Abstract
Complete mineralization of dye effluent was realized through sequential anaerobic followed by aerobic processes. The aim of this study was to evaluate and compare the degradation of wastewater of four remazol dyes with integrated anaerobic/aerobic (SBR1) and aerobic (SBR2) processes under sequencing batch reactor operation. The ORP profiles show that the SBR1 was in anaerobic condition during the 15 h React mode and turned to aerobic condition through air aeration for 3 h. The SBR1 and SBR2 achieved almost similar performance in the removal of organic compounds with average 95% COD removal. The SBR1 performed better in the color removal with 93% removal efficiency while the SBR2 only attained 50% removal efficiency. The biodegradation rate of the remazol dyes was well described by the first-order kinetic model with the sequence degradation rate remazol pink > remazol violet > remazil yellow > remazol green.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Textile industries develop rapidly and have contributed to the economy growth to our country, Malaysia. The discharge of colored effluent to the environment must be prohibited because the dye molecules and intermediate compounds that produce from the partially treated dye effluent in wastewater treatment plants are toxic to the biological activities in the surface waters such as lakes and rivers. The conventional treatments of dye effluent and organic pollutants include adsorption, coagulation and flocculation, advanced oxidation processes, filtration and biological processes (Yaacob et al. 2016; El-Sheekh et al. 2016; El-Zaher et al. 2016). The selection of the treatment techniques normally is based on the availability of technology, knowledge, financial, environmental concern, maintenance, etc.
Biological treatment of dye effluent has some merits such as low cost, less maintenance and is environmental friendly compared to the physical and chemical treatment techniques. The aerobic and anaerobic processes for treatment of dye effluent are reported by many groups of researchers and the anaerobic process has shown better results compared to aerobic process (Wijetunga et al. 2010; Khalid et al. 2009; Sirianuntapiboon et al. 2007; Venkata Mohan et al. 2007; Ong et al. 2008). The anaerobic process could reduce the azo bond of dye molecule and the intermediates compounds that generate could be further mineralized through aerobic process. Thus, a sequential anaerobic followed by aerobic treatment technique is considered as very efficient in mineralization of dye effluent. The combination of anaerobic and aerobic processes can be realized either using two separated treatment tanks or in a single treatment tank (Işık and Sponza 2006; Bonakdarpour et al. 2011; Ong et al. 2012).
The objective of this study was to evaluate and compare the treatment performance between integrated anaerobic/aerobic SBR and aerobic SBR for removing four remazol dyes containing wastewater. A first-order kinetic model was employed to compare the biodegradation rate among the four remazol dyes in batch study.
2 Materials and methods
Four types of remazol dyes, remazol brilliant green, remazol brilliant pink, remazol Violet and remazol yellow, which are commonly used for batik dye in Malaysia, were employed in this study. 1 g of each remazol dyes was dissolved in a 1 L volumetric flask with ultrapure water and stirred to ensure the dye stuff was well mixed. The solution was used in stock solution for preparing the synthetic wastewater. The synthetic wastewater used throughout this study was containing 580 mg/L NaCl, 680 mg/L KH2PO4, 280 mg/L NH4Cl, 1000 mg/L sucrose and 80 mg/L of each remazol dye.
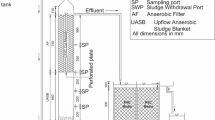
Two laboratory scale sequencing batch reactors (SBR), namely integrated anaerobic/aerobic SBR (SBR1) and aerobic SBR (SBR2), were developed to simulate the biological process for removing the remazol dyes containing wastewater. The experimental setup for the SBRs is shown in Fig. 1. The SBRs were constructed from polyvinyl chloride (PVC) cylinders (760 mm H × 106 mm D) with working volume of 6 L. Sampling ports were provided along the reactors. 4.5 L of synthetic wastewater was fed into the SBRs during Fill mode using a peristaltic pump. The seeding of activated sludge was done by adding 1.5 L of concentrated biological sludge collected from Shorubber (M) Sdn. Bhd., Kangar, Malaysia. Air was supplied by diffuser located at the middle level of SBR1 and at the bottom level for SBR2. The operation of peristaltic pumps, diffuser and stirrer was controlled by timers that have been setup according to the operation mode as shown in Table 1. Both the SBRs were operated in 24 h per cycle with fill mode 1 h, react mode 18 h, Settle mode 4 h, Decant mode 45 min and idle mode 15 min. In SBR 1, the aeration with diffuser only operated during the last 3 h of React mode, whereas the air was provided for all 18 h of react mode in SBR2. The SBRs were operated in two phases with the first phase for determining the operating mode for integrated anaerobic/aerobic (SBR1) and aerobic (SBR2) processes, whereas the second phase for evaluating the performance of both SBRs in remazol dye removal.
