Abstract
In this work, we characterized a novel acrylamide-degrading bacterium with the ability to degrade diesel. Tentatively, the isolate was identified as Burkholderia sp. strain DRY27 and was shown to have increased growth rate on media supplemented with 0–3 % (v/v) diesel. We showed that sodium nitrate is the best nitrogen source for the bacterium growth on diesel. The optimal temperature and optimal pH supporting growth on diesel were between 10 and 40 °C and pH 7.5–8.5, respectively. Growth kinetics modeling showed that the Haldane model gave a correlation coefficient value of 0.99 and was better than other kinetic models such as Luong or Monod. Using the Haldane model, the maximum growth rate (µ max) was 0.305 h−1, while the saturation constant or half-velocity constant K s and inhibition constant K i, were 1.171 % (v/v) or 9.95 g/L and 3.215 % (v/v) or 27.32 g/L diesel, respectively. Microbial adhesion to hydrocarbon assay showed that after extraction, 65 % of the bacterium was found in the hexadecane phase indicating that the bacterium was hydrophobic. We showed that diesel components were completely removed based on the reduction in the hydrocarbon peaks monitored by solid-phase microextraction gas chromatography analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pollutions of soils and aquatic bodies are usually caused by industrials’ wastes. The physical and chemical treatments that exist nowadays are expensive and are not able to remove trace quantities of pollutant. There are a few successful remediation techniques that have been developed and used in petroleum-contaminated sites (Mohammed et al. 2007). Indigenous bioremediation plays an important part in diesel pollution because diesel produces damaging vapors and intolerable smell that have to remediate immediately (Mohammed et al. 2007; Claassens et al. 2006). Other advantages include easy to maintain, can be apply to large area, affordable, and completely degrade the contaminant (Bento et al. 2005).
In Malaysia, oil and grease are ranked as the highest industrial pollution (DOE 2007). Since Malaysia is one of the oil and gas producer in the world, oil pollution could not be avoided especially since Malaysia owns the Straits of Malacca—the busiest waterway in the world. Contamination occurs in Malaysia mostly caused by human error. For instance, when two oil tankers collided with each other in the coastal areas of the Straits of Malacca spilling, almost 150 tons of diesel making the case to be one of the largest hydrocarbon spills to be reported (Berita Harian Online 1997). Other reports include an overturned lorry tanker spilling almost 15 tons of diesel contaminating the soils in Seremban (The New Straits Times 2000) and spilling of one ton of diesel into the soils in Gelugor from a 1,000 kW-mobile generator unit (The New Straits Times 2001). A locally isolated bacterial consortium that can effectively degrade diesel has been reported (Ghazali et al. 2004). Even though there are a lot of reports on the isolation of diesel-degrading bacteria, the search for the best degrader is still going at full speed to isolate bacteria with better properties to improve diesel remediation.
In this work, we report on the isolation of a diesel-degrading bacterium that is shown to be able to optimally degrade diesel at a broad range of temperature from 10 to 40 °C. Based on the characteristics of this bacterium, it is practical to be used as a bioremediation agent in the tropics.
2 Experimental work
2.1 Isolation of diesel-degrading bacteria
10 g of soil sample was taken randomly from a depth of 15–20 cm from topsoil and stored in sterile screw-capped vials. The soil samples were taken in the year 2004 near Bukit Ekspo (Universiti Putra Malaysia). The soil samples were resuspended in 10 mL of sterile saline solution (0.9 % NaCl) and were vigorously shaken for 5 min. A basal salt medium consists of diesel as sole carbon source was used as the enrichment culture media. A modified basal salt medium composed of; KH2PO4, 1.360 g; Na2HPO4, 1.388 g; KNO3, 0.5 g; MgSO4, 0.01 g; CaCl2, 0.01 g; (NH4)2SO4, 7.7 g; and 100 mL of a mineral solution containing 0.01 g of ZnSO4·7H2O, MnCl2·4H2O, H3BO4, CoCl2·6H2O, Fe2SO4·2H2O, CuCl2·2H2O, and NaMoO4·2H2O (Michaud et al. 2004). The flasks were then incubated at 30 °C and were shaken at 150 rpm (YIH DER Taiwan) for 7 days. Isolation and enumeration were performed using the spread plate technique. The cultures were then incubated at 30 °C. Isolates showing a distinct colonial morphologies were isolated by repeated sub-culturing into basal salt medium and solidified basal salt medium to obtain purified strains. Identification was performed using Biolog GN MicroPlate (Biolog, Hayward, CA, USA) according to the manufacturer’s instructions and molecular phylogenetic studies.
