Abstract
Since integrins were first described as cell adhesion receptors over two decades ago, our understanding of their binding specificity and functional capacity has evolved dramatically. A number of in vitro cell culture experiments have suggested that integrins may play a role in the response of bone cells to mechanical stimuli. To determine whether the loss of integrins in bone cells affects mechanical adaptation in vivo, we used an ulnar loading model in mice with an osteocyte-specific β1 integrin deficiency. Using a Cre-loxP strategy in which Cre was driven by the 2.3 kb ColI(α1) promoter, the β1 integrin subunit was deleted from cortical osteocytes in mature (16 week old) mice. While there was no observable skeletal phenotype as a result of β1 integrin deletion, we found that conditional knockout mice exhibited a significant reduction in bone formation rates at the ulnar midshaft in response to three consecutive days of cyclic loading compared to floxed control mice. Further, there was a greater increase in periosteal expansion in control vs. conditional knockout mice in response to loading. While there are likely multiple signaling pathways involved in the cellular response to physical stimuli, our results suggest that β1 integrins play a role in mechanically induced bone formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone loss due to prolonged bed-rest, immobilization, paralysis, and spaceflight results from an imbalance between the processes of bone formation and bone resorption. Mechanical loading can influence this balance by increasing the activity of bone-building osteoblasts, decreasing the formation of bone-resorbing osteoclasts, or both. These processes may be regulated by mechanosensitive osteocytes, which are terminally differentiated bone cells within the mineralized matrix. Understanding how mechanical forces at the tissue level are transduced into a cellular response is important in the development of new therapies to treat skeletal conditions, such as disuse bone loss and osteoporosis.
Integrin heterodimers are transmembrane adhesion proteins that form cellular focal adhesions and may initiate mechanosensitive signaling in osteocytes.4,15,34 They consist of α and β subunits that bind to extracellular matrix (ECM) ligands and to intracellular signaling proteins. The β1 integrin subunit forms dimers with twelve α isoforms, creating the largest subgroup of integrin heterodimers,4 which are the only known integrins to bind type I collagen, the major structural protein in bone.10 Integrin-based focal adhesions tether osteocytes, via their cellular processes, to canalicular walls.20,34 Load-induced fluid movement through the canalicular space is believed to deform integrin attachments2 which modulates downstream osteogenic signaling targets, such as focal adhesion kinase (FAK) and the MAPK signaling cascade.15,18 Cultured osteoblasts exposed to cyclic strain respond via the Ras/Raf/MEK signaling pathway that is initiated via integrins,3 and blocking focal adhesion assembly in osteoblasts decreases the osteogenic response to steady fluid flow and to cyclic compression.25,31 In vivo data show that mice with a β1 integrin deficiency in cortical osteocytes exhibit increased whole bone strength and stiffness, expansion of bone and marrow compartments, and increased inertial properties in response to short-duration disuse.24 Additionally, an osteoblast-specific conditional deletion of FAK in mice attenuated the osteogenic response of bone marrow cells to mechanical stimuli.19 While accumulating evidence suggests that integrins play a key role in bone cell mechanotransduction, the role of β1 integrins in mechanically induced adaptation of bone remains unclear.
The goal of this study was to determine the precise role of β1 integrins in load-induced cortical bone formation in adult mice. Here, we generated mice with an osteocyte-specific β1 integrin deficiency by mating β1 integrin floxed (β1integrinfl/fl) mice with 2.3 kb ColI(α1)-Cre recombinase transgenic mice.6,24 Sixteen-week-old β1integrinfl/fl; ColI(α1)-Cre− (controls) and β1integrinfl/fl; ColI(α1)-Cre+ (cKO) offspring were subjected to three consecutive days of in vivo ulnar loading. Cortical bone formation rates and cross-sectional geometry of loaded and nonloaded ulnae were analyzed. Osteocyte-specific β1 cKO mice exhibited significantly lower load-induced bone formation rates compared to controls, suggesting β1 integrins are involved in osteocyte-driven mechanical adaptation of bone.
