Abstract
Silver nanoparticles were synthesized by bio-inspired route, a cost effective and fast synthesis method. Structural and morphological characterization of nanoparticles was performed by UV–visible absorption spectroscopy, Fourier transform infrared spectroscopy, X-ray diffraction and transmission electron microscopy. The cytotoxic activity of both nanoparticles and Plantago major extract containing nanoparticles against a human breast cancer cell (MCF-7) was studied in vitro. MTT (3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assays were performed using various concentrations of P. major extract (alone) and extract containing AgNPs ranging from 0.5 to 2.5 µg/ml. Data analysis showed significant level of cytotoxic activity. The potential cytotoxicity of silver nanoparticles in the treatment of breast cancer is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Synthesis of nanoparticles (NPs) has been one of the branches of technology that exhibits a fast development in the area of nanotechnology (Hashemi et al. 2016; Mahmoudvand et al. 2014; Abedi et al. 2011; Sattarahmady et al. 2013, 2015; Khatami et al. 2017a). Specifically, major attention has been paid to investigate different controlled shape, size and dispersion degree of NPs which focused on bio-inspired methods (Shimoshige et al. 2017; Zare et al. 2017; Hamedi et al. 2017a; Mortazavi et al. 2017; Khatami et al. 2017b; Singh et al. 2016a, 2017a; Ahn et al. 2017; Beitollahi et al. 2016; Jahani and Beitollahi 2016; Seddighi et al. 2017). Among NPs, technology for the synthesis of silver nanoparticles (AgNPs) can be highlighted, due to its important activities such as anti-microbial, anti-leishmania activities and optical property (SudhaLakshmi 2011; Moradi et al. 2016; Hamedi et al. 2016, 2017b; De Sio et al. 2015; Meymandi et al. 2010; Singh et al. 2016b; Markus et al. 2016; Niroomand et al. 2017). Several methods have been used in the synthesis of NPs such as physical, chemical and biological methods (Bankara et al. 2010; Balasooriya et al. 2017; Nejad et al. 2017; Ghodselahi et al. 2014, 2015; Moussavi et al. 2014; Samadi et al. 2015; Beitollahi and Nekooei 2016). Among these methods, chemical method is simple, but reducing agents in it such as Citrate, Polyvinylpyrrolidone, Borohydride and other inorganic compounds are very toxic and are not appropriate for medical purposes (Kemp et al. 2009; Khodashenas and Ghorbani 2016; Moghaddam et al. 2017; Ahmadrajabi et al. 2016; Sobhanipoor et al. 2017). On the other hand, the nanoparticle produced by biological method is more valuable than the one produced by chemical method due to the non-existence of organic toxic residuals, production of minimum wastages, high volume of production and repeatability (Soshnikova et al. 2017). Regardless of wide applications of AgNPs, there is a serious lack of information regarding their biological influences on human cells (Singh et al. 2017b). The toxic potential of biological synthesis of AgNPs on human breast cancer cell (MCF-7 cell line) has been evaluated. Breast cancer is one of the most widespread malignant cancers in women all over the world and is the most important cause of death arising from cancer among women (Malorni et al. 2006; Castro-Aceituno et al. 2016). Some researchers have reported the effects of plant extracts on cancer cells including breast cancer. Herbal compounds as anti-cancer agents were used for the first time by Hartwell et al. in the late 1960’s. They used Podophyllotoxin and its derivatives as anti-cancer agents (Srivastava et al. 2005). The plant used in this research is fleawort (Plantago major L.) from the family of Plantaginaceae which has various medicinal purposes (Samuelsen 2000a). This plant is considered as an important medicinal plant due to the presence of compounds such as phenol, flavonoids, alkaloids, terpenoids and Vitamin C (Samuelsen 2000b). Traditionally, P. major has played a role in the treatment of cancer due to the presence of high amounts of phenolic compounds and flavonoids. Kanda et al. studied 8 types of flavonoids of P. major and indicated that Luteolin-7-O-β-glucoside is the major compound of flavonoids in most species of P. major (Kanda et al. 1994). Results of researches by Galves et al. showed that Luteolin-7-O-β-glucoside has a strong anti-cancer activity in inhibiting breast adenocarcinoma (Galvez et al. 2003).