2.2 Diesel analysis using gas chromatography
The fingerprint of the individual diesel residues and the intermediate products produced was quantified using Varian 2900 (Varian, USA) Gas Chromatograph equipped with a flame ionization detector (FID) fitted with a Chrompack Capillary Column, WCOT Fused Silica 30 m × 0.39 (film thickness 0.25 μm) (Varian). The column temperature parameters were set at an initial temperature of 50 °C for 5 min followed by a 10 °C increment per minute to 30 °C and the isothermal held for 10 min. Carrier gas velocity was 30 mL/min, and makeup gas velocity was 30 mL/min with a total run time of 35 min.
2.3 Solid-phase microextraction (SPME)
An SPME [Polydimethylsiloxane(PDMS), 7 μm thickness, Supelco, USA] was used as a hydrocarbon compounds extraction device. Since diesel fuel is volatile, it is well suited for sampling with SPME fibers (Eriksson et al. 1998). To analyze the aromatic hydrocarbons during the biodegradation process, 1.5 mL of homogenized culture was extracted from the incubated growth medium and filtered through 0.45 μm (Milipore) membrane and stored in 1.5 mL Eppendorf tube. For GC analysis, 100 μL of the diesel constituents was transferred into 1.5 mL glass vials and heated on a hot plate. Teflon septum was pierced through by a SPME fiber coated with a 7 μm polydimethylsiloxane layer (Supelco USA), and was pushed down into the middle of the static headspace using SPME holder Supelco (Bellefonte, PA, USA). The fiber was then retracted after extraction (headspace) at 110 °C for 10 min and immediately inserted manually into the injector for GC analysis.
2.4 16S rDNA gene sequencing
Alkaline lysis was performed to extract genomic DNA from bacterial colonies. PCR amplification was performed using a thermal cycler (Biometra, Gottingen, Germany). The PCR mixture with a final volume of 50 µL consists of 0.5 pM of each primer, 200 µM of each deoxynucleotide triphosphate, 1X reaction buffer, 2.5 U of Taq DNA polymerase (Promega). The 16S rDNA gene from the genomic DNA was amplified using the following primers: 5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-AAGGAGGTGATCCAGCCGCA-3′ corresponding to the forward and reverse primers of 16S rDNA, respectively (Devereux and Wilkinson 2004). PCR was performed under the following conditions: initial denaturation at 94 °C for 3 min; 25 cycles of 94 °C for 1 min, 50 °C for 1 min, and 72 °C for 2 min; and a final extension at 72 °C for 10 min. Cycle sequencing was subsequently performed with the Big Dye terminator kit (Perkin-Elmer Applied Biosystems) as recommended by the manufacturer.
2.5 Sequence analysis
The BLAST 2 sequences algorithm using the BLASTN option with the matrix turned off and default parameters available from the server NCBI (http://www.ncbi.nlm.nih.gov/blast/) was used to analyze the pairwise comparisons to measure the level of homology between the two nucleotide sequences of the forward and the reverse complement of the reverse primer sequence. The two sequences were compared and checked for errors and omissions of bases especially at the overlapped region based on the overlapped region between the forward and reverse complement of the reverse primer sequence using the CHROMAS software Version 1.45. The sequences were combined at bases giving the least ambiguous characters and gaps. The comparison of the combined 16S RNA gene sequence, and the resultant 758 bases were compared with the GenBank database using the Blast server at NCBI (Altschul et al. 1990). This analysis showed that the sequence is closely related to rrs from Gammaproteobacteria. The partial 16S rRNA ribosomal gene sequences for this isolate have been deposited in GenBank under the following accession number DQ851856. The Blast server at NCBI (http://www.ncbi.nlm.nih.gov/BLAST/) was used to compare the resultant 852 bases. This analysis showed that this sequence is similar to Burkholderia species with 99 % similarity. The results obtained from Biolog™ identification system showed that Isolate 27 was similar to Burkholderia cepacia with 75 % similarity. Together with the Biolog™ results, for now, isolate 27 is assigned tentatively as Burkholderia sp. strain DRY27 (Gusmanizar et al. 2008).
