Abstract
With increasing industrialization and increasing population, excess accumulation of heavy metals such as Cd2+, Cr6+, Cu2+, Ni2+, Pb2+ and Zn2+ in soils have caused the disruption of the terrestrial ecosystem. Different chemical, physical and biological techniques are being explored for the remediation and restoration of such heavy metal-polluted soil. This study aims to evaluate the tolerance capacity of Tagetes erecta for heavy metals Pb2+ and Ni2+ with the objective of using this plant for phytoremediation of heavy metal-polluted soil. The plant is found to tolerate up to 200 mmol of both the test heavy metals. Dry weight, root length, proline content and antioxidant activity increased significantly in all the treatments, whereas shoot length, wet weight and chlorophyll content of plantlets were adversely affected after 21 days of metal treatment in hydroponic culture condition. This study suggests that Tagetes erecta has developed a defense mechanism against heavy metal stress, suggesting its acceptability for phytoremediation in soil conservation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sudden population explosion in the last few years along with exponentially growing industrialization has caused severe heavy metal contamination of air and water as well as soil. This has been a reason for many lethal effects on humans and the complete ecosystem. Besides, it also alters the composition and activity of soil microbial communities (Singh et al. 2014). According to a study conducted by the European Environment Agency (EEA) in 2007, heavy metals are suspected to be the most frequent among the soil contaminants (EEA environmental statement 2007 with data from 2006). Similar trends of heavy metal contaminants in soil were also reported in countries like the USA, England, China, Poland and India (Yahaya et al. 2009). The significant contribution to such soil contamination by heavy metals is a result of mining, energy and fuel production, power transmission, intensive agricultural practices, sludge and industrial effluent dumping and military operations. This leads to significant disparity between the average yield of crops and the record yield. Moreover, the elevated concentrations of both essential and non-essential heavy metals in soil and water can lead to toxicity and growth inhibitions in most plants proving to be dangerous as they also tend to bioaccumulate (Singh et al. 2014; Slater et al. 2006). Such metal toxicity reduces the overall vigor and growth of plants and can operate as a stress factor; which can not only reduce the strength, but also totally inhibit plant growth, converting fertile lands into non-productive barren lands and leading to serious economic losses (Shaikh et al. 2013; McBride et al. 2016).
Ghosh and Singh (2005) have discussed about remediation of metal-contaminated soil by chemical, physical and biological techniques. Removal of contaminated soil for treatment on or off site, and returning the treated soil to the resorted site by excavation, detoxification and/or destruction of the contaminant physically or chemically are a few options available. However, none of these proves to be appropriate because they merely shift the contamination problem elsewhere. Hence, plants capable of tolerating heavy metals are explored, as they decrease the amount of soil disturbance compared to conventional methods, decrease the spread of contaminant via air and also reduce the amount of waste to be landfilled (up to 95%), which can also further be utilized as bio-ore of heavy metals. Most species suitable for phytoremediation purposes belong to the families Brassicaceae and Fabaceae (Raskin et al. 1994), but consumption/utilization of contaminated plant biomass is a cause of alarm (Ghosh and Singh 2005). To embark upon this problem, in this study, we have tested a non-edible, ornamental plant Tagetes erecta, which is not used for consumption by humankind. This can not only clean the soil and render the land useful again, but may also make this phytoremediation economically productive as the ornamental plants used for this purpose can also be marketed without any concern of consumption-related threats.
2 Materials and methods
2.1 Plant material and pretreatment of seeds
Seeds of Tagetes erecta were procured from Anant Seeds and Agro Ltd India. Seed were surface sterilized prior to actual testing to eliminate the contaminants in the following tests. For this, seeds were washed with distilled water three to four times to remove all unwanted particles attached, followed by soaking in a solution of few drops of TWEEN 20 dissolved in distilled water for 20 min after treatment. The seeds were washed with sterile water and dipped in 70% ethanol solution for 2–3 min. The ethanol solution was then drained out and a short treatment of 2% NaOCl2 for 15 s followed by treatment of 1% NaOCl2 for 15 s was given. After this treatment, the seeds were thoroughly washed with sterile distilled water to remove any sterilizer attached to seeds. The seeds were then ready for inoculation.
