Abstract
Low back pain is associated with intervertebral disc degeneration. One of the main signs of degeneration is the inability to maintain extracellular matrix integrity. Extracellular matrix synthesis is closely related to production of adenosine triphosphate (i.e. energy) of the cells. The intervertebral disc is composed of two major anatomical regions: annulus fibrosus and nucleus pulposus, which are structurally and compositionally different, indicating that their cellular metabolisms may also be distinct. The objective of this study was to investigate energy metabolism of annulus fibrosus and nucleus pulposus cells with and without dynamic compression, and examine differences between the two cell types. Porcine annulus and nucleus tissues were harvested and enzymatically digested. Cells were isolated and embedded into agarose constructs. Dynamically loaded samples were subjected to a sinusoidal displacement at 2 Hz and 15% strain for 4 h. Energy metabolism of cells was analyzed by measuring adenosine triphosphate content and release, glucose consumption, and lactate/nitric oxide production. A comparison of those measurements between annulus and nucleus cells was conducted. Annulus and nucleus cells exhibited different metabolic pathways. Nucleus cells had higher adenosine triphosphate content with and without dynamic loading, while annulus cells had higher lactate production and glucose consumption. Compression increased adenosine triphosphate release from both cell types and increased energy production of annulus cells. Dynamic loading affected energy metabolism of intervertebral disc cells, with the effect being greater in annulus cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Degeneration of the intervertebral disc (IVD) is a common spine disorder which is closely related to low back pain (LBP),38,49 affecting millions of people.32 Disc degeneration has been attributed to aging, genetic factors, nutrition, and mechanical factors. The cause of this pathology is still to be determined.1,17,29
IVDs are strong, yet deformable, soft tissue found between the vertebral bodies, and are biomechanically important because their main functions are to transfer loads1 and permit proper movement of the spine, allowing compression, torsion and bending.6,19 IVDs are composed of two major anatomical regions, annulus fibrosus (AF) and nucleus pulposus (NP).29 AF is typically composed of approximately 15–25 distinct layers of collagen fiber lamellae organized into concentric rings around the NP.6,39 At the cellular level, AF cells originate from the mesenchyme during embryonic development16 and vary from being elongated to more spherical and chondrocyte-like, depending on their location within the annulus tissue of the disc.56 NP is characterized by randomly oriented collagen fibrils embedded in a proteoglycan gel. NP cells originate from the notochord and in humans notochordal NP cells disappear at maturity. At age 10, maturity begins and notochordal NP cells are replaced by mature NP cells, which have been compared to chondrocytes.1,16 Notochordal and mature NP cells differ in structure and function.15 Notochordal cells have faster biosynthesis rates, which corresponds to a higher energy demand.40 Notochordal NP has a water content of 80–88%, while mature NP has a water content of approximately 70%.24 As discs mature, these changes in the NP may contribute to degeneration.3,15,16 NP has a lower collagen content than AF but higher proteoglycan content, indicating differences in metabolism between AF and NP cells.8,45
Maintenance of extracellular matrix (ECM) integrity in IVDs is imperative, otherwise disc degeneration may occur due to mechanical destruction.17 Synthesis of ECM components is a highly energy-demanding process. The major source of energy in cells is adenosine triphosphate (ATP). IVD is the largest avascular tissue in the human body50 and utilizes diffusion as the main mechanism of nutrition transport. In the fetal stage, the discs are vascular, but within a few years, vascularization of the disc decreases and is limited to the outer annulus tissue.33 Poor nutrient supply, which limits cellular energy production, has been attributed as a major factor of IVD degeneration. However, few studies have investigated the energy metabolism of IVD cells and its possible relation to disc degeneration. Furthermore, various studies have demonstrated that mechanical loading modulates nutrient supply to IVD cells and its ECM synthesis.21,26,27,30,31,34,44,55 It also has been shown that mechanical loading affects glucose and oxygen consumption rates and ATP released from chondrocytes.10,36 Changes in ECM synthesis by mechanical loading may be due to a combination of extrinsic (i.e., nutrient supply) and intrinsic (i.e., metabolism) effects on energy production. Therefore, the objective of this study was to investigate energy metabolism of AF and NP cells with and without dynamic compression and examine differences between the two cell types.