2.6 Determination of kinetic parameters for growth on diesel
Determination of intrinsic growth kinetic parameters can be modeled based on several substrate inhibition models available such as Haldane and Luong compared to the commonly used Monod model due to the known inhibitory effect of high concentration of diesel to growth (Mulchandani et al. 1989; Zhukov et al. 2007; Abdel Megeed and Mueller 2009). The formulas for the above model are shown in Table 1 where u, u max, K s, K i, S, S m, and n are specific growth rate (h−1), maximum growth rate (h−1), half-saturation constant (mg/L), inhibition constant (mg/L), substrate concentration (mg/L), critical substrate concentration above which growth completely stops (mg/L) and the exponent representing the impact of the substrate to u max, respectively. The values of the specific growth rate coefficient µ at each initial diesel concentration could be obtained by plotting In X (bacterial numbers) vs. time. When these values were plotted against substrate concentrations, a nonlinear curve will be obtained and modeling could be done to determine the constants. CurveExpert Professional software (Version 1.6) with custom equation algorithm was used to find the constants.
2.7 MATH assay
A modified method of Rosenberg (1984) and Zoueki et al. (2010) was used for the MATH assay. The bacterial suspension was adjusted to an absorbance of 1.0 at 600 nm on a spectrophotometer (Shimadzu) with the addition of NaCl to a final concentration of about 0.2 M. About 300 µL of hexadecane was added to 5 mL of bacterial suspension in a clean borosilicate round-bottom glass tube (16 × 150 mm, Pyrex). The tube was vortexed for 2 min and then was set aside resting for 15 min to allow for the phases to separate. About 2 mL of the bacterial suspension was removed carefully using Pasteur pipet and transferred to a quartz cuvette for absorbance measurement at 600 nm. Bacterial adhesion to the hydrocarbons was evaluated using the formula FPC = 1−A f/A o where FPC is a fraction partitioned to the hydrocarbon phase, A f is final absorbance and A o is initial absorbance.
3 Results and discussion
3.1 Temperature and pH effects on growth
Based on Fig. 1, strain DRY27 grew optimally in a wide range of temperatures ranging from 10 to 40 °C and the growth rate grew dramatically at higher temperatures. The optimum temperature for strain DRY27 growth was 30 °C. Temperature of 30 °C was reported to be the most optimum temperature for diesel degradation by other microorganisms (Cavalca et al. 2000; Mukherji et al. 2004; Hong et al. 2005; Lee et al. 2006; Kwapisz et al. 2008). In Ma and Herson (2000), growth of Burkholderia sp. was reported to be at 37 °C. Growth at a lower temperature optima in between 10 and 15 °C (Shukor et al. 2009b), 10 and 25 °C (Margesin 2000), at 20 °C (Chapman and Shelton 1995; Lee et al. 2005; Ueno et al. 2007), at 27 °C (Rajasekar et al. 2007) and in between 27 and 37 °C (Shukor et al. 2009a) has also been reported. Diesel degradation at a higher temperature by a bacterium has been described by Márquez-Rocha et al. (2005). Rhodococcus ruber and Rhodococcus erythropolis also have been shown to be able to degrade diesel at 37 °C (Bicca et al. 1999). Even though these bacteria were reported to grow well at 37 °C, they were not able to grow in a wide temperature range as shown by strain DRY27. This is an advantage since Malaysia has a tropical climate where soil temperature can vary from 24 to 35 °C year-round (Sinnakkannu et al. 2004).