In the light of the above cited results, the present study attempted to design a green, high cost/benefit ratio and nontoxic method for synthesizing AgNPs using seed extract of P. major for the first time. Both the anti-cancer properties of plant extract and biosynthesized AgNPs on MCF-7 cancer cells were investigated.
2 Materials and methods
2.1 Preparation of the extract
The seeds were washed under running tap water. The clean seeds were powdered. 20 grams of obtained powder was added to an Erlenmeyer flask containing 100 ml of boiling deionized water and was boiled for 15 min. Next the extract was filtered by Whatman filter paper No. 1.
2.2 Synthesis of silver nanoparticle
Briefly, 0.1 M aqueous solution of silver nitrate (AgNO3) was prepared. A 10 ml of P. major extract was added into 20 ml of aqueous solution of 1 mM silver nitrate for reduction of Ag+ ions and was incubated overnight at room temperature. The resultant yellowish brown solution indicates the formation of AgNPs (Khatami et al. 2016a).
2.3 UV–vis spectra analysis
The AgNPs formation was monitored using UV visible spectrophotometry. Absorption wavelength was studied between the range of 200–700 nm.
2.4 Powder X-ray diffraction (XRD)
The red solid product was separated by repeated centrifugation at 14,000 rpm for 10 min followed by re-dispersion of the agglomerate of AgNPs into deionized water three times. After this procedure, the remaining sediment was dried at 30 °C for 48 h. Panalitical, X Pert Pro, from Netherland was used for XRD analysis. XRD analysis was also applied to determine the particle size using Scherer’s formula:
where, d is the average crystal size, is the X-ray wavelength (0.1541 nm), β is the full-width at half-maximum (FWHM) and θ is the diffraction angle (Azizi et al. 2016).
2.5 TEM analysis
TEM (Carl ZIESS) was using to determine the size, shape and distribution of biosynthesized AgNPs (Darroudi et al. 2014).
2.6 FTIR
FTIR was performed before and after the biosynthesis of AgNPs to detect the possible functional groups in biomolecules present in the plant extract (Khatami et al. 2016b).
3 Anti-cancer test
3.1 Treatment of cancer cells by the extract
Twenty-four hours after seeding the cells in 96-well microtiter plates, the medium was changed and the cells were treated with doses of 1–15 μg/ml of AgNPs or of the extract and incubated for 24 h (Alishah et al. 2017; Oh et al. 2017). The MTT assay was performed as previously described (Castro-Aceituno et al. 2016).
Data were subjected to One-way Analysis of Variance to determine the significance of individual differences at p < 0.05 level. Significant means were compared by Duncan’s multiple range test. All statistical analyses were conducted using SPSS statistical software package (SPSS, Version 10.0, Chicago, USA).
4 Results and discussion
The visual inspection of extract containing synthesized AgNPs showed a clear change in color from yellowish to reddish brown. This color evolution indicates the formation of metallic AgNPs. The Ag0 nanoparticle was synthesized through the reduction process of Ag+ ions using plant extract of Plantago as a major reducing agent to synthesize silver nanoparticles (Fig. 1).
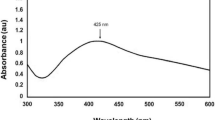
The UV–visible absorption peak of P. major extract treated with silver nitrate is detected at around 432 nm indicating the formation of AgNPs (Fig. 1). Spectrum of the mixture containing seed extract of P. major treated with silver nitrate was measured at different times. It was observed that AgNPs are present for more than 4 months, in samples with the concentration of 5 mM of silver nitrate.