Daily water sampling was collected for the influent and effluent from SBR1 to SBR2. The water sample was filtered with 0.45 µm filter paper and then analyzed for COD, color, DO and pH. The COD was measured with DR2800 reactor whereas the color was measured using UV–Vis spectrophotometer (HITACHI U-2810). For color measurement, the water sample was scanned with the UV–Vis spectrophotometer from 200 to 700 nm and it was observed the maximum absorbance peak at 544 nm. The percentage of color removal from the synthetic wastewater can be measured by reference to the absorbance reduction at 544 nm. The ORP and pH were measured using Hanna ORP and pH meter, respectively. The MLSS of the SBRs also analyzed using APHA method.
3 Results and discussion
The biodegradation of dye is a co-metabolism process where the presence of organic co-substrate is very important as electron donor for the reduction of dye. Sucrose is a highly biodegradable organic compound and used as electron donor in this study, and contributed with average 1200 mg/L of COD. As shown in Fig. 2a, the COD removal from both SBR1 and SBR2 was far different at the initial stage. The aerobic process normally has better performance in the degradation of highly biodegradable organic compound like sucrose compared to the anaerobic process. However, as the operating time of the SBRs proceeded, it was observed that the COD removal efficiency in SBR1 improved gradually and attained similar performance as SBR2. Actually, a few of the operating conditions was studied by changing the aeration duration in SBR1 to obtain similar removal efficiency as SBR2. This shows that the operating condition as shown in Table 1 was the optimum for integrated anaerobic/aerobic in SBR1 in bio-oxidation of organic compound.
Once the optimum operating condition was achieved, the performance of SBRs on the removal of remazol dye synthetic wastewater was evaluated for about 60 days. As shown in Fig. 2b, the COD removal in SBR1 improved gradually throughout the study with approximately 80% removal efficiency at initial stage and attained 95% removal efficiency at the end of the experiment. On the other hand, the COD removal efficiency in SBR2 was deteriorated at the initial stage followed by stable removal efficiency and then slightly improved at the final stage. The different trend of COD removal efficiency could be ascribed to the different processes involved in the SBR1 and SBR2. In the SBR1, the aeration only provided during the last 3 h in the React mode and the microorganisms in the bioreactor were in anaerobic condition, which promotes the reduction of color in remazol dye and the intermediate products generated could be further mineralized by aerobic microbes during the last 3 h of air aeration in React mode. Thus, the integrated anaerobic/aerobic processes may contribute to the mineralization of remazol dye once the microorganisms in the SBR1 could adapt to the remazol dye synthetic wastewater. As shown in Fig. 3, the color removal efficiency in SBR1 was much higher than SBR2, which shows that the integrated anaerobic/aerobic processes in SBR1 could remove the color of remazol dye effectively. Overall, the average percentage of color removal in SBR1 and SBR2 was 92 and 50%, respectively. The higher removal efficiency of color in SBR1 shows that the 15 h of anaerobic condition was enough for decolorization of remazol dye.