Materials and Methods
Intervertebral Disc Cell Isolation
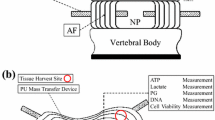
Lumbar spines were harvested from 4 to 6 month-old pigs, which were obtained within 2 h of sacrifice from the slaughterhouse (Cabrera Farms, Hialeah, FL). Young pigs were chosen to ensure that NP tissue contained notochordal cells because the loss of notochordal cells in the NP region has been associated with the initiation of disc degeneration.7,25 Tissues were harvested from AF and NP regions and digested in a collagenase-pronase solution of Dulbecco’s Modified Eagle Media (DMEM; Invitrogen Corp., Carlsbad, CA) containing 1 mg/mL collagenase (Worthington Biochemical Corp., Lakewood, NJ), and 0.6 mg/mL pronase (Sigma Chemical, St. Louis, MO) overnight at 37 °C. After 24 h, the cell-enzyme solution was purified by filtration through a 70 μm strainer, and then cells were isolated by centrifugation.
Agarose Construct Preparation
IVD cells were resuspended in DMEM containing 10% FBS, and 1% antibiotics, at a density of 107 cells/mL. Cell suspension and 4% agarose were combined in equal amounts to obtain cell-agarose constructs (8 mm in diameter and 2 mm in thickness) in which IVD cells were encapsulated in 2% agarose at a density of 5 × 106 cells/mL. The constructs were made by casting the same volume (i.e., 100 μL) of cell-agarose mixture into the same custom-made mold (8 mm in diameter) to ensure consistency in the thickness of the constructs (2.07 ± 0.08 mm). Cell-agarose constructs were cultured overnight prior to conducting experiments.
Bioreactor
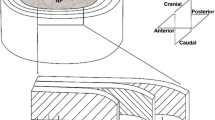
A custom bioreactor (Fig. 1) was modified from a previous design.22 Each specimen was individually placed in a compression chamber. After placing the sample and media into the chambers, a cap was used to close each chamber and guide each compressive rod’s motion. Compression plugs were initially set to rest on the samples and quickly locked in place by position nuts to prevent unintended compression of samples during experiment set-up. The light weight (~1.5 g) of the compression plug caused a minimal deformation (<1%) based on the mechanical properties of agarose reported in our previous study.14 Then the compression plugs were attached to the dynamic loading system to establish the initial position. A cam-follower system was used to apply the same sinusoidal displacement (magnitude: 200 μm) to samples in compression chambers though a compression plate (Fig. 1), and a linear variable differential transformer (LVDT) was used to measure the displacement. To obtain 5% preloading, a 100 μm thick shim (Small Parts Inc., Seattle, WA) was inserted between the cam and the follower. The bioreactor performed compressive loading experiments at 37 °C, 21% O2 and 5% CO2 in a conventional humidified tissue culture incubator.
Dynamic Loading
Cell-agarose constructs were divided into four groups: NP control, AF control, NP compression, and AF compression. The sample size (n = 15) was the same for all four groups. The compression samples were pre-loaded with 5% static compressive strain, and then subjected to sinusoidal compressive loading of 10% strain (i.e., loading strain between 5 and 15%), at 2 Hz for 4 h. The control samples were cultured in the chambers without plugs or compression rods (i.e., without any loading) (Fig. 1), and were placed inside the incubator for the same period of time. Since the consumption rates of glucose of IVD cells are low16 and high glucose concentration was used, differences in glucose concentration between the samples in the chambers with and without the compressive plug were less than 1% (i.e., a negligible effect on glucose consumption) after a 4 h experiment according to our theoretical analysis using a finite element software (COMSOL, Inc., Burlington, MA).21 This was also verified by our preliminary study which showed no significant difference in glucose consumption between the samples with and without the plugs. However, the compressive plug may hinder release of lactate and ATP from the sample whereas dynamic compressive loading may promote their release by inducing convective flow. This could introduce another factor in comparison between the control and loading groups. Thus, in order to minimize this factor and facilitate release of ATP and lactate from the samples, as occurring during dynamic compression, the compressive plug was not used in control group. DMEM (Invitrogen), without FBS or antibiotics was used in all experiments. After experiments, each sample was homogenized with 1 mL of lysis buffer containing 0.225 M NaCl (Sigma), 5 mM EDTA (Sigma) pH 8, 1% Triton X-100 (Sigma), and 10 mM Tris (Sigma) pH 7.4, and then heated at 65 °C for 15 min. After centrifugation, supernatant was collected for measurements of ATP and DNA contents. Media were also collected for ATP, nitric oxide (NO), lactate, and glucose measurements. The evaporation of the media (~10% reduction in volume) was also evaluated and taken into account in measurements after 4-h experiments.