Materials and Methods
Animals
All procedures were approved by the Veterans Affairs Department of Laboratory Animal Medicine Institutional Animal Care and Use Committee. The 2.3 kb ColI(α1)-Cre recombinase transgenic mice6,24 were bred with mice containing loxP sites flanking the coding exon of the β1 integrin monomer in both alleles (β1fl/fl).26 As reported previously, a promoterless LacZ gene was engineered into β1fl/fl mice downstream of the 3′ loxP site such that β1 integrin deletion by genetic recombination resulted in LacZ expression driven by the endogenous β1 integrin promoter.26 Offspring included β1fl/fl; ColI(α1)-Cre+ conditional knockout (cKO) mice with an osteocyte-specific β1 deletion, and β1fl/fl; ColI(α1)-Cre− mice, which served as controls. Genotyping of mice was performed by PCR using custom primers specific for ColI(α1)-Cre and the β1 floxed gene.6,24,26 All mice were maintained on a C57BL/6 background. Animal body weight was measured on the first day of loading and at euthanasia.
Confirmation of β1 Integrin Deletion
To confirm gene deletion of β1 integrins, femora and tibiae from cKO and control mice were incubated with 2 mg mL−1 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (X-gal) in DMEM/10%FBS for 20 h for detection of β-galactosidase activity initiated by recombination.24 To confirm β1 protein deficiency, immunohistochemistry was performed in ulnae and calvaria obtained from 16-week-old mice. Briefly, bone specimens were fixed in 3% paraformaldehyde, permeabilized in 0.5% Triton X-100, and blocked for 2 h at 37 °C in a blocking solution of 1% bovine serum albumin in phosphate-buffered saline. Samples were incubated in 10 μg mL−1 monoclonal anti-integrin β1 primary antibody (Chemicon, MAB1997) for 1 h at 37 °C, followed by a 1:20 dilution of FITC-conjugated goat anti-rat IgG (Chemicon, AP136F) with a 1:100 dilution of DAPI (Roche, Switzerland) for 30 min at 37 °C in the dark. Embedded osteocytes were imaged at 100× on a Nikon TE2000/C1 laser scanning confocal microscope (Nikon Instruments, Melville, NY).
In Vivo Ulnar Loading
Cyclic axial loads were delivered to the right distal forearm of 16-week-old virgin female cKO and control mice under 2.5% isoflurane-induced anesthesia. Ulnae were loaded 3 consecutive days at 120 cycles day−1 with a 2-Hz sine waveform using an EnduraTEC ELF 3200 electromechanical loading system (Bose, Eden Prairie, MN). A 0.1 N preload fixed the forearm into place prior to loading. A maximum load of 3 N was applied, which was designed to generate peak deformations of approximately 3000 microstrain (με) in the ulna mid-diaphysis.28 The contralateral limb served as an internal control. Mice experienced normal cage activity between loading sessions. Subcutaneous injections of calcein (10 mg kg−1 body mass; Sigma, St. Louis, MO) and Alizarin Red S (50 mg kg−1 body mass; Sigma, St. Louis, MO) were administered 4 and 8 days after the first day of loading, respectively. Animals were euthanized 13 days after the first day of loading and bones were immediately dissected and fixed in 70% ethanol for processing.