pH plays an important role in bacterial growth. pH in a media can be changed simply by the production and accumulation of bacterial waste products (Shukor et al. 2009b). This is why it is very important to optimize the environmental conditions for the enhancement of bacterial growth. To design an effective bioremediation strategy, identification of the pH optima is important (Davey 1994). The optimal pH that supported growth of the bacterium was between pH 7.5 and 8.5 in borate, and Tris–HCl buffer (Fig. 2). There are a lot of other bacterial strains that need the neutral or near neutrality for the optimal growth of bacteria on diesel (Espeche et al. 1994; Chapman and Shelton 1995; Bicca et al. 1999; Margesin 2000; Cavalca et al. 2000; Mukherji et al. 2004; Hong et al. 2005; Márquez-Rocha et al. 2005; Lee et al. 2005, 2006; Rajasekar et al. 2007; Ueno et al. 2007; Kwapisz et al. 2008). However, Burkholderia sp. has been reported to grow at pH 4.9 (Somtrakoon et al. 2008).
The effect of pH on the growth of strain DRY27 using three overlapping buffers. The buffer systems used were phosphate (open circles), carbonate (filled circles) and tris (diamonds). Growth was carried out at room temperature for 5 days on an orbital shaker (150 rpm). Data represent mean ± SEM, n = 3
3.2 The effect of carbon source
This experiment was performed to study the optimum diesel concentration as a carbon source for strain DRY27. Based on Fig. 3, the optimum carbon source (diesel) concentration for the growth of strain DRY27 was 3 % (v/v). We also showed that strain DRY27 was able to grow on 2 % (v/v), 4 % (v/v) and 5 % (v/v) of diesel with a declining bacterial growth (Fig. 4). Even though diesel is needed as a carbon source, at a high concentration, it can be considered as toxic to microorganisms due to the effect of solvent in diesel that damages bacterial cell membrane (Shukor et al. 2009b). This is why a lot of biodegradation studies on diesel are carried out using lesser diesel concentrations ranging from 0.5 to 1.5 % (Mukherji et al. 2004; Lee et al. 2005, 2006; Hong et al. 2005; Rajasekar et al. 2007; Ueno et al. 2007). Concentration higher than 1 or 1.5 % has been proven to cause retardedness in degradation (Espeche et al. 1994; Bicca et al. 1999; Lee et al. 2005, 2006). Degradation of diesel at a much higher concentration (6 % v/v) is possible but it requires glucose (0.2 % w/v) and yeast extract (0.1 % w/v) (Kwapisz et al. 2008). DRY27 displays high cell surface hydrophobicity when cultured on aliphatic hydrocarbons or diesel as the sole carbon source (Mohanti and Mukherji 2007). Burkholderia sp. can also degrade a wide variety of alkanes, toluene, benzoate, m-, p- and O-toluate as sole carbon (Ma and Herson 2000).
3.3 Effect of nitrogen sources
Growth of strain DRY27 on various inorganic nitrogen sources including ammonium sulphate, ammonium chloride, sodium nitrate, sodium nitrite, acrylamide, iodoacetamide, nicotinamide, methacrylamide and propionamide was tested. Sodium nitrate gave the highest growth rate on diesel compared to the other carbon sources (p < 0.05) followed by ammonium sulfate and ammonium chloride (p < 0.05). The other inorganic nitrogen sources tested showed a significant reduction in growth rate compared to sodium nitrate, ammonium sulfate and ammonium chloride (Fig. 5). It was reported that the best nitrogen source in diesel biodegradation works is mostly either ammonium or nitrate salts (Espeche et al. 1994; Chapman and Shelton 1995; Bicca et al. 1999; Margesin 2000; Cavalca et al. 2000; Mukherji et al. 2004; Hong et al. 2005; Márquez-Rocha et al. 2005; Lee et al. 2005, 2006; Rajasekar et al. 2007; Ueno et al. 2007; Kwapisz et al. 2008). The use of nitrite in bioremediation has to be handled carefully since it is known to inhibit cellular growth during hydrocarbon biodegradation (Chayabutra and Ju 2000). The study of nitrogen sources is important as the decrease of nitrogen in the microbial environment will limit the rate of hydrocarbon degradation (Atlas and Cerniglia 1995).