XRD analysis showed four distinct diffraction lines positioned at 38.03°, 44.38°, 64.54° and 77.64°, which were indexed to (hkl) planes 111, 200, 220 and 311, respectively, characteristic of metallic silver with cubic face-centered symmetry (Fig. 2). The unassigned diffraction line at 2θ = 54.6° in Fig. 2 is thought to be related to crystalline or semi-amorphous organic phases. The average size of the AgNPs was calculated using Scherer’s formula being equal to 26 nm.
TEM image of the AgNPs showed that particles are spherical in shape with an average size ranging from 1 to 30 nm (Fig. 3). Given that the applications for AgNPs are highly dependent on the shape, size, and average particle size distribution of the particles, the strategy of using seed extract of P. major seems promising. Therefore, in this study for the first time, we have reported the simple and facile green synthesis of AgNPs using seed extract of P. major.
FTIR spectrum shows absorption bands at 3422, 2921, 2856, 1743, 1631, 1450, 1377, 1240, 1043 and 596 cm−1 indicating the presence of capping agent with the nanoparticles (Fig. 4).
According to Fig. 4, the bands centered at 3422, 2921, 1743, 1631 and 1450 cm−1 in spectra are assigned to O–H, C–H, C–C, C–N and N–H, respectively. This assignment suggests a possible role of alcohol, phenol and proteins in the synthesis of AgNPs (Bankar et al. 2010).
The effects of different doses of seed extract of P. major and seed extract of P. major containing silver nanoparticles on cellular survival of MCF-7 breast cancer cells have been investigated. First, we examined the effect of seed extracts of P. major on MCF-7 cells survival using the MTT test. Twenty-four hours after seeding the cells into 96 well microliter plates, the effect of 1.0, 1.5, 2.0, 2.5, 5, 10 and 15 μg/ml of P. major extract on cell growth was analyzed. Extract concentrations of 1.0, 1.5 and 2 μg/ml did not show a considerable toxic effect on MCF-7 cancer cells, however, a significant toxic effect was detected with doses from 5 to 15 μg/ml. With the latter dose the toxic effect was highest (Fig. 5). Further analysis of the organic compounds and of their concentrations present in the extract of P. major seeds are needed. The results obtained by treating the MCF-7 cancer cells with extracts containing silver nanoparticles were quite different. We observed a toxic effect with concentrations of 1.5 μg/ml.
Krishnaraj et al. (2016) and Kulandaivelu et al. (2014) also studied the toxic effects of AgNPs on the MCF-7 cancer cell line. In those studies, however, AgNPs had significant cytotoxic effects at concentrations of 100 μg/ml (Krishnaraj et al. 2016; Kulandaivelu et al. 2014). Sonbaty studied the cytotoxic effect of fungi-synthesized AgNPs (8–20 nm) using Agaricus bisporus on MCF-7 breast cancer cell and reported its LD50 at 50 μg/ml (El-Sonbaty 2013), In addition, Murugan et al. (2016) reported that AgNPs IC50 is 60–80 μg/ml on MCF-7. Therefore, the anti-cancer activity of biosynthesized AgNPs using P. major observed in the present study was greater than that observed in the above cited studies. Higher anti-cancer activity of biosynthesized AgNPs using P. major can be especially useful in developing green synthesized NPs with greater anticancer potential.
5 Conclusion
Green synthesis of silver nanoparticles using seed extract of P. major plant was achieved. The UV–visible absorption peak of biosynthesized AgNPs is detected at around 432 nm. XRD analysis showed four distinct diffraction lines positioned at 38.03°, 44.38°, 64.54° and 77.64°, which were indexed to planes 111, 200, 220 and 311, respectively. Cytotoxic and anti-cancer effect of P. major seed extract on MCF-7 cancer cell was determined. Both the extract and the AgNPs exhibited a cytotoxic effect on the MCF-7 breast cancer cells. The biosynthesized AgNPs showed a stronger anti-cancer effect than that showed in similar studies performed.