Histomorphometry
Ulnae were measured for total length, and the midsection of each ulna was marked using a lead pencil. Bones were fixed overnight in 70% ethanol at 4 °C and then dehydrated at room temperature in graded ethanol, infiltrated with 50:50 ethanol:methyl methacrylate (Fisher Scientific, Pittsburgh, PA), and embedded at 40 °C in methyl methacrylate polymerized with benzoyl peroxide. Embedded bones were sectioned at midshaft with a Buehler IsoMet 1000 saw (Buehler, Lake Bluff, Illinois) and imaged at 10× magnification with a Nikon TE2000/C1 laser scanning confocal microscope (Nikon Instruments, Melville, NY). Static histomorphometric parameters were measured at the periosteal surface using ImageJ (U.S. National Institutes of Health, Bethesda, Maryland) including total bone perimeter (B.Pm); single labeled perimeter (sL.Pm); double labeled perimeter, measured along the first label (dL.Pm), and double label area (dL.Ar). Standard measures of bone formation were calculated at the periosteal surface, including: mineral apposition rate [MAR (μm day−1) = (dL.Ar/dL.Pm)/4 days]; mineralizing surface [MS/BS (%) = (½ sL.Pm + dL.Pm)/B.Pm × 100]; and bone formation rate [BFR (μm3 μm−2 yr−1) = MAR × MS/BS × 365].23 Adobe Photoshop CS3 (Adobe, San Jose, CA) and ImageJ software were used to analyze geometric parameters of loaded and nonloaded ulnae from cKO and control mice. The following were calculated at the midshaft of each ulna: total area (μm2), cross-sectional area (μm2), the minimum second moment of area (I min, μm4), and the section diameter (Se.Dm, μm) along the I min axis, which represents the direction of bending. The endocortical area (μm2) was calculated by subtracting the cross-sectional area from the total area for each ulna. Bone formation parameters for individual mice were normalized by section modulus to account for differences in bone cross-sectional geometry and achieved peak strain during loading. Section modulus was estimated as 2/3 × Se.Dm/I min.13,29
Microcomputed Tomography
Bone morphologic assessment of cKO and control mice was performed using an in vivo microcomputed tomography (μCT) scanner (vivaCT40, Scanco Medical, Bassersdorf, Switzerland). Cortical cross-sectional area (μm2) and the maximum and minimum second moments of area (I max and I min, respectively; μm4) were measured at the tibial midshaft. Trabecular bone measurements at the proximal tibia metaphysis, the distal femur metaphysis, and the lumbar vertebrae (L2, L3, and L5) were obtained by separating the cancellous bone from cortical bone with semi-automatically drawn contour areas. Trabecular microarchitecture analysis of the proximal tibia included bone volume fraction (BV/TV, %), trabecular number (Tb.N, mm−1), trabecular spacing (Tb.Sp, mm), trabecular thickness (Tb.Th, mm), and connectivity density (Conn.D, mm−3).
Statistical Methods
All data are presented as mean ± standard error of the mean (SEM). For measures of bone formation (MAR, MS/BS, and BFR), a two-factor ANOVA was used to analyze the interaction effect between loading and genotype (Statview, CiteWise.com, Action, MA). Differences between bone formation parameters in loaded (right) and nonloaded (left) ulnae were detected using a one-way ANOVA followed by paired t-tests with a Bonferroni correction for multiple comparisons. For two-sample comparisons of percent change in cortical area, a student’s t test was used. Statistical significance was considered at p < 0.05.
Results
Genetic Recombination and Deletion of the β1 Integrin Protein in Cortical Osteocytes
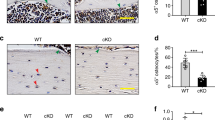
β1 integrin cKO mice were normal in appearance, both at birth and throughout development. There was no significant difference in body weight between genotypes (data not shown). Long bones from 4-month-old cKO mice were positive for β-galactosidase expression at the bone surface, indicating genomic deletion of β1 integrins in mature bone tissue (Fig. 1a). Bones isolated from control animals exhibited only background staining. Immunostaining showed protein expression of β1 integrin was absent in embedded osteocytes in cKO mice (Fig. 1b) compared with robust expression of β1 integrins along the cell body and processes of embedded osteocytes in control mice.