However, in the SBR2, the aeration was provided throughout the React mode and this promoted the growth of aerobic microorganisms in the bioreactor. As reported by other researchers, most of the color removal in dye wastewater was achieved in the anaerobic condition (Spagni et al. 2010; Ong et al. 2013; Gnanapragasam et al. 2011). Thus, the aerobic condition in SBR2 did not promote the degradation of remazol dye and the average 50% of color removal (Fig. 3) could be due to the adsorption of the dye onto the activated sludge, and subsequently reduced the COD in effluent. However, once the surface area of the activated sludge was saturated with the remazol dye molecule, the color removal efficiency decreased and the COD increased in the effluent. As reported by Sirianuntapiboon and Srisornsak (2007), the high adsorption ability of resting bio-sludge could be used as an adsorbent of both organic matter and disperse dye. The removal of dyestuffs, Acid Yellow 17 and Maxilon Red BL-N, by adsorption on to live activated sludge was also reported by Chu and Chen (2002).
The oxidation–reduction potential (ORP) can be used as a measure of the aerobic and anaerobic conditions of aquatic environment. Redox potentials greater than 100 mV are commonly interpreted as aerobic environment, whereas those less than −100 mV are considered as anaerobic environment (Suthersan 2002). As shown in Fig. 4, it was observed that the SBR1 and SBR2 show different pattern in ORP monitoring during React mode. Generally, the SBR1 was operated in integrated anaerobic–aerobic condition, and the ORP was in negative value, while the SBR2 which was operated in aerobic condition showed a positive value for ORP. The ORP values for both SBR1 and SBR2 were increased until they reached a stable state at approximately -350 and +145 mV, respectively. The ORP value shows that the condition of SBR1 and SBR2 was perfectly maintained under anaerobic and aerobic condition, respectively. The development of reducing environment in the integrated anaerobic/aerobic SBR was very crucial for decolorization of remazol dye and it recorded about 92% of color removal efficiency.
The kinetic removal of four remazol dyes was conducted in batch study with initial concentration of 100 mg/L. A simple first-order model was used to describe and compare the biodegradation rate of the four remozal dyes. There are quite a number of reports that show that the decolorization of dyes through bioactivity followed the first-order model (Van der Zee et al. 2001; Carliell et al. 1995; Sponza and Işık 2004). As shown in Fig. 5, the experimental data collected for biodegradation of four remazol dyes were well fitted with the first-order kinetic model with correlation coefficients greater than 0.90. It was observed that the degradation rate varies with the types of remazol dye. The biodegradation of remazol pink was the most rapid, whereas the remazol green was the slowest being degraded by the microorganisms. The sequence of the biodegradation rate of the dyes was remazol pink > remazol violet > remazol yellow > remazol green. The different degradation rate could be due to their different molecular structure of the dye molecule. Sponza and Işık (2004) reported the decolorization of Direct Black 38 followed a first-order model with kinetic constants in the ranges of 0.0532–0.0688 h−1 at concentrations of 200 and 3200 mg/L. On the other hand, Van der Zee et al. (2001) investigated the biodegradation of 20 selected azo dyes and the degradation of the dyes also followed a first-order model with kinetic constants varying between 0.01 and 10 day−1.
4 Conclusions
The integrated anaerobic/aerobic SBR performed better than aerobic SBR in terms of COD and color removal efficiency. The OPR profiles showed that the anaerobic and aerobic conditions were well developed during the React mode in integrated anaerobic/aerobic SBR. Consequently, the removal of color was realized in 13 h of anaerobic condition, whereas the aromatic compounds generated may be further mineralized during the last 3 h of air aeration. The data obtained from the biodegradation of four remazol dyes could be expressed by a first-order kinetic model. From the kinetic constants, the biodegradation rate of remazol pink was the highest, whereas vice versa in the case of remazol green.