Assays
Lactate
A reaction mix was prepared containing 5 mg/mL of β-Nicotineamide Adenine Dinucleotide (Sigma), 0.2 M glycine buffer (Sigma), and 22.25 units/mL of l-Lactic Dehydrogenase (Sigma). A standard curve (12.5–800 μM) was made using a standard lactate solution (Trinity Biotech, Wicklow, Ireland). Equal parts of the reaction mix and each sample were mixed in a 96-well plate. Absorbance at 340 nm was measured using a plate reader (model: DTX880, Beckman Coulter, Brea, CA). Lactate concentration of each sample was then calculated using the standard curve.
Nitric Oxide
The assay used to detect nitric oxide is based on the Griess principle to detect nitrates.25 The Griess reagent was 1% sulfanilamide (Sigma) and 0.1% naphthylethylenediamine (Sigma) in 20% orthophosphoric acid (Sigma). A reaction mix was prepared, containing 1 M Tris (Sigma), 0.02 mM NADPH (Sigma), 5 mM glucose-6-phosphate (Sigma), 10 unts/mL glucose-6-phosphate dehydrogenase (Roche Diagnostics), and 1 unit/mL nitrate reductase (Sigma). A standard curve (from 2.5–160 μM) was made using sodium nitrite (Sigma). Equal parts of the reaction mix and each sample were mixed in a 96-well plate using a shaker for 30 min. Then Griess reagent was added in equal parts to the previous mixture and placed on the shaker for another 10 min. Finally, absorbance was measured at 550 nm using the plate reader.
ATP
ATP was measured using the luciferin-luciferase method (Sigma). Immediately after each experiment, aliquots from media were mixed in equal parts with ATP stabilizing solution, which is a 3 mM EDTA solution, and then boiled for 2 min. The ATP Bioluminescent Assay Kit (Sigma) was used as instructed by manufacturer. Luminescence was measured using the plate reader at 1000 ms. Total ATP was calculated by summing the ATP measurements from the media and the lysed samples.
DNA Measurements
DNA content was measured from the lysed samples using a QubitTM fluorometer (Invitrogen) and its corresponding Quant-iT dsDNA HS Assay Kit (Invitrogen).
Glucose Measurements
The Cobas C System (Roche Diagnostics, Indianapolis, IN) was used to determine the glucose concentration in medium samples. Glucose concentration was also measured from unused media as a reference point and used to calculate consumption by taking the difference between this and the samples.
Statistical Analyses
The measurements of ATP release, ATP content, NO content, lactate content, and glucose consumption were normalized to DNA content. Student t-test analyses, using SPSS (SPSS Inc., Chicago, Illinois), were performed to examine differences in all measurements between AF and NP cells and effects of dynamic loading on energy metabolism. When comparing AF and NP, all measurements were normalized to AF measurements, and when comparing control and compression, all measurements were normalized to respective controls. A p ≤ 0.05 was considered statistically significant.
Results
Comparison Between NP and AF Cells
Without compression there were no significant differences between the ATP release from NP and AF cells (Fig. 2). However, under dynamic loading the ATP release of NP cells was significantly higher than that of AF cells (Fig. 3). NP cells had a significantly higher total ATP than AF cells both without compression (Fig. 2), and under dynamic loading (Fig. 3). Without dynamic loading, there were no significant differences between the lactate productions of AF and NP cells (Fig. 2). Conversely, under dynamic loading the lactate production of AF cells was higher than that of NP cells (Fig. 3). Without dynamic loading there were no significant differences in NO production among cell types (Fig. 2), but under dynamic loading, NO content was significantly higher in AF compared to NP (Fig. 3). Glucose consumption without compression and under dynamic loading was significantly higher for AF cells than for NP cells (Figs. 2 and 3). The rates of glucose consumption and lactate production by NP and AF cells are listed in Table 1.