References
Abedi G, Sotoudeh A, Soleymani M, Shafiee A, Mortazavi P, Aflatoonian MR (2011) A collagen–poly(vinyl alcohol) nanofiber scaffold for cartilage repair. J Biomater Sci Polym Ed 22(18):2445–2455
Ahmadrajabi R, Shakibaie MR, Iranmanesh Z, Mollaei HR, Sobhanipoor MH (2016) Prevalence of mip virulence gene and PCR-base sequence typing of Legionella pneumophila from cooling water systems of two cities in Iran. Virulence 7(5):602–609
Ahn S, Singh P, Castro-Aceituno V, Yesmin Simu S, Kim Y-J, Mathiyalagan R, Yang D-C (2017) Gold nanoparticles synthesized using Panax ginseng leaves suppress inflammatory—mediators production via blockade of NF-κB activation in macrophages. Artif Cells Nanomed Biotechnol 45(2):270–276
Alishah H, Pourseyedi S, Ebrahimipour SY, Mahani SE, Rafiei N (2017) Green synthesis of starch-mediated CuO nanoparticles: preparation, characterization, antimicrobial activities and in vitro MTT assay against MCF-7 cell line. Rend Fis Acc Lincei 28(1):65–71
Azizi Z, Pourseyedi S, Khatami M, Mohammadi H (2016) Stachys lavandulifolia and Lathyrus sp. mediated for green synthesis of silver nanoparticles and evaluation its antifungal activity against Dothiorella sarmentorum. J Clust Sci 27(5):1613–1628
Balasooriya ER, Jayasinghe CD, Jayawardena UA, Ruwanthika RWD, Mendis de Silva R, Udagama PV (2017) Honey mediated green synthesis of nanoparticles: new era of safe nanotechnology. J Nanomater 2017:10
Bankar A, Joshi B, Kumar AR, Zinjarde S (2010) Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf A 368(1):58–63
Bankara A, Joshi B, Kumara AR, Zinjarde S (2010) Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Physicochem Eng Aspects 2(4):58–63
Beitollahi H, Nekooei S (2016) Application of a modified CuO nanoparticles carbon paste electrode for simultaneous determination of isoperenaline, acetaminophen and N-acetyl-l-cysteine. Electroanalysis 28(3):645–653
Beitollahi H, Tajik S, Jahani S (2016) Electrocatalytic determination of hydrazine and phenol using a carbon paste electrode modified with ionic liquids and magnetic core-shell Fe3O4@SiO2/MWCNT nanocomposite. Electroanalysis 28(5):1093–1099
Castro-Aceituno V, Ahn S, Simu SY, Singh P, Mathiyalagan R, Lee HA, Yang DC (2016) Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed Pharmacother 84:158–165
Darroudi M, Sarani M, Oskuee RK, Zak AK, Amiri MS (2014) Nanoceria: gum mediated synthesis and in vitro viability assay. Ceram Int 40(2):2863–2868
De Sio L, Caracciolo G, Placido T, Pozzi D, Comparelli R, Annesi F, Curri ML, Agostiano A, Bartolino R (2015) Applications of nanomaterials in modern medicine. Rend Fis Acc Lincei 26(2):231–237
El-Sonbaty SM (2013) Fungus-mediated synthesis of silver nanoparticles and evaluation of antitumor activity. Cancer Nanotechnol 4(4–5):73–79
Galvez M, Marti C, Lopez-Lazaro M, Cortes F, Ayuso J (2003) Cytotixic effect of Plantago spp. oncanceralllins. J Ethopharmacol 88:125–130
Ghodselahi T, Arsalani S, Neishaboorynejad T (2014) Synthesis and biosensor application of Ag@Au bimetallic nanoparticles based on localized surface plasmon resonance. Appl Surf Sci 301:230–234
Ghodselahi T, Neishaboorynejad T, Arsalani S (2015) Fabrication LSPR sensor chip of Ag NPs and their biosensor application based on interparticle coupling. Appl Surf Sci 343:194–201