References
Bonakdarpour B, Vyrides I, Stuckey DC (2011) Comparison of the performance of one stage and two stage sequential anaerobic–aerobic biological processes for the treatment of reactive-azo-dye-containing synthetic wastewaters. Int Biodeterior Biodegrad 65(4):591–599
Carliell CM, Barclay SJ, Naidoo N, Buckley CA, Mulholland DA, Senior E (1995) Microbial decolourasition of a reactive azo dye under anaerobic conditions. Water SA 21(1):61–69
Chu HC, Chen KM (2002) Reuse of activated sludge biomass: II. The rate processes for the adsorption of basic dyes on biomass. Process Biochem 37(10):1129–1134
El-Sheekh MM, Farghl AA, Galal HR, Bayoumi HS (2016) Bioremediation of different types of polluted water using microalgae. Rend Fis Acc Lincei 27(2):401–410
El-Zaher EHFA, Abou-Zeid AM, Mostafa AA, Arif DM (2016) Industrial oil wastewater treatment by free and immobilized Aspergillus niger KX759617 and the possibility of using it in crop irrigation. Rend Fis Acc Lincei. doi:10.1007/s12210-016-0578-7
Gnanapragasam G, Senthilkumar M, Arutchelvan V, Velayutham T, Nagarajan S (2011) Bio-kinetic analysis on treatment of textile dye wastewater using anaerobic batch reactor. Bioresour Technol 102(2):627–632
Işık M, Sponza DT (2006) Biological treatment of acid dyeing wastewater using a sequential anaerobic/aerobic reactor system. Enzyme Microb Technol 38(7):887–892
Khalid A, Arshad M, Crowley DE (2009) Biodegradation potential of pure and mixed bacterial cultures for removal of 4-nitroaniline from textile dye wastewater. Water Res 43(4):1110–1116
Ong SA, Toorisaka E, Hirata M, Tadashi H (2008) Combination of adsorption and biodegradation processes for textile effluent treatment using a granular activated carbon-bioflm confgured packed column system. J Environ Sci 20(8):952–956
Ong SA, Ho LN, Wong YS, Raman K (2012) Performance and kinetic study on bioremediation of diazo dye (reactive black 5) in wastewater using spent GAC-biofilm sequencing batch reactor. Water Air Soil Pollut 223(4):1615–1623
Ong SA, Ho LN, Wong YS, Komalathevi R, Bahari R (2013) Mineralization of Methyl Orange-containing wastewater by integrated anaerobic and aerobic processes using spent granular activated carbon-biofilm under sequencing batch reactor operation. Desalination Water Treat 51(13–15):2813–2819
Sirianuntapiboon S, Srisornsak P (2007) Removal of disperse dyes from textile wastewater using bio-sludge. Biores Technol 98(5):1057–1066
Sirianuntapiboon S, Sadahiro O, Salee P (2007) Some properties of a granular activated carbon-sequencing batch reactor (GAC-SBR) system for treatment of textile wastewater containing direct dyes. J Environ Manage 85(1):162–170
Spagni A, Grilli S, Casu S, Mattioli D (2010) Treatment of a simulated textile wastewater containing the azo-dye reactive orange 16 in an anaerobic-biofilm anoxic–aerobic membrane bioreactor. Int Biodeterior Biodegradation 64(7):676–681
Sponza DT, Işık M (2004) 0. Decolorization and inhibition kinetic of Direct Black 38 azo dye with granulated anaerobic sludge. Enzyme Microb Technol 34(2):147–158
Suthersan SS (2002) Natural and enhanced remediation systems. Acradis, Lewis Publisher, Washington, DC
Van der Zee EP, Lettinga G, Field JA (2001) Azo dye decolorization by anaerobic granular sludge. Chemosphere 44:1169–1176
Venkata Mohan S, Chandrasekhara Rao N, Sarma PN (2007) Simulated acid azo dye (Acid black 210) wastewater treatment by periodic discontinuous batch mode operation under anoxic–aerobic–anoxic microenvironment conditions. Ecol Eng 31(4):242–250
Wijetunga S, Li XF, Jian C (2010) Effect of organic load on decolourization of textile wastewater containing acid dyes in upflow anaerobic sludge blanket reactor. J Hazard Mater 177(1–3):792–798
Yaacob NS, Mohamad R, Ahmad SA, Abdullah H, Ibrahim AL, Ariff AB (2016) The influence of different modes of bioreactor operation on the efficiency of phenol degradation by Rhodococcus UKMP-5M. Rend Fis Acc Lincei 27(4):749–760
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ong, SA., Ho, LN., Wong, YS. et al. Comparative study on the biodegradation of mixed remazol dyes wastewater between integrated anaerobic/aerobic and aerobic sequencing batch reactors. Rend. Fis. Acc. Lincei 28, 497–501 (2017). https://doi.org/10.1007/s12210-017-0622-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-017-0622-2