Comparison of ATP release, total ATP content, lactate production, glucose consumption, and NO production between the AF and NP control groups. Differences were only observed for total ATP content and glucose consumption, where total ATP content was higher for NP cells, and glucose consumption was higher for AF cells. *p < 0.05
Comparison of ATP release, total ATP content, lactate production, glucose consumption, and NO production between the AF and NP loading groups. Differences were observed in all measurements, where NP had higher total ATP content and higher ATP release, and AF had higher glucose consumption, lactate production, and NO production. *p < 0.05
Effect of Dynamic Loading on Energy Metabolism
ATP release significantly increased with compression for both AF and NP cells (Figs. 4 and 5). Dynamic compression significantly promoted ATP production, glucose consumption, and lactate production of AF cells (Fig. 4). Although no significant effects of dynamic loading were seen on ATP production, glucose consumption, and lactate production of NP cells, a slight increase was seen in these measurements. NO production increased as a result of compression in both AF and NP cells.
Discussion
As disc degeneration advances, NP tissue becomes granular and stiffer, cells begin to resemble chondrocytes, and the tissue becomes devoid of its gel-like properties.45,51 Integrity of IVDs is dependent on the ability of its cells to produce and maintain ECM.54 Dynamic compression has been shown to modulate ECM synthesis in chondrocytes9 and in IVD cells.35 Since ECM synthesis requires energy, given the limited supply of nutrients to the IVD, energy production may play an important role in ECM synthesis of IVD cells. This is the first study to demonstrate (1) the differences in energy metabolism between AF and notochordal NP cells under dynamic compression and (2) the effects of dynamic compressive loading on energy metabolism of IVD cells.
Glycolysis is the process by which glucose is oxidized into pyruvate and ATP is produced. It rapidly occurs upon glucose availability. Pyruvate may be oxidized aerobically or anaerobically, depending on the cell type and presence of oxygen. In aerobic oxidation, pyruvate enters the mitochondria of cells and more ATPs are produced through the Krebs cycle and electron transport chain. Aerobic oxidation produces significantly higher amounts of ATP than glycolysis alone. In the anaerobic respiration pathway, pyruvate is oxidized into lactate without ATP production. Previous studies have suggested that glycolysis is the main source of energy in IVD cells.5,16,50 This study found that notochordal NP cells had much higher quantities of total ATP compared to AF cells, although glucose consumption of AF cells was significantly higher than that of notochordal NP cells. These differences suggest that the major pathway for energy production is different for notochordal NP and AF cells. NP cells consume less glucose and produce much more ATP, suggesting that notochordal NP cells may also produce ATP through aerobic respiration in addition to glycolysis. This suggestion is supported by our previous study23 that showed the oxygen consumption rate of notochordal NP cells was higher than that of AF cells. More recently, Guehring et al.16 suggested notochordal NP cells rely on glycolysis for ATP production based on the rates of oxygen consumption, glucose consumption, lactate production, and CO2 production. The ratio between the rates of glucose consumption and lactate production for notochordal NP cell was found to be less than 2 in the study of Guehring et al.16 and the current study. Aerobic oxidation can produce ATP much more efficiently than glycoslysis (30 ATP vs. 2 ATP). Hence, if a small amount of pyruvate enters the mitochondria, much more ATP could be produced compared with glycolysis. ATP measurements in our study provided a more direct indication for energy production than the other measurements. Higher ATP content of notochordal NP cells found in this study suggests that metabolism of notochordal NP cells demand more ATP than AF cells. Therefore, because of its efficiency and higher ATP output, aerobic respiration would be the best option to supply sufficient ATP for metabolically active NP cells when oxygen is available. Nevertheless, cellular energy metabolism is strongly influenced by the local levels of oxygen and glucose in the experiments. Differences between the current study and the study of Guehring et al.16 may be due to different experimental set-ups and resulting differences in the local levels of oxygen and glucose. For example, the initial glucose concentration in the experiments was 25 mM in the current study and 5 mM in the study of Guehring et al.16
On the other hand, the major pathway for ATP production in AF cells is glycolysis.43,50 The difference in energy metabolism between AF and NP cells may be due to their different embryonic origins and/or their distinct environmental conditions. AF cells originate from the mesenchyme and have been histologically analyzed and characterized as chondrocyte-like cells,43 while NP used in this study are notochordal cells, which have a distinct molecular phenotype compared to AF.16 Furthermore, although glycolysis and aerobic respiration do not occur exclusively to produce ATP in these cells, increased glucose consumption in AF cells by dynamic loading suggested that glycolysis is promoted in AF cells via dynamic loading. The latter was also indicated by the increase in lactate production and total ATP of AF cells, under dynamic loading.