Hamedi S, Ghaseminezhad M, Shokrollahzadeh S, Shojaosadati SA (2016) Controlled biosynthesis of silver nanoparticles using nitrate reductase enzyme induction of filamentous fungus and their antibacterial evaluation. Artif Cells Nanomed Biotechnol 14:1–9. doi:10.1080/21691401.2016.1267011
Hamedi S, Shojaosadati SA, Shokrollahzadeh S, Hashemi-Najafabadi S (2017) Mechanism study of silver nanoparticle production using Neurospora intermedia. IET Nanobiotech 11:157–163
Hamedi S, Shojaosadati SA, Mohammadi A (2017b) Evaluation of the catalytic, antibacterial and anti-biofilm activities of the Convolvulus arvensis extract functionalized silver nanoparticles. J Photochem Photobiol, B 167:36–44
Hashemi S, Asrar Z, Pourseyedi S, Nadernejad N (2016) Green synthesis of ZnO nanoparticles by olive Olea europaea. IET Nanobiotechnol 10(6):400–404
Jahani S, Beitollahi H (2016) Selective detection of dopamine in the presence of uric acid using NiO nanoparticles decorated on graphene nanosheets modified screen-printed electrodes. Electroanalysis 28(9):2022–2028
Kanda S, Wani C, Middletion E (1994) Free scavenging and antioxidant activity of plant flavonoids. Adv Exp Med Biol 366:354–376
Kemp MM, Kumar A, Mousa S, Park TJ, Ajayan P, Kubotera N, Mousa SA, Linhardt RJ (2009) Synthesis of gold and silver nanoparticles stabilized with glycosaminoglycans having distinctive biological activities. Biomacromol 10(3):589–595
Khatami M, Mehnipor R, Poor MHS, Jouzani GS (2016a) Facile biosynthesis of silver nanoparticles using Descurainia sophia and evaluation of their antibacterial and antifungal properties. J Clust Sci 27(5):1601–1612
Khatami M, Nejad MS, Salari S, Almani PGN (2016) Plant-mediated green synthesis of silver nanoparticles using Trifolium resupinatum seed exudate and their antifungal efficacy on Neofusicoccum parvum and Rhizoctonia solani. IET Nanobiotech 10:237–243
Khatami M, Heli H, Jahani PM, Azizi H, Nobre MAL (2017a) Copper/copper oxide nanoparticles synthesis using Stachys lavandulifolia and its antibacterial activity. IET Nanobiotech 11:709–713
Khatami M, Mortazavi SM, Kishani-Farahani Z, Amini A, Amini E, Heli H (2017b) Biosynthesis of silver nanoparticles using pine pollen and evaluation of the antifungal efficiency. Iran J Biotechnol 15(2):95–101
Khodashenas B, Ghorbani HR (2016) Optimisation of nitrate reductase enzyme activity to synthesise silver nanoparticles. IET Nanobiotechnol 10(3):158–161
Krishnaraj C, Muthukumaran P, Ramachandran R, Balakumaran MD, Kalaichelvan PT (2014) Acalypha indica Linn: biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol Rep 4:42–49
Kulandaivelu B, Gothandam KM (2016) Cytotoxic effect on cancerous cell lines by biologically synthesized silver nanoparticles. Braz Arch Biol Technol 59:1–8. doi:10.1590/1678-4324-2016150529
Mahmoudvand H, Fasihi Harandi M, Shakibaie M, Aflatoonian MR, ZiaAli N, Makki MS, Jahanbakhsh S (2014) Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int J Surg 12(5):399–403
Malorni L, Cacace G, Cuccurullo M, Pocsfalvi G, Chambery A, Farina A, Di Maro A, Parente A, Malorni A (2006) Proteomic analysis of MCF-7 breast cancer cell line exposed to mitogenic concentration of 17β-estradiol. Proteomics 6(22):5973–5982