β1 integrin deletion was confirmed in cKO mice. (a) Histochemical detection of β-galactosidase expression in the tibia confirmed genetic recombination in cKO mice (left), while the controls (right) exhibited only background staining. (b) Immunohistochemistry shows protein expression of the β1 integrin (green) in cortical osteocytes of control mice (top panel), and depletion of β1 integrin protein in cKO mice (bottom panel). Bar = 10 μm
Cortical and Trabecular Bone Structure
Trabecular bone analysis by μCT showed no statistically significant difference between cKO and control mice for BV/TV, Tb.N, Tb.Sp, Tb.Th, or Conn.D at the proximal tibia, the distal femur, or lumbar vertebrae (Table 1). There was no significant difference in cortical cross-sectional area or inertial properties (I max and I min) at the tibia midshaft, and the mean length of both loaded and nonloaded ulnae was the same for cKO and control mice (data not shown).
Load-Induced Bone Formation
Load-induced bone formation was localized to the medial and lateral quadrants of the periosteal and endosteal surfaces, as shown by the area between fluorochrome labels (Fig. 2). Mineralizing surface (MS/BS), mineral apposition rate (MAR), and bone formation rate (BFR) were significantly greater in loaded ulnae compared to contralateral nonloaded ulnae for both cKO and control mice; however, there was no significant interaction effect between loading and genotype for MS/BS and MAR, either before (Table 2) or after normalization (data not shown). There was a significant interaction effect (loading * genotype) on BFR, both before (Table 2) and after (Fig. 3) normalization, indicating that there was significantly less periosteal bone formation in cKO mice compared to controls. Total cortical area at the ulnar midshaft increased in both cKO and control mice in response to loading, although the percent change in control mice was significantly greater than that of cKO mice (Fig. 4). There was a decrease in endocortical area in both cKO and control mice with loading, but the difference between genotypes did not reach significance. The cross-sectional area of both cKO and control mice increased as a result of loading; however, there was no difference due to genotype in changes in cross-sectional area during the four-day interlabel period.
Fluorochrome labels were used to measure parameters of bone formation and changes in cross-sectional at the ulna midshaft in response to loading. Robust bone formation response is evident on the medial and lateral surfaces of the loaded ulnae (right) compared to nonloaded ulnae (left). The osteogenic response was significantly greater in control (top) mice compared to β1 cKO (bottom) mice
Discussion
The goal of this study was to determine whether β1 integrins play a role in the anabolic response of bone to mechanical loading. Osteocytes, which are embedded within mineralized bone matrix, are ideally situated to sense external mechanical stimuli and to transduce these signals into intracellular signaling cascades. Integrins anchor osteocytes, via focal adhesions, to the mineralized bone matrix within individual lacunae and are believed to deform in the presence of fluid flow within the canalicular space.34 We show that mice with a β1 integrin deficiency in cortical osteocytes have significantly less mechanically-induced bone formation, demonstrating an in vivo role for integrins in osteocyte mechanosensitivity.
These data are consistent with previous work in which integrin function was inhibited in mice by expression of a function-perturbing β1 integrin tail in osteoblasts.36 Young transgenic mice with impaired integrin function exhibited increased cortical porosity and decreased thickness of parietal bones. At 35 days of age, transgenic mice experienced a 40% lower rate of bone formation,36 and adult transgenic mice had decreased femoral strength.7 While adult mice expressing the function-perturbing β1 integrin tail exhibited mildly reduced bone mass and altered tibial curvature,7 in the current study we found no significant difference in bone structure between cKO and control mice, including ulnar length and cross-sectional geometry. This indicates that phenotypic differences may depend on the extent and timing of integrin inhibition or deletion. Also consistent with results presented in the current study, cre-mediated deletion of focal adhesion kinase (FAK), an integrin-mediated protein tyrosine kinase, using the 2.3 kb ColI(α1) promoter abrogates the response of bone marrow cells to mechanical stimuli and the subsequent deposition of matrix proteins by osteoblasts.19 Further, distraction osteogenesis induces localization of FAK and c-Src to regions of loading.27,32 Taken together with previous results, our data suggest that β1 integrins contribute not only to bone formation associated with normal growth, but also with the anabolic response of bone to externally applied mechanical loads.