ATP production of notochordal NP cells was found to be much higher than the AF cells, indicating the notochordal NP cells are more metabolically active and rely on sufficient nutrient supply to maintain their normal function. Therefore, disappearance of notochordal cells from the NP region of the IVD could result from reduction of nutrient supply during the maturation of disc.25 The cartilage endplates (CEP) are a 1 mm-thick layer of hyaline cartilage which enables fluid exchange and nutrient transport between the IVD and the vertebral body, through osmosis and diffusion.24 CEPs have been found to calcify with aging.43 A previous study of rat endplate has shown that endplate calcification started as early as one week of age which was earlier than the time (i.e., 8 week of age) when disc degeneration occurred.20 Therefore, calcification of CEPs may diminish transport of nutrients into IVD,42 resulting in disappearance of notochordal cells and initiating IVD degeneration.
Inorganic phosphates and inorganic pyrophosphates have been identified to cause chondrocalcinosis or calcification of chondrocytes, which appears as two different mineral formations: calcium pyrophosphate dehydrate (CPPD) and basic calcium phosphate (BCP).46,48 Calcification can cause enzymatic damage to cartilage matrix. Matrix vesicles are membrane-limited structures that bind to calcium and contain enzymes that generate inorganic pyrophosphates from ATP,2,12,18 which promotes CPPD and BCP formation.28 Extracellular ATP can be hydrolyzed by the ecto-ATPase family of enzymes (e.g. ecto-nucleoside triphosphate diphosphohydrolases, and ecto-nucleoside triphosphate pyrophosphohydrolase) and release inorganic pyrophosphates and phosphates.28,48 Therefore, extracellular ATP may promote calcification of cartilage.46 The current study found that ATP release from IVD cells was promoted by dynamic loading in IVD cells. A study showed that permeability of the CEP is limited to its central portion basically limiting nutrient exchange to and from NP and inner AF regions.41 An increase in ATP release from NP cells under dynamic loading may contribute to CEP calcification, diminishing nutrient transport to NP. However, it has been suggested that inorganic pyrophosphates may inhibit hydroxyapatite formation, while promoting CPPD crystal deposition.41 Concurrently, inorganic pyrophosphates can be reduced by alkaline phosphatases into inorganic phosphates, which may in turn form BCP crystals.53 Inorganic pyrophosphates and phosphates seem to have an antagonistic relationship affecting calcification,48 and generation of both is ATP-dependent.28 Therefore, investigating metabolism of extracellular ATP in the IVD may provide a better understanding of CEP calcification. In addition, recent studies demonstrated that ATP release from chondrocytes under mechanical loading mediated cellular proteoglycan synthesis through a purinergic pathway.10 Therefore, ATP release promoted by dynamic loading may have a similar effect on regulating extracellular matrix biosynthesis of IVD cells.
A previous study showed that NO production was inhibited in chondrocytes encapsulated in high-density-cross-linked PEG gels as these were dynamically loaded at 0.3 Hz.52 In the current study, the contrary was found for IVD cells, where dynamic loading promoted NO production in both AF and NP. These differences may be due to the differences in cell type and culture conditions. However, our findings are consistent with another previous study on cartilage explants, where NO production was promoted by dynamic loading at 0.5 Hz.11 Furthermore, since NO is known to inhibit aerobic oxidation of pyruvate after glycolysis, by blocking the complex C in mitochondrial electron transport chain,37 NO could regulate mitochondrial respiration in cells.46 Although increased NO production of AF cells via dynamic loading may reduce mitochondrial activity, no inhibitory effects were seen on ATP production of AF cells under dynamic loading. This supports the suggestion that glycolysis is the main pathway of ATP production in AF cells. In NP cells, NO production was significantly promoted via dynamic loading. However, energy metabolism of NP cells was not affected by dynamic compression (i.e., no significant changes were observed in total ATP, glucose consumption, and lactate production). This suggests that dynamic loading may counteract possible inhibitory effect of increased NO production on mitochondrial respiration of NP cells.
