santos AFS, Luz LA, Argolo ACC, Teixeira JA, Paiva PMG, Coelho LCBB (2009) Isolation of a seed coagulant Moringa oleifera lectin. Process Biochem 44(4):504–508
Sikora RA, Fernandez E (2005) Nematode parasites of vegetables. In: Luc M, Sikora RA, Bridge J (eds) Plant-parasitic nematodes in subtropical and tropical agriculture, 2nd edn. CABI Publishing, Wallingford, pp 319–392
Sokal RR, Rohlf FJ (1995) Biometry: The Principles and practice of statistics in biological research, 3rd edn. W.H. Freeman and Co., New York
Zhao L, Feng C, Wu K, Chen W, Chen Y, Hao X, Wu Y (2017) Advances and prospects in biogenic substances against plant virus: a review. Pestic Biochem Physiol 135:15–26
Acknowledgements
We gratefully acknowledge Dr. Assma Abd Ella, University of Sadat City, for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Ansary, M.S.M., Al-Saman, M.A. Appraisal of Moringa oleifera crude proteins for the control of root-knot nematode, Meloidogyne incognita in banana. Rend. Fis. Acc. Lincei 29, 631–637 (2018). https://doi.org/10.1007/s12210-018-0692-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-018-0692-9