3.4 Growth kinetic studies
Data from experimental value were fitted to three kinetic models of growth i.e., Monod, Luong and Haldane using CurveExpert Professional software (Version 1.6) custom equation algorithm that minimizes sums of square of residuals (Haldane 1930; Monod 1949). The correlation coefficient value for the substrate inhibition model of Haldane was 0.999 indicating good fitting, while the Luong model gave a slightly lower value of 0.997. The Monod model gave the lowest correlation coefficient value of 0.91 indicating poor fitting (Fig. 6). The value of specific growth rate μ tends to increase as the substrate concentration is increased and rises to a peak value and finally decreases. According to the best model, Haldane, the maximum growth rate(µ max) was 0.305 h−1, while the saturation constant or half-velocity constant K s and inhibition constant K i, were 1.171 % (v/v) or 9.95 g/L and 3.215 % (v/v) or 27.32 mg/L diesel, respectively. There are few data on diesel degradation and utilization kinetics in the literature. The µ max value is higher than values reported for alkane-degrading P. frederiksbergensis and R. erythropolis at 0.0154 and 0.0125 (h−1), respectively (Abdel Megeed and Mueller 2009), and lower than Rhodococcus rubber and Rhodococcus erythropolis grown on diesel in another study that showed maximum growth rates of 0.086 and 0.123 h−1, respectively (Zhukov et al. 2007). A lower value of K s indicates a high affinity of biomass and degradative enzymes to substrate, while a high K i value indicates good tolerance towards high diesel concentrations.
3.5 MATH assay
After extraction with hexadecane, 65 % of the bacterium was found in the hexadecane phase indicating that the bacterium was hydrophobic. This value is close to MATH values of between 70 and 80 % found in several hydrocarbon-degrading strains (Zoueki et al. 2010; Mara et al. 2012). The vortexing part produces fine hydrocarbon droplets that the bacteria can adhere to. The inclusion of high salt concentration prevents attraction due to charge surfaces and adhesion is only due to hydrophobic interaction—the more hydrophobic the bacterial surface the more likely for binding and biodegradation.
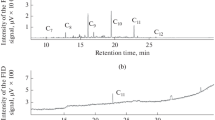
After achieving all of the optimization results on strain DRY27, biodegradation of diesel was performed. The reduction in hydrocarbon peaks was shown to be 96.5 % after 7 days of incubation (Fig. 7). In Mohanti and Mukherji, B. cepacia is able to degrade only 51.37 % of diesel for 15 days which is a longer time compared to DRY27 (Mohanti and Mukherji 2007).
4 Conclusions
This work served as an introductory study for the actual bioremediation works on the polluted site using the autochthonous strain DRY27. Crude and processed hydrocarbons are known to contain significant amount of heavy metals that could inhibit bioremediation of the diesel polluted site. This is why study of resistant of strain DRY27 to heavy metal is performed. Work is also underway to characterize the enzymes and genes involved in diesel degradation. We are also working on bioaugmentation studies using this strain to remediate hydrocarbon sludge from a petroleum-processing plant as part of a bioremediation study using allochthonous bacterium.