Markus J, Mathiyalagan R, Kim Y-J, Abbai R, Singh P, Ahn S, Perez ZEJ, Hurh J, Yang DC (2016) Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic Lactobacillus kimchicus DCY51T isolated from Korean kimchi. Enzyme Microb Technol 95:85–93
Meymandi SS, Bahmanyar M, Dabiri S, Aflatonian MR, Bahmanyar S, Meymandi MS (2010) Comparison of cytologic giemsa and real-time polymerase chain reaction technique for the diagnosis of cutaneous Leishmaniasis on scraping smears. Acta Cytol 54(4):539–545
Moghaddam HM, Beitollahi H, Tajik S, Jahani S, Khabazzadeh H, Alizadeh R (2017) Voltammetric determination of droxidopa in the presence of carbidopa using a nanostructured base electrochemical sensor. Russ J Electrochem 53(5):452–460
Moradi M, Sattarahmady N, Rahi A, Hatam GR, Sorkhabadi SMR, Heli H (2016) A label-free, PCR-free and signal-on electrochemical DNA biosensor for Leishmania major based on gold nanoleaves. Talanta 161:48–53
Mortazavi SM, Khatami M, Sharifi I, Heli H, Kaykavousi K, Sobhani Poor MH, Kharazi S, Nobre MAL (2017) Bacterial biosynthesis of gold nanoparticles using Salmonella enterica subsp. enterica serovar Typhi isolated from blood and stool specimens of patients. J Clust Sci 2:1–10. doi:10.1007/s10876-017-1267-0
Moussavi SP, Ehrampoush MH, Mahvi AH, Rahimi S, Ahmadian M (2014) Efficiency of multi-walled carbon nanotubes in adsorbing humic acid from aqueous solutions. Asian J Chem 26(3):821–826
Murugan K, Dinesh D, Kavithaa K, Paulpandi M, Ponraj T, Alsalhi MS, Devanesan S, Subramaniam J, Rajaganesh R, Wei H et al (2016) Hydrothermal synthesis of titanium dioxide nanoparticles: mosquitocidal potential and anticancer activity on human breast cancer cells (MCF-7). Parasitol Res 115(3):1085–1096
Nejad MS, Bonjar GHS, Khatami M, Amini A, Aghighi S (2017) In vitro and in vivo antifungal properties of silver nanoparticles against Rhizoctonia solani, a common agent of rice sheath blight disease. IET Nanobiotech 11:236–240
Niroomand S, Khorasani-Motlagh M, Noroozifar M, Jahani S, Moodi A (2017) Photochemical and DFT studies on DNA-binding ability and antibacterial activity of lanthanum(III)-phenanthroline complex. J Mol Struct 1130:940–950
Oh KH, Soshnikova V, Markus J, Kim YJ, Lee SC, Singh P, Castro-Aceituno V, Ahn S, Kim DH, Shim YJ et al (2017) Biosynthesized gold and silver nanoparticles by aqueous fruit extract of Chaenomeles sinensis and screening of their biomedical activities. Artif Cells Nanomed Biotechnol 6:1–8. doi:10.1080/21691401.2017.1332636
Samadi MT, Zolghadrnasab H, Godini K, Poormohammadi A, Ahmadian M, Shanesaz S (2015) Kinetic and adsorption studies of reactive black 5 removal using multi-walled carbon nanotubes from aqueous solution. Der Pharma Chemica 7(5):267–274
Samuelsen AB (2000a) The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J Ethnopharmacol 71(1–2):1–21
Samuelsen AB (2000b) The traditional uses, chemical constituents and biological activities of Plantago major L. J Ethnopharmacol 71:1–21
Sattarahmady N, Heli H, Moradi SE (2013) Cobalt hexacyanoferrate/graphene nanocomposite—application for the electrocatalytic oxidation and amperometric determination of captopril. Sens Actuators B Chem 177:1098–1106