The current study suggests that β1 integrins contribute to the anabolic response of bone to mechanical signals. However, there is also evidence that β1 integrins can regulate the catabolic response to skeletal disuse. Transgenic mice expressing a function-perturbing β1 integrin tail in osteoblasts exhibit a greater increase in cancellous resorption surfaces in response to hindlimb unloading, suggesting that integrin signaling may normally suppress the osteoclastic response to disuse.17 Mice with bone-specific deletion of β1 integrins using Cre-loxP with the 2.3 kb ColI(α1) promoter experience greater changes in tibia cross-sectional geometry during hindlimb unloading. In response to 1 week of disuse, β1 integrin cKO mice exhibited increased erosion of the endosteal surface and increased expansion at the periosteal surface of the tibia,24 similar to what occurs in aging bone and during long-duration spaceflight. In contrast to the increased periosteal apposition that occurs in cKO mice during hindlimb unloading,24 we found that β1 integrin cKO mice have significantly decreased periosteal apposition in response to ulnar loading. This suggests that integrins play distinct roles in the response of bone to loading vs. unloading and may initiate different molecular signaling pathways under different mechanical stimuli. One significant difference between ulnar loading and hindlimb unloading is the distinct populations of specialized cells recruited in each case. Ulnar loading activates pre-osteoblasts on bone surfaces and recruits new osteoblast progenitors from the mesenchymal stem cell niche to affect new bone formation,33 whereas hindlimb unloading recruits osteoclasts, which are formed from fused macrophages and are of hematopoietic origin, to carry out bone resorption.1 Although the regulatory signaling mechanisms involved in each of these processes are not well known, our and others’ in vivo data suggest that osteocyte-specific signaling via β1 integrins normally enhances osteoblast activity while inhibiting osteoclastogenesis and/or osteoclast activity. Several in vitro studies contradict this latter concept, however, by showing a relationship between β1 integrin engagement with ECM proteins and osteoclast formation. In primary osteoblasts, attachment of β1 integrins to type I collagen increases RANKL expression, which induces formation of tartrate resistant acid phosphatase-positive multinuclear cells in a coculture of osteoblasts and peripheral monocytes.11,22 These data suggest that β1 integrin activation in osteoblasts may initiate osteoclastogenesis in bone. It may be that under normal daily loading, β1 integrins mediate osteoclastogenesis as part of healthy bone remodeling, but under reduced mechanical loading, β1 integrins inhibit overall bone resorption.
β1 integrin deficiency was targeted to bone using the 2.3 kb ColI(α1) promoter, which is expressed in differentiated osteoblasts and osteocytes.5,30 While 2.3 kb ColI(α1) driven-Cre expression has been demonstrated extensively,30 the degree to which protein deletion occurs in osteoblasts and osteocytes has not been widely characterized. It has been shown that a 2.3 kb ColI(α1)-Cre-mediated deletion of the β1 integrin gene is confined to bone tissue and results in protein deficiency from cortical osteocytes, but not cancellous osteocytes or surface osteoblasts in adult mice.24 While it is possible that both copies of the β1 integrin floxed allele were not deleted effectively except in cortical osteocytes, which have a longer life span than cancellous osteocytes or surface osteoblasts, this is unlikely since β-gal activity at the bone surface demonstrated widespread gene deletion in osteoblastic cells. A second possible explanation described previously by Phillips et al. 24 is that β1 integrin proteins may exhibit a long half-life in vivo, and the relatively short life span of osteoblasts35 may not enable complete degradation of β1 protein. As noted by Phillips et al.,24 the presence of non-osteoblast lineage cells, such as chondrocytes and bone marrow cells, likely contributed to residual floxed β1 integrin in cKO bone samples. We confirmed protein deletion of β1 integrins in embedded osteocytes by immunostaining in long bones isolated from mature mice (Fig. 1b). Because axial load was applied to the mouse ulna, which contains minimal cancellous bone, the use of the 2.3 kb ColI(α1) promoter was ideal to examine the role of β1 integrins in mechanosensing by cortical osteocytes. The use of different promoters, such as 3.6 kb ColI(α1), osterix, osteocalcin, or dentin matrix protein-1 (DMP-1) may be beneficial for future studies to examine the role of β1 integrins in other stages during osteoblast lineage differentiation.