The main limitation in the current study is that the experiments were performed at higher concentrations of oxygen (i.e., 0.204 mM) and glucose (i.e., 25 mM) compared to in vivo conditions. In this study, high oxygen and glucose concentrations were chosen to eliminate the influence of concentration change on consumption of oxygen and glucose due to experimental set-up (e.g., enhanced transport caused by dynamic loading). Consumption of oxygen or glucose can be described by Michaelis–Menten kinetics.16,23 When the oxygen or glucose level is much higher than the Michaelis constant (e.g., for mature NP cells, 0.000147 mM for oxygen and 0.194 mM for glucose16), the consumption rate of oxygen or glucose is close to the maximum reaction rate and insensitive to small concentration changes. Therefore, high concentrations of oxygen and glucose used in the current study can eliminate the extrinsic effects of dynamic compressive loading which promotes solute transport, and allow us examine its intrinsic effect on energy metabolism of IVD cells. Since the energy metabolism of IVD cells strongly depends on the concentrations of oxygen and glucose, the data obtained in the current study may not exactly represent the in vivo behaviors of IVD cells. Steep concentration gradients of oxygen and glucose exist in the disc due to its avascularity. These conditions may alter both absolute metabolic rates as well as relative activities of AF and NP cells. For example, metabolically active notochordal NP cells may exhibit higher glucose consumption (i.e., glycolysis) than the AF cells under in vivo hypoxic condition.
In summary, this study demonstrated the differences in the energy metabolism between AF and notochordal NP cells. Higher ATP content found in notochordal NP cells indicates that NP cells are more metabolically active and may produce ATP through aerobic respiration in addition to glycolysis. Dynamic loading caused an increase in ATP release from both cell types, and had a greater effect on AF cells, resulting in elevated glucose consumption, lactate production, NO production, and ATP production. Moreover, ATP release due to dynamic loading may be a cause for cartilage endplate calcification, leading to malnourishment of the IVD and thus degeneration.
References
Adams, M. A., P. Dolan, and D. S. McNally. The internal mechanical functioning of intervertebral discs and articular cartiladge, and its relevance to matrix biology. Matrix Biol. 28:384–389, 2009.
Anderson, H. C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 5:222–226, 2003.
Antoniou, J., T. Steffen, F. Nelson, et al. The human intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Invest. 98:996–1003, 1996.
Bernick, S., and R. Cailliet. Vertebral end-plate changes with aging of human vertebrae. Spine 7:97–102, 1982.
Bibby, S. R., D. A. Jones, R. M. Ripley, et al. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine 30:487–496, 2005.
Bogduk, N. Clinical Anatomy of the Lunbar Spine and Sacrum. Livingstone: Elsevier Churchill, 2005.
Boos, N., S. Weissbach, H. Rohrbach, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 27:2631–2644, 2002.
Bron, J. L., M. N. Helder, H.-J. Meisel, et al. Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur. Spine J. 18:301–313, 2009.
Buschmann, M. D., Y. A. Gluzband, A. J. Grodzinsky, et al. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J. Cell. Sci. 108:1497–1508, 1995.
Chowdhury, T. T., and M. M. Knight. Purinergic pathway suppresses the release of NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J. Cell. Physiol. 209:845–853, 2006.
Fermor, B., J. B. Weinberg, D. S. Pisetsky, et al. The influence of oxygen tension on the induction of nitric oxide and prostaglandin E2 by mechanical stress in articular cartilage. Osteoarthr. Cartil. 13:935–941, 2005.
Golub, E. E. Role of matrix vesicles in biomineralizationBiochim. Biophys. Acta 1790:1592–1598, 2009.