2.2 Culturing of seeds to check germination potential
Five sterilized seeds were inoculated in triplicate in each Petri dish containing pre-soaked absorbent cotton with ½ Murashige and Skoog’s (1962) components with full-strength vitamins and sugar and half-strength inorganic nutrients. Test metal salts NiSO4·6H2O and Pb (NO3)2 were added separately to achieve the final concentration of range 50–300 mmol of 50 mmol intervals each in the media. 20 ml of each sterile media was spread over the cotton layer of each set of the Petri plate, respectively. The cultures were incubated in 8-h light and 16-h dark photoperiod at 32-μE m−2 s−1 light intensity in a culture room maintained at 25 ± 2 °C (Abraham et al. 2013). The concentrations of salts; in which seed germination was observed, were selected for hydroponic culture test.
2.3 Screening of metal tolerance in hydroponic cultures
The hydroponic medium was prepared using ½ Murashige and Skoog’s (1962) components. The test metal NiSO4·6H2O was added to achieve a final concentration in the range of 50–250 mmol, whereas Pb (NO3)2 was added in the range 100–200 mmol. Ten seeds of similar size and uniform health for each treatment were cultured in 100 ml of hydroponic medium containing the respective concentration of metal in triplicate. Cultures were incubated in light in a culture room at 25 ± 2 °C with 8-h light and 16-h dark photoperiod at 32 μE m−2 s−1-light intensity. Solutions were continuously replenished at 7-day intervals with the respective concentrations of heavy metal-containing media. After 21 days of treatment, the plants were washed two to three times with deionized water and used for further analysis including root length, shoot length, fresh/wet weight, dry weight, proline content, chlorophyll content and ROS scavenging potential analysis.
2.4 Proline estimation
Proline extraction and determination was performed according to Bates et al. (1973). 100 mg of plant tissue was homogenized in 1.2 ml 3% aqueous sulfosalicylic acid and centrifuged at 13,000 rpm for 10 min. 500 µl of supernatant was taken in a test tube and was made up to 1 ml with distilled water and reacted with 1 ml of glacial acetic acid and 1 ml of ninhydrin (2% in acetone). The mixture was incubated at 90 °C for 1 h. The samples were cooled in ice bath and 2 ml of toluene was added and vortexed for 2 min. The upper phase was aliquoted to read the absorbance at 520 nm using a spectrophotometer. Proline content (mg/100 mg wt) was quantified using the ninhydrin acid reagent method using l-proline.
2.5 Chlorophyll content estimation
The treated plantlets were blotted on tissue paper and homogenized with 100 ml of 90% acetone. The homogenate was centrifuged at 10,000 rpm for 10 min. The extract was diluted five times with 90% acetone and used for chlorophyll estimation using spectrophotometer at 652 nm using the formula given by Arnon (1949).
2.6 Determination of ROS scavenging potential
The activity of different concentrations of metal-treated Tagetes erecta (marigold) plantlet extracts was checked for its antioxidant production using the method described by Choi et al. (2002). All plant extracts were diluted in methanol to make up a total 4 ml volume. 1 ml of 0.3 mmol DPPH solution was added to 4 ml of the test plant extract. A blank solution consisting of 5 ml methanol and 1 ml sample solution of different concentrations was used as the negative control. These solutions were allowed to react at RT for 30 min. The absorbance values were measured at 517 nm. The same procedure was applied with ascorbic acid, which was used as a standard to be compared as a superoxide scavenging agent.
2.7 Statistical analysis
Each experiment was performed in triplicate. From individual experiments, the obtained data were statistically analyzed by calculating the standard deviation and error bars based on the standard deviations. Dunnett’s test was conducted by computing the t test between the mean value of treatment and the control. Mean values higher than the Dunnett table value were considered significant in comparison to the control and marked accordingly.
3 Results
All the results are given in Table 1.
Seed germination potential was analyzed to select those concentrations of metals which are normally tolerated by the Tagetes erecta seeds at the time of germination. In the case of both, the metal tolerance capacity was found to decrease with increase in the metal concentration in the medium. In the case of Ni2+, 80% seeds geminated in 100 and 150 mmol metal concentrations within 5 days, whereas 60–70% seeds germinated within 7 days in the 200 and 250 mmol metal-containing media. In 300 mmol metal concentrations in medium, only 20% seeds out of ten germinated in a week. Similar results were obtained for 100–200 mmol concentration of lead. However, seeds germinated at very less frequency in 250 and 300 mmol concentration of lead in the medium. The range of 100–250 mmol concentration of Ni2+ and 100–200 mmol concentration of Pb2+ was selected for further studies.
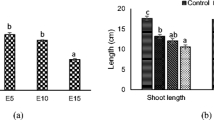
The total fresh weight, dry weight, shoot length and root length of the treated plantlets were recorded after 21 days of treatment (Figs. 1, 2). Plantlets survived up to 250 mmol concentration of Ni2+ with a reduction in the overall growth. The fresh weight, dry weight, shoot length and root length were affected significantly at all concentrations of Ni2+. The wet weight and shoot length decreased, whereas root length and dry weight increased at 100–250 mmol of Ni2+ concentration in media, as compared to control plants. Similar observations were noted in case of Pb2+ (100–200 mmol)-treated plantlets.
The proline content in the metal-treated plantlets had increased significantly with increase in concentration of Ni2+ (100–250 mmol) and Pb2+ (100–200 mmol) as compared to that of the control plantlets. In fact, Pb2+-treated plantlets showed higher proline accumulation as compared to Ni2+-treated plantlets (Fig. 3). The test plantlets showed symptoms of chlorosis at all the different concentrations of the metal. However, the chlorophyll content had equally decreased at all concentrations significantly (Fig. 4). The ROS scavenging potential of both the metals in the lower concentration (100 mmol)-treated plantlet extracts of the test plant was much more comparable to that of the control, but it was seen to significantly increase with increase in the concentration of both the metals in the medium (Fig. 5).
4 Discussion
The reduced shoot length of Tagetes erecta plantlets in metal treatments can be attributed to the reduction in meristematic cells present in this region as also evidenced by Sharifah and Hishashi (1992). These meristematic cells and some enzymes contained in the cotyledon and endosperm cell become active and begin to digest and store food, which is converted into soluble form and transported to the radical and plumule tips: e.g., enzyme amylase converts starch into sugar and protease acts on protein (Sharifah and Hishashi 1992). So when enzymatic activities were affected by heavy metal treatment, the food did not reach the plumule and thus the rates of seed germination and plant growth were affected. Although heavy metals generally affect the overall growth of the plant, our results reported increase in root length and dry weight, which have also been reported by Acharya and Sharma (2014) and Aldoobie and Beltagi (2013). This increase in dry weight and root length may be attributed to metal hyperaccumulation in the plantlet and the struggle for nutrient availability from the soil.
Proline, an amino acid, plays a highly beneficial role in plants exposed to various stress conditions. Proline is advocated not only as an excellent osmolyte, but also plays three major roles during stress, i.e., as a metal chelator, an antioxidative defense molecule and a signaling molecule. (Hayat et al. 2012). Proline accumulation under heavy metal stress condition has also been reported in some higher plants; like Cajanus cajan, Vigna mungo, Triticum aestivum and Phaseolus vulgaris, as well as in marine algae like Salicornia brachiate, as an important protective tool (Alia et al. 1991; Bassi and Sharma 1993; Sharma et al. 1998, 2010). Proline hyperaccumulation under stress condition plays a major role in osmoregulation, which is the general requirement of plants to overcome almost all abiotic stresses. Along with this, the antioxidative activity of proline is also reported to protect plants against injury caused by heavy metals (Slater et al. 2006). As plants are subjected to stress condition during heavy metal exposure, proline scavenges singlet oxygen and free radical-induced damages and performs an important role in the protection of proteins against denaturation (Alia et al. 1991).
Chlorophyll decrease is one of the regular symptoms of heavy metal toxicity in plants which generally relates to poor synthesis of chlorophyll pigments in stressful conditions (Gopal et al. 2002). This may occur during heavy metal treatment due to the intervention of those in different chlorophyll biosynthesis pathways.
Abiotic stresses like heavy metals exert a reactive oxygen species (ROS) stress which may lead to a variety of damaging effects. These ROS, being hyperactive, rapidly attack important biomolecules including proteins, carbohydrates, nucleic acid, etc., of the cell leading to irreversible damage to it (Slater et al. 2006). The antioxidant defense system can counteract these dangerous effects of ROS. Antioxidant enzymes prevent conversion of O2 − radical to more deleterious OH− (Liszkay et al. 2003). The increasing concentration of ROS scavenging potential in test plants thus can be attributed to increased antioxidant production, giving them the ability to tolerate increasing concentration of heavy metal.
Based on hydroponics assays, Tagetes erecta can tolerate heavy metal Pb2+ and Ni2+ stress in soil. Their tolerance capacity can be attributed to the antioxidants produced naturally within the plant to tackle the stress and its related effects. Being the first report for Tagetes erecta for heavy metal Pb2+ and Ni2+ tolerance, this study opens new avenues for research for improved phytoremediation. This study advocates the plant’s natural ability to resist heavy metal stress, which if improved through different ways like introduction of stress-resistant genes can establish it as one of the good tools for phytoremediation.