The mild phenotype of β1 integrin deletion targeted to bone may be due, in part, to the timing and extent of gene deletion, both of which have been shown to be key factors in resulting phenotypic differences.14,16 Additionally, β3 and β5 integrins are expressed in cells of the osteoblastic lineage,8,9 and in situ immunostaining for β3 integrins in osteocytes shows punctuate expression along the canalicular wall.20 Thus, functional redundancy between integrin subunits may mitigate the phenotypic manifestation of β1 integrin deletion.12,21 While β3 or β5 integrins may partially compensate for loss of β1 integrins in cKO mice, bone formation was significantly reduced in cKO mice, demonstrating that β1 integrins do indeed play a role in the response of cortical osteocytes to a mechanical stimulus. Nevertheless, the relatively mild loading effect in cKO mice indicates that additional proteins and signaling pathways are likely involved in initiating the osteogenic response to mechanical loading. These may include other integrin subunits, for example β3 and β5, as well as non-integrin proteins such as G-protein coupled receptors, ion channels, primary cilia, and cadherins.
Our data show that β1 integrins are involved in mechanically-induced bone formation. Bone formation and changes in cross-sectional geometry are significantly reduced in mice with a bone-specific deletion of β1 integrins. An interesting dichotomy is beginning to arise between the molecular mechanisms that govern bone resorption during disuse and those that drive mechanically-induced bone formation in vivo. Specifically, it appears that integrins may attenuate the response of bone to reduced mechanical loading, while they enhance mechanical stimuli during increased mechanical loading. Nevertheless, it is evident that integrins play a significant role in osteocyte mechanosensitivity and may be a promising molecular target for prevention and treatment of certain bone conditions including osteoporosis and disuse bone loss.
Abbreviations
- cKO:
-
Conditional knockout
- ColI(α1):
-
Type I collagen α1(I)
- MAR:
-
Mineral apposition rate
- MS/BS:
-
Mineralizing surface
- BFR:
-
Bone formation rate
References
Aguirre, J. I., L. I. Plotkin, S. A. Stewart, R. S. Weinstein, A. M. Parfitt, S. C. Manolagas, and T. Bellido. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 21(4):605–615, 2006.
Arcangeli, A., and A. Becchetti. Complex functional interaction between integrin receptors and ion channels. Trends Cell Biol. 16(12):631–639, 2006.
Boutahar, N., A. Guignandon, L. Vico, and M. H. Lafage-Proust. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in erk activation. J. Biol. Chem. 279(29):30588–30599, 2004.
Brakebusch, C., and R. Fassler. Beta 1 integrin function in vivo: adhesion, migration and more. Cancer Metastasis Rev. 24(3):403–411, 2005.
Dacic, S., I. Kalajzic, D. Visnjic, A. C. Lichtler, and D. W. Rowe. Col1a1-driven transgenic markers of osteoblast lineage progression. J. Bone Miner. Res. 16(7):1228–1236, 2001.
Dacquin, R., M. Starbuck, T. Schinke, and G. Karsenty. Mouse alpha1(i)-collagen promoter is the best known promoter to drive efficient cre recombinase expression in osteoblast. Dev. Dyn. 224(2):245–251, 2002.