Granger, D. L., N. M. Anstey, W. C. Miller, et al. Measuring nitric oxide production in human clinical studies. Meth. Enzymol. 301:49–61, 1999.
Gu, W. Y., H. Yao, C.-Y. Huang, and H. S. Cheung. New insight into deformation-dependent hydraulic permeability and dynamic compressive behavior of agarose gels. J. Biomech. 36:593–598, 2003.
Guehring, T., A. Nerlich, M. Kroeber, et al. Sensitivity of notochordal disc cells to mechanical loading: an experimental animal study. Eur. Spine J. 19:113–121, 2010.
Guehring, T., G. Wilde, M. Sumner, et al. Notochordal intervertebral disc cells: sensitivity to nutrient deprivation. Arthritis Rheum. 60:1026–1034, 2009.
Hadjipavlou, A. G., M. N. Tzermiadianos, N. Bogduk, et al. The pathophysiology of disc degeneration. J. Bone Joint Surg. Br. 90-B:1261–1270, 2008.
Hashimoto, S., R. L. Ochs, F. Rosen, et al. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc. Ann. Natl Acad. Med. Sci. 95:3094–3099, 1998.
Hickey, D. S., and D. W. L. Hukins. Relation between the structure of the annulus fibrosus and the function and failure of the intervertebral disc. Spine 5:106–116, 1980.
Higuchi, M., K. Kaneda, and K. Abe. Postnatal histogenesis of the cartilage plate of the spinal column: electron microscopic observations. Spine 7:89–96, 1982.
Huang, C.-Y., and W. Y. Gu. Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc. J. Biomech. 41:1184–1196, 2008.
Huang, C.-Y., K. L. Hagar, L. E. Frost, et al. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 22:313–323, 2004.
Huang, C.-Y., T.-Y. Yuan, A. R. Jackson, L. Hazbun, C. Fraker, and W. Y. Gu. Effects of low glucose concentrations on oxygen consumption rates of intervertebral disc cells. Spine 32:2063–2069, 2007.
Humzah, M. D., and R. W. Soames. Human intervertebral disc: structure and function. Anat. Rec. 220:337–356, 1988.
Hunter, C. J., J. R. Matyas, and N. A. Duncan. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 9:667–677, 2003.
Hutton, W. C., W. A. Elmer, S. D. Boden, et al. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine 24:1507–1515, 1999.
Jackson, A. R., T.-Y. Yuan, C.-Y. Huang, et al. Effect of compression and anisotropy on the diffusion of glucose in annulus fibrosus. Spine 33:1–7, 2008.
Johnson, K., and R. Terkeltaub. Inorganic pyrophosphate (PPi) in pathologic calcification of articular cartilage. Front. Biosci. 10:988–997, 2005.
Kandel, R., S. Roberts, and J. P. Urban. Tissue engineering and the intervertebral disc: the challenges. Eur. Spine 17:S480–S491, 2008.
Kasra, M., V. Goel, J. Martin, et al. Effect of dynamic hydrostatic pressure on rabbit intervertebral disc cells. J. Orthoptera Res. 21:597–603, 2003.
Kasra, M., W. D. Merryman, K. N. Loveless, et al. Frequency response of pig intervertebral disc cells subjected to dynamic hydrostatic pressure. J. Orthoptera Res. 24:1967–1973, 2006.
Kelsey, J. L., A. L. Golden, and D. J. Mundt. Low back pain/prolapsed lumbar intervertebral disc. Rheum. Dis. Clin. North Am. 16:699–716, 1990.
Kliskey, K., K. Williams, J. Yu, et al. The presence and absence of lymphatic vessels in the adult intervertebral disc: relation to disc pathology. Skeletal Radiol. 38:1169–1173, 2009.
Korecki, C. L., C. K. Kuo, R. S. Tuan, et al. Intervertebral disc cell response to dynamic compression is age and frequency dependent. J. Orthoptera Res. 27:800–806, 2009.
Korecki, C. L., J. J. MacLean, and J. C. Iatridis. Dynamic compression effects of intervertebral disc mechanics and biology. Spine 33:1403–1409, 2008.
Lee, R. B., R. J. Wilikins, S. Razaq, et al. The effect of mechanical stress on cartilage energy metabolism. Biorheology 39:133–143, 2002.