Sattarahmady N, Tondro GH, Gholchin M, Heli H (2015) Gold nanoparticles biosensor of Brucella spp. genomic DNA: visual and spectrophotometric detections. Biochem Eng J 97:1–7
Seddighi NS, Salari S, Izadi AR (2017) Evaluation of antifungal effect of iron-oxide nanoparticles against different Candida species. IET Nanobiotech 12:1–6. doi:10.1049/iet-nbt.2017.0025
Shimoshige H, Nakajima Y, Kobayashi H, Yanagisawa K, Nagaoka Y, Shimamura S, Mizuki T, Inoue A, Maekawa T (2017) Formation of core-shell nanoparticles composed of magnetite and samarium oxide in Magnetospirillum magneticum strain RSS-1. PLoS ONE 12(1):e0170932
Singh P, Singh H, Ahn S, Castro-Aceituno V, Jiménez Z, Simu SY, Kim YJ, Yang DC (2016) Pharmacological importance, characterization and applications of gold and silver nanoparticles synthesized by Panax ginseng fresh leaves. Artif Cells Nanomed Biotechnol 18:1–10. doi:10.1080/21691401.2016.1243547
Singh P, Kim Y-J, Zhang D, Yang D-C (2016b) Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol 34(7):588–599
Singh P, Kim YJ, Singh H, Ahn S, Castro-Aceituno V, Yang DC (2017a) In situ preparation of water-soluble ginsenoside Rh2-entrapped bovine serum albumin nanoparticles: in vitro cytocompatibility studies. Int J Nanomed 12:4073–4084
Singh P, Singh H, Castro-Aceituno V, Ahn S, Kim YJ, Farh ME-A, Yang DC (2017b) Engineering of mesoporous silica nanoparticles for release of ginsenoside CK and Rh2 to enhance their anticancer and anti-inflammatory efficacy: in vitro studies. J Nanopart Res 19(7):257
Sobhanipoor MH, Ahmadrajabi R, Karmostaji A, Saffari F (2017) Molecular characterization of nasal methicillin resistant Staphylococcus aureus isolates from workers of an automaker company in southeast Iran. APMIS. doi:10.1111/apm.12732
Soshnikova V, Kim YJ, Singh P, Huo Y, Markus J, Ahn S, Castro-Aceituno V, Kang J, Chokkalingam M, Mathiyalagan R et al (2017) Cardamom fruits as a green resource for facile synthesis of gold and silver nanoparticles and their biological applications. Artif Cells Nanomed Biotechnol 14:1–10. doi:10.1080/21691401.2017.1296849
Srivastava V, Negi AS, Kumar JK, Gupta MM, Khanuja SPS (2005) Plant-based anticancer molecules: a chemical and biological profile of some important leads. Bioorg Med Chem 13:5892–5908
SudhaLakshmi GY (2011) Green synthesis of silver nanoparticles from Cleome viscosa: synthesis and antimicrobial activity. Int Conf Biosci Biochem Bioinform 5(1):334–337
Zare E, Pourseyedi S, Khatami M, Darezereshki E (2017) Simple biosynthesis of zinc oxide nanoparticles using nature’s source, and it’s in vitro bio-activity. J Mol Struct 1146:96–103
Acknowledgement
The authors thank Bam University of Medical Sciences, Bam, Iran.
Author information
Authors and Affiliations
Contributions
The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that this article content has no competing interests.
Rights and permissions
About this article
Cite this article
Poor, M.H.S., Khatami, M., Azizi, H. et al. Cytotoxic activity of biosynthesized Ag Nanoparticles by Plantago major towards a human breast cancer cell line. Rend. Fis. Acc. Lincei 28, 693–699 (2017). https://doi.org/10.1007/s12210-017-0641-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-017-0641-z