Globus, R. K., D. Amblard, Y. Nishimura, U. T. Iwaniec, J. B. Kim, E. A. Almeida, C. D. Damsky, T. J. Wronski, and M. C. van der Meulen. Skeletal phenotype of growing transgenic mice that express a function-perturbing form of beta1 integrin in osteoblasts. Calcif. Tissue Int. 76(1):39–49, 2005.
Gronthos, S., K. Stewart, S. E. Graves, S. Hay, and P. J. Simmons. Integrin expression and function on human osteoblast-like cells. J. Bone Miner. Res. 12(8):1189–1197, 1997.
Grzesik, W. J., and P. G. Robey. Bone matrix rgd glycoproteins: Immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J. Bone Miner. Res. 9(4):487–496, 1994.
Gullberg, D. E., and E. Lundgren-Akerlund. Collagen-binding i domain integrins—what do they do? Prog. Histochem. Cytochem. 37(1):3–54, 2002.
Hirai, F., S. Nakayamada, Y. Okada, K. Saito, H. Kurose, A. Mogami, and Y. Tanaka. Small gtpase rho signaling is involved in beta1 integrin-mediated up-regulation of intercellular adhesion molecule 1 and receptor activator of nuclear factor kappab ligand on osteoblasts and osteoclast maturation. Biochem. Biophys. Res. Commun. 356(1):279–285, 2007.
Horton, M. A., H. M. Massey, N. Rosenberg, B. Nicholls, U. Seligsohn, and A. M. Flanagan. Upregulation of osteoclast alpha2beta1 integrin compensates for lack of alphavbeta3 vitronectin receptor in iraqi-jewish-type glanzmann thrombasthenia. Br. J. Haematol. 122(6):950–957, 2003.
Hsieh, Y. F., T. Wang, and C. H. Turner. Viscoelastic response of the rat loading model: Implications for studies of strain-adaptive bone formation. Bone 25(3):379–382, 1999.
Huang, Z., K. Shimazu, N. H. Woo, K. Zang, U. Muller, B. Lu, and L. F. Reichardt. Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J. Neurosci. 26(43):11208–11219, 2006.
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110(6):673–687, 2002.
Imai, T., M. Jiang, P. Chambon, and D. Metzger. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid x receptor alpha mediated by a tamoxifen-inducible chimeric cre recombinase (cre-ert2) in adipocytes. Proc. Natl Acad. Sci. USA 98(1):224–228, 2001.
Iwaniec, U. T., T. J. Wronski, D. Amblard, Y. Nishimura, M. C. van der Meulen, C. E. Wade, M. A. Bourgeois, C. D. Damsky, and R. K. Globus. Effects of disrupted beta1-integrin function on the skeletal response to short-term hindlimb unloading in mice. J. Appl. Physiol. 98(2):690–696, 2005.
Katsumi, A., A. W. Orr, E. Tzima, and M. A. Schwartz. Integrins in mechanotransduction. J. Biol. Chem. 279(13):12001–12004, 2004.
Leucht, P., J. B. Kim, J. A. Currey, J. Brunski, and J. A. Helms. Fak-mediated mechanotransduction in skeletal regeneration. PLoS ONE 2(4):e390, 2007.
McNamara, L. M., R. J. Majeska, S. Weinbaum, V. Friedrich, and M. B. Schaffler. Attachment of osteocyte cell processes to the bone matrix. Anat. Rec. Hoboken 292(3):355–363, 2009.
Mercurio, A. M. Lessons from the alpha2 integrin knockout mouse. Am. J. Pathol. 161(1):3–6, 2002.
Nakayamada, S., Y. Okada, K. Saito, M. Tamura, and Y. Tanaka. Beta1 integrin/focal adhesion kinase-mediated signaling induces intercellular adhesion molecule 1 and receptor activator of nuclear factor kappab ligand on osteoblasts and osteoclast maturation. J. Biol. Chem. 278(46):45368–45374, 2003.