References
Abdel Megeed A, Mueller R (2009) Degradation of long chain alkanes by a newly isolated Pseudomonas frederiksbergensis at low temperature. Biorem Biodiv Bioavail 3:55–60
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Atlas RM, Cerniglia CE (1995) Bioremediation of petroleum pollutants. Bioscience 45:1–10
Bento FM, Camargo FAO, Okeke BC et al (2005) Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresource Technol 96:1049–1055
Berita Harian Online (1997) 150 tonnes of diesel spilled in the Straits of Malacca, 6 August 1997
Bicca FC, Fleck LC, Antonio M et al (1999) Production of biosurfactant by hydrocarbon degrading Rhodococcus ruber and Rhodococcus erythropolis. Rev Microbiol 30:231–236
Cavalca L, Gennaro PD, Colombo M et al (2000) Distribution of catabolic pathways in some hydrocarbon-degrading bacteria from a subsurface polluted soil. Res Microbiol 151:877–887
Chapman PJ, Shelton M (1995) Fossil fuel biodegradation, laboratory studies. Environ Health Perspect Supplement 1035:1–7
Chayabutra C, Ju LK (2000) Degradation of n-hexadecane and its metabolites by Pseudomonas aeruginosa under microaerobic and anaerobic denitrifying conditions. Appl Environ Microbiol 66:493–498
Claassens S, Van Rensburg L, Riedel KJ et al (2006) Evaluation of the efficiency of various commercial products for the bioremediation of hydrocarbon contaminated soil. Environmentalist 26:51–62
Davey KR (1994) Review paper, modelling the combined effect of temperature and pH on the rate coefficient for bacterial growth. Int J Food Microbiol 23:295–303
Devereux R, Wilkinson SS (2004) Amplification of ribosomal RNA sequences. In: Kowalchuk GA, de Brujin FJ, Head IM, Akkermans ADL, van Elsas JD (eds) Molecular microbial ecology manual, 2nd edn. Kluwer, Dordrecht, pp 509–522
DOE: Malaysia Environmental Quality Report 2006 (2007) Department of Environment, Ministry of Natural Resources and Environment, Malaysia, ISSN 0127-6433
Eriksson M, Swartling A, Dalhammar G (1998) Biological degradation of diesel fuel in water and soil monitored with solid-phase microextraction and GC-MS. Appl Microbiol Biotechnol 50:129–134
Espeche ME, MacCormack WP, Fraile ER (1994) Factors affecting growth of an n-hexadecane degrader Acinetobacter species isolated from a highly polluted urban river. Int Biodeter Biodegr 33:187–196
Ghazali FM, Rahman RNZA, Salleh AB et al (2004) Biodegradation of hydrocarbons in soil by microbial consortium. Int Biodeter Biodegr 54:61–67
Gusmanizar N, Shukor Y, Ramli J et al (2008) Isolation and characterization of an acrylamide-degrading Burkholderia sp. STRAIN DR.Y27. J Riset Kimia 2(1):34–44
Haldane JBS (1930) Enzymes. Longman Green, London
Hong JH, Kim J, Choi OK et al (2005) Characterization of a diesel-degrading bacterium, Pseudomonas aeruginosa IU5, isolated from oil-contaminated soil in Korea. World J Microbiol Biotechnol 21:381–384
Kwapisz E, Wszelaka J, Marchut O et al (2008) The effect of nitrate and ammonium ions on kinetics of diesel oil degradation by Gordonia alkanivorans S7. Int Biodeter Biodegr 61:214–222
Lee M, Kim MK, Kwon MJ et al (2005) Effect of the synthesized mycolic acid on the biodegradation of diesel oil by Gordonia nitida strain LE31. J Biosci Bioeng 100:429–436
Lee M, Kim MK, Singleton I et al (2006) Enhanced biodegradation of diesel oil by a newly identified Rhodococcus baikonurensis EN3 in the presence of mycolic acid. J Appl Microbiol 100:325–333
Ma Y, Herson DS (2000) The cathecol 2, 3- deoxygenase gene and toluene monooxygenase genes from burkholderia sp. AA1, an isolate capable of degrading aliphatic hydrocarbons and toluene. J Ind Microbiol Biotechnol 25:127–131