Lodish, H., A. Berk, P. Matsudaira, et al. Molecular Cell Biology (5th ed.). New York: W. H. Freeman and Company, 2004.
Luoma, K., H. Riihimaki, R. Luukkonen, et al. Low back pain in relation to lumbar disc degeneration. Spine 25:487–492, 2000.
Marchand, F., and A. M. Ahmed. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine 15:402–410, 1990.
Miyazaki, T., S. Kobayashi, K. Takeno, et al. A phenotypic comparison of proteoglycan production of intervertebral disc cells isolated from rats, rabbits, and bovine tails: which animal model is most suitable to study tissue engineering and biological repair of human disc disorders? Tissue Eng. Part A. 15:3835–3846, 2009.
Nachemson, A., and G. Elfstrom. Intravital dynamic pressure measurements in lumbar discs: a study of common movements, maneuvers and exercises. Scand. J. Rehabil. Med. Supplement 1:1–40, 1970.
Nachemson, A., T. Lewin, A. Maroudas, et al. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop. Scand. 41:589–607, 1970.
Oegema, T. R. Biochemistry of the intervertebral disc. Clin. Sports Med. 12:419–439, 1993.
Ohshima, H., J. P. Urban, and D. H. Bergel. Effect of static load on matrix synthesis rates in the intervertebral disc measured in vitro by a new perfusion technique. J. Orthoptera Res. 13:22–29, 1995.
Raj, P. P. Intervertebral disc: anatomy-phisiology-pathophisiology-treatment. Pain Pract. 8:18–44, 2008.
Ryan, L. M., I. V. Kurup, B. A. Derfus, et al. ATP-induced chondrocalcinosis. Arthritis Rheum. 35:1520–1525, 1992.
Ryan, L. M., and A. K. Rosenthal. Metabolism of extracellular pyrophosphate. Curr. Opin. Rheumatol. 15:311–314, 2003.
Terkeltaub, R. A. Inorganic pyrophosphate generation and disposition in pathophysiology. Am. J. Physiol. Cell Physiol. 281:C1–C11, 2001.
Urban, J. P., and S. Roberts. Degeneration of the intervertebral disc. Arthritis Res. Ther. 5:120–130, 2003.
Urban, J. P., S. Smith, and J. C. T. Fairbank. Nutrition of the intervertebral disc. Spine 29:2700–2709, 2004.
Vernon-Roberts, B., and C. J. Pirie. Degenerative changes in the intervertebral discs of the lumbar spine and their sequelae. Rheumatol. Rehabil. 16:13–21, 1977.
Villanueva, I., D. S. Hauschulz, D. Mejic, et al. Static and dynamic compressive strains influence nitric oxide production and chondrocyte bioactivity when encapsulated in PEG hydrogels of different cosslinking densities. Osteoarthr. Cartil. 16:909–918, 2008.
Wang, W., J. Xu, B. Du, et al. Role of the progressive ankylosis gene (ank) in cartilage mineralization. Mol. Cell. Biol. 25:312–323, 2005.
Yang, X., and X. Li. Nucleus pulposus tissue engineering: a brief review. Eur. Spine J. 18:1564–1572, 2009.
Yuan, T. Y., A. R. Jackson, C.-Y. Huang, et al. Strain-dependent oxygen diffusivity in bovine annulus fibrosus. J. Biomech. Eng. 131:074503, 2009.
Zhao, C.-Q., L.-M. Wang, L.-S. Jiang, et al. The cell biology of intervertebral disc aging and degeneration. Ageing Res. Rev. 6:247–261, 2007.
Acknowledgements
This study was supported by grants from NIH NIAMS (AR056101 and AR050609). The authors would like to thank Dr. Armando Mendez from the Diabetes Research Institute for his assistance in obtaining glucose measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Marc Levenston oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Czamanski Salvatierra, J., Yuan, T.Y., Fernando, H. et al. Difference in Energy Metabolism of Annulus Fibrosus and Nucleus Pulposus Cells of the Intervertebral Disc. Cel. Mol. Bioeng. 4, 302–310 (2011). https://doi.org/10.1007/s12195-011-0164-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-011-0164-0