Parfitt, A. M., M. K. Drezner, F. H. Glorieux, J. A. Kanis, H. Malluche, P. J. Meunier, S. M. Ott, and R. R. Recker. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the asbmr histomorphometry nomenclature committee. J. Bone Miner. Res. 2(6):595–610, 1987.
Phillips, J. A., E. A. Almeida, E. L. Hill, J. I. Aguirre, M. F. Rivera, I. Nachbandi, T. J. Wronski, M. C. van der Meulen, and R. K. Globus. Role for beta1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol. 27:609–618, 2008.
Ponik, S. M., and F. M. Pavalko. Formation of focal adhesions on fibronectin promotes fluid shear stress induction of cox-2 and pge2 release in mc3t3-e1 osteoblasts. J. Appl. Physiol. 97(1):135–142, 2004.
Potocnik, A. J., C. Brakebusch, and R. Fassler. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity 12(6):653–663, 2000.
Rhee, S. T., and S. R. Buchman. Colocalization of c-src (pp60src) and bone morphogenetic protein 2/4 expression during mandibular distraction osteogenesis: In vivo evidence of their role within an integrin-mediated mechanotransduction pathway. Ann. Plast. Surg. 55(2):207–215, 2005.
Robling, A. G., J. Li, K. L. Shultz, W. G. Beamer, and C. H. Turner. Evidence for a skeletal mechanosensitivity gene on mouse chromosome 4. FASEB J. 17(2):324–326, 2003.
Robling, A. G., and C. H. Turner. Mechanotransduction in bone: Genetic effects on mechanosensitivity in mice. Bone 31(5):562–569, 2002.
Rossert, J., H. Eberspaecher, and B. de Crombrugghe. Separate cis-acting DNA elements of the mouse pro-alpha 1(i) collagen promoter direct expression of reporter genes to different type i collagen-producing cells in transgenic mice. J. Cell Biol. 129(5):1421–1432, 1995.
Sanchez, C., O. Gabay, C. Salvat, Y. E. Henrotin, and F. Berenbaum. Mechanical loading highly increases il-6 production and decreases opg expression by osteoblasts. Osteoarthr. Cartilage 17:473–481, 2009.
Tong, L., S. R. Buchman, M. A. Ignelzi, Jr., S. Rhee, and S. A. Goldstein. Focal adhesion kinase expression during mandibular distraction osteogenesis: Evidence for mechanotransduction. Plast. Reconstr. Surg. 111(1):211–222, 2003; discussion 23–24.
Turner, C. H., I. Owan, T. Alvey, J. Hulman, and J. M. Hock. Recruitment and proliferative responses of osteoblasts after mechanical loading in vivo determined using sustained-release bromodeoxyuridine. Bone 22(5):463–469, 1998.
Wang, Y., L. M. McNamara, M. B. Schaffler, and S. Weinbaum. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc. Natl. Acad. Sci. USA 104(40):15941–15946, 2007.
Weinstein, R. S., R. L. Jilka, A. M. Parfitt, and S. C. Manolagas. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Invest. 102(2):274–282, 1998.
Zimmerman, D., F. Jin, P. Leboy, S. Hardy, and C. Damsky. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev. Biol. 220(1):2–15, 2000.
Acknowledgments
The authors thank Dr. Ruth Globus, Dr. Jonathan Phillips, and Rose Mojarrab at NASA Ames Research Center, and Sara Temiyasathit at Stanford University for significant technical assistance and intellectual contributions. Funding sources included NIH Grant AR45989; NIH Grant AR54156; the US Department of Veterans Affairs; a National Science Foundation Graduate Research Fellowship; and a Veterans Affairs Pre-Doctoral Associated Health Rehabilitation Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Litzenberger, J.B., Tang, W.J., Castillo, A.B. et al. Deletion of β1 Integrins from Cortical Osteocytes Reduces Load-Induced Bone Formation. Cel. Mol. Bioeng. 2, 416–424 (2009). https://doi.org/10.1007/s12195-009-0068-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-009-0068-4