References
Abraham KR, Sridevi B, Suresh, Damodharam T (2013) Effect of heavy metals (Cd, Pb, Cu) on seed germination of Arachis hypogeae L. Asian J Plant Sci Res 3(1):10–12
Acharya S, Sharma DK (2014) Study on the effects of heavy metals on seed germination and plant growth on Jatropha curcas. Int J Agric Sci Res 3(3):31–34
Aldoobie NF, Beltagi MS (2013) Physiological, biochemical and molecular responses of common bean (Phaseolus vulgaris L.) plants to heavy metals stress. Afr J Biotechnol 12(29):4614–4622
Alia PSP, Pardha SP, Mohanty P (1991) Proline enhances primary photochemical activities in isolated thylakoid membranes of Brassica juncea by arresting photoinhibitory damage. Biochem Biophys Res Commun 181:1238–1244
Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenoxidase in beta vulgaris. Plant Physiol 24:1–15
Bassi R, Sharma SS (1993) Proline accumulation in wheat seedling exposed to zinc and copper. Phytochem 33:1339–1342
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–209
Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY, Park SH, Kim SK (2002) Antioxidant activity and free radical scavenging capacity between Korean medicinal plant and flavonoids by assay-guided comparison. Plant Sci 163:1161–1168
EEA environmental statement 2007 with data from 2006. Office for Official Publications of the European Communities, Copenhagen K Denmark, Luxembourg
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of its byproducts. Appl Ecol Environ Res 3(1):1–18
Gopal R, Mishra KB, Zeeshan M, Prasad SM, Joshi MM (2002) Laser-induced chlorophyll fluorescence spectra of mung plants growing under nickel stress. Curr Sci 83:880–884
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments—a review. Plant Signal Behav 7(11):1456–1466
Liszkay A, Kenk B, Schopfer P (2003) Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta 217:658–667
McBride MB, Martinez CE, Kim B (2016) Zn, Cd, S and trace metal bioaccumulation in willow (Salix spp.) cultivars grown hydroponically. Int J Phytoremediat 8(12):1178–1186
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:431–497
Raskin I, Kumar NPBA, Dushenkov V, Salt DE (1994) Bioconcentration of heavy metals by plants. Curr Opin Biotechnol 5:285–290
Shaikh IR, Shaikh PR, Shaikh RA, Shaikh AA (2013) Phytotoxic effects of heavy metals (Cr, Cd, Mn and Zn) on wheat (Triticum aestivum L) seed germination and seedlings growth in black cotton soil of Nanded. Indian J Chem Technol 3(6):14–23
Sharifah BA, Hishashi O (1992) Effect of lead, cadmium and zinc on the cell elongation of impatiens balsmina. Environ Exp Bot 32:439–448
Sharma SS, Schat H, Vooijs R (1998) In vitro alleviation of heavy metal induced enzyme inhibition by proline. Phytochemistry 46:1531–1535
Sharma A, Gontia I, Agarwal PK, Jha BA (2010) Accumulation of heavy metals and its biochemical responses in Salicornia brachiata, an extreme halophyte. Mar Biol Res 6:511–518
Singh J, Hembram P, Basak J (2014) Potential of Vigna unguiculata as a phytoremediation plant in the remediation of Zn from contaminated soil. Am J Plant Sci 5:1156–1162
Slater A, Scott NW, Fowler MR (2006) Plant biotechnology—the genetic manipulation of plants, 2nd edn. Oxford University Press, New York
Yahaya MI, Mohammad S, Abdullahi BK (2009) Seasonal variations of heavy metals concentration in abattoir dumping site soil in Nigeria. J Appl Sci Environ Manag 13(4):9–13
Acknowledgements
The authors are thankful to Dr. Sanjay Nagar for his constant support during working of the project and to Dr. Iti Gontia-Mishra for her valuable suggestions in the manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bardiya-Bhurat, K., Sharma, S., Mishra, Y. et al. Tagetes erecta (marigold), a phytoremediant for Ni- and Pb-contaminated area: a hydroponic analysis and factors involved. Rend. Fis. Acc. Lincei 28, 673–678 (2017). https://doi.org/10.1007/s12210-017-0636-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-017-0636-9