Mara K, Decorosi F, Viti C et al (2012) Molecular and phenotypic characterization of Acinetobacter strains able to degrade diesel fuel. Res Microbiol 163:161–172
Margesin R (2000) Potential of cold-adapted microorganisms for bioremediation of oil-polluted Alpine soils. Int Biodeter Biodegr 46:3–10
Márquez-Rocha FJ, Olmos-Soto J, Concepción Rosano-Hernández M et al (2005) Determination of the hydrocarbon-degrading metabolic capabilities of tropical bacterial isolates. Int Biodeter Biodegr 55:17–23
Michaud L, Di Cello F, Brilli M et al (2004) Biodiversity of cultivable Antarctic psychrotrophic marine bacteria isolated from Terra Nova Bay (Ross Sea). FEMS Microbiol Lett 230:63–71
Mohammed D, Ramsubhag A, Beckles DM (2007) An assessment of the biodegradation of petroleum hydrocarbons in contaminated soil using non-indigenous, commercial microbes. Water, Air, Soil Poll 182:349–356
Mohanti G, Mukherji S (2007) Effect of an emulsifying surfactant on diesel degradation by cultures exhibiting inducible cell surface hydrophobicity. J Chem Technol Biotechnol 82:1004–1011
Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3:371–394
Mukherji S, Jagadevan S, Mohapatra G et al (2004) Biodegradation of diesel oil by an Arabian Sea sediment culture isolated from the vicinity of an oil field. Bioresour Technol 95:281–286
Mulchandani A, Luong JHT, Groom C (1989) Substrate inhibition kinetics for microbial growth and synthesis of poly-β-hydroxybutyric acid by Alcaligenes eutrophus ATCC 17697. Appl Microbiol Biotechnol 30:11–17
Rajasekar A, Babu TG, Pandian ST et al (2007) Role of Serratia marcescens ACE2 on diesel degradation and its influence on corrosion. J Ind Microbiol Biotechnol 34:589–598
Rosenberg M (1984) Bacterial adherence to hydrocarbon: a useful technique for studying cell surface hydrophobicity. FEMS Microbiol Lett 22:289–295
Shukor MY, Dahalan FA, Jusoh AZ et al (2009a) Characterization of a diesel-degrading strain isolated from a hydrocarbon-contaminated site. J Environ Biol 30:145–150
Shukor MY, Hassan NAA, Jusoh AZ et al (2009b) Isolation and characterization of a Pseudomonas diesel-degrading strain from Antarctica. J Environ Biol 30:1–6
Sinnakkannu S, Abdullah AR, Tahir NM et al (2004) Degradation of metsulfuron methyl in selected Malaysian agricultural soils. Fresenius Environ Bull 13:258–261
Somtrakoon K, Suanjit S, Pokethitiyook P et al (2008) Phenanthrene stimulates the degradation of pyrene and fluoranthene by Burkholderia sp. VUN10013. World J Microbiol Biotechnol 24:523–531
The New Straits Times (2000) Oil Spill closes road for six hours, 3 February 2000
The New Straits Times (2001) Tenaga Nasional moves quickly to avert disaster after diesel spill, 23 May 2001
Ueno A, Ito Y, Yumoto I et al (2007) Isolation and characterization of bacteria from soil contaminated with diesel oil and the possible use of these in autochthonous bioaugmentation. World J Microbiol Biotechnol 23:1739–1745
Zhukov DV, Murygina VP, Kalyuzhnyi SV (2007) Kinetics of the degradation of aliphatic hydrocarbons by the bacteria Rhodococcus rubber and Rhodococcus erythropolis. Appl Biochem Microbiol 43:587–592
Zoueki CW, Tufenkji N, Ghoshal S (2010) A modified microbial adhesion to hydrocarbons assay to account for the presence of hydrocarbon droplets. J Colloid Interface Sci 344:492–496
Acknowledgments
This project was supported by the funds from the ScienceFund, Malaysia, Project No: 02-01-04-SF1473.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, S.A., Ku Ahamad, K.N.E., Wan Johari, W.L. et al. Kinetics of diesel degradation by an acrylamide-degrading bacterium. Rend. Fis. Acc. Lincei 25, 505–512 (2014). https://doi.org/10.1007/s12210-014-0344-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-014-0344-7