Abstract
Parkin-related mitophagy is vital for endothelial cell viability and the development of atherosclerosis, although the upstream regulatory factor underlying Parkin-mediated mitophagy in endothelial apoptosis and atherosclerosis progression remains unknown. In the present study, we demonstrated that nuclear receptor subfamily 4 group A member 1 (NR4A1) is actually expressed in aortic endothelial cells (AECs) under oxidized low-density lipoprotein (ox-LDL) treatment in vitro or isolated from high-fat treated mice in vivo. Higher NR4A1 levels were associated with AEC apoptosis, mitochondrial dysfunction, and energy disorder. At the molecular level, ox-LDL stimulation increased NR4A1 expression, which evoked Parkin-mediated mitophagy. Excessive mitophagy overtly consumed mitochondrial mass, leading to an energy shortage and mitochondrial dysfunction. However, loss of NR4A1 protected AECs against ox-LDL induced apoptosis by inhibiting excessive mitophagy. Furthermore, we also identified that NR4A1 regulated Parkin activation via post-transcriptional modification by Ca2+/calmodulin-dependent protein kinase II (CaMKII). Activated CaMKII via NR4A1 induced the phosphorylated activation of Parkin. In summary, our data support the role of NR4A1/CaMKII/Parkin/mitophagy in AEC apoptosis and atherosclerosis formation and provide new insights into treating atherosclerosis with respect to endothelial viability, mitophagy, and NR4A1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of atherosclerosis (AS) has declined significantly worldwide over the past half-century (Hamilton et al. 2017). Nevertheless, AS remains a leading health issue worldwide. The development and progression of AS are thought to be a multistep process and endothelial cell injury is the requisite for the development of AS (Camare et al. 2017; Wang et al. 2016). Damage to endothelial cells allows lipid deposition in the intima (Okamoto and Suzuki 2017). Unfortunately, the molecular mechanisms underlying endothelial cell damage in response to high-fat injury are poorly understood.
Mitochondria, which are organelles that are present in all cells of the human body except erythrocytes, play a pivotal role in energy production (Caja and Enriquez 2017; Vendrov et al. 2017). In addition to this vital function, mitochondria are involved in other complex processes, such as cellular energy metabolism, oxidative stress regulation, calcium signaling modification, cellular proliferation, and cellular death (especially mitochondria-dependent caspase 9 apoptosis) (Chen et al. 2017; Wang et al. 2017a; Zhou et al. 2014, 2015a). Notably, previous studies have indicated that endothelial viability under high-fat stimulation is related to mitochondrial apoptosis (Chattopadhyay et al. 2017; Zhang et al. 2016; Zhou et al. 2017a). Excessive oxidative stress, increased inflammatory response, and cellular calcium overload are upstream activators of mitochondrial apoptosis (Chen et al. 2016a; Zhao et al. 2017; Zhu et al. 2017), which can be accompanied by the collapse of the vascular endothelial barrier (Zhou et al. 2017c). Interestingly, a recent study has further demonstrated that mitochondrial apoptosis can be derived from mitophagy (mitochondrial autophagy) (Zhou et al. 2017e). Excessive mitophagy extensively consumes mitochondrial mass, leading to an energy undersupply (Jin et al. 2018; Zhou et al. 2018b). Meanwhile, excessive mitophagy was shown to be associated with the activation of pro-apoptotic pathways (Bravo-San Pedro et al. 2017). However, whether mitophagy has a role in endothelial viability in the context of high-fat stimulation is unclear.
Previous studies have illustrated that mitophagy is primarily activated through the Parkin pathway in cardiac endothelial cells (Zhou et al. 2017d). Activated Parkin may label mitochondria via LC3II and overtly deliver mitochondria to lysosomes, leading to mitochondrial mass loss and energy disorder. However, the upstream signaling regulating Parkin-associated mitophagy in endothelial cells under high-fat injury are yet unknown.
NR4A1, the subfamily of NR4A orphan receptors, is of importance in cellular proliferation, cancer migration, chronic metabolic disease, immune inflammatory reaction, and cell death (Pawlak et al. 2015; Ranhotra 2015). Functional studies have indicated that NR4A1 is actually rich in the fatty or diabetic liver (Min et al. 2014). NR4A1 primarily exerts pro-apoptotic effects that contribute to liver cell death via induction of mitochondrial damage. Based on this finding, we questioned whether NR4A1 is involved in the endothelial damage that occurs under high-fat attack via mitochondrial homeostasis.
Several researchers have also found that NR4A1 overexpression is involved in the cellular calcium imbalance and adverse cardiac remodeling in cardiomyocytes (Abdou et al. 2013; Medzikovic et al. 2015). Interestingly, the elevation of cellular calcium is the upstream activator for Parkin-related mitophagy via Ca2+/calmodulin-dependent protein kinase II (CaMKII) pathways (Sato et al. 2006). Thus, we aimed to determine whether NR4A1 has the ability to increase the vulnerability of endothelial cells in response to high-fat injury via a modification of mitophagy by regulating calcium signaling. Our results indicated that NR4A1 is upregulated in endothelial cells in the context of high-fat injury and that higher levels of NR4A1 triggered apoptosis in endothelial cells via excessive Parkin-related mitophagy. Further, NR4A1 promoted a cellular calcium overload that signaled CaMKII; the latter activated Parkin through post-phosphorylation modification. Therefore, we discovered that NR4A1/Ca2+/CaMKII/Parkin/mitophagy pathways are involved in the pathogenesis of endothelial apoptosis induced by high-fat injury.
Material and methods
Animal study
The animal study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). The animal study was approved by the Ethics Committee of Beijing Chaoyang Hospital Affiliated to Capital Medical University. Wild type (WT) and ApoE(−/−) mice at 8 weeks old were purchased from the Jackson Laboratory. These mice were treated with a low-fat diet and/or high-fat diet for approximately 3 months (Kalyanaraman 2017). Accordingly, the animals used in our study were divided into two groups: low-fat diet (LDF) group and high-fat diet (HFD) group. The diets were purchased from Research Diets (New Brunswick, NJ, USA) according to previous study (Han et al. 2017).
Endothelial cell culture and high-fat treatment in vitro
Aortic endothelial cells (AECs) were purchased from the American Type Culture Collection (ATCC) Company (Rockville, MD, USA). The L-DME medium supplemented with the 10% fetal bovine serum was used to culture the AECs at 37 °C with 5% CO2. To induce high-fat injury in vitro, AECs were cultured with 100 μM oxidized low-density lipoprotein (ox-LDL, Selleck Chemicals, Houston, TX, USA) for approximately 12 h (Das et al. 2017). To induce the mitophagy, FCCP (5 μm, Selleck Chemicals, Houston, TX, USA) was applied for about 2 h with AECs and to inhibit the activity of CaMKII, the KN93 (10 μm, Selleck Chemicals, Houston, TX, USA) was used for about 45 min (Kozlov et al. 2017; Lee and Back 2017).
Immunocytochemistry and immunofluorescence
Samples were permeabilized by 0.1% Triton X-100 at 4 °C for 30 min. Subsequently, samples were washed three times with PBS. Primary antibodies were then added to the samples overnight at 4 °C (Tallman et al. 2017). The following primary antibodies were purchased either from Abcam or Cell Signaling Technology: Tomm20 (Abcam, No. ab78547), LAMP1 (Abcam, No. ab24170), F-actin (Abcam, No. ab205), and cyt-c (Abcam, No. ab133504). After washing three times with PBS, the samples were incubated with secondary antibody for 1 h at room temperature. The samples were then observed using an Olympus BX 50 microscope (Xiao et al. 2017).
Immunoblotting
Western blots were used to analyze protein changes. Samples were washed three times with PBS. Samples were then lysed in RIPA buffer to extract proteins and were then centrifuged for 10 min at 4 °C (Xu et al. 2017). The supernatant was then collected and subjected to immunoblotting analysis (Zhou et al. 2015b). The primary antibodies used for immunoblotting were as follows: pro-caspase 3 (1:1000, Cell Signaling Technology, No. 9662), cleaved caspase 3 (1:1000, Cell Signaling Technology, No. 9664), Bad (1:2000, Abcam, No. ab90435), Bax (1:2000, Cell Signaling Technology, No. 5023), Bcl2 (1:1000, Cell Signaling Technology, No. 3498), caspase 9 (1:1000, Abcam, No. ab32539), LC3II (1:1000, Cell Signaling Technology, No. 3868), p62 (1:1000, Abcam, No. ab56416), Beclin1 (1:1000, Cell Signaling Technology, No. 3495), Atg5 (1:1000, Cell Signaling Technology, No. 12994), p-Parkin (1:1000, Abcam, No. ab73015), Parkin (1:1000, Abcam, No. ab15954), CaMKII (1:1000, Cell Signaling Technology, No. 3362), p-CaMKII (1:1000, Cell Signaling Technology, No. 12716), NR4A1 (1:1000, Cell Signaling Technology, No. 3960), PARP (1:1000, Cell Signaling Technology, No. 9532), complex III subunit core (CIII-core2, 1:1000, Invitrogen, No. 459220), complex II (CII-30, 1:1000, Abcam, No. ab110410), complex IV subunit II (CIV-II, 1:1000, Abcam, No. ab110268), and complex I subunit NDUFB8 (CI-20, 1:1000, Abcam, No. ab110242) (Zhou et al. 2017f).
JC-1 staining for mitochondrial potential and ATP measurement
JC-1 staining was used to observe mitochondrial changes. Samples were washed three times with cold-PBS and then 10 mg/ml of JC-1 were added into the medium for approximately 10 min at 37 °C in the dark (Hu et al. 2017b; Zhou et al. 2015a). Subsequently, cold-PBS was used to wash the samples three times at room temperature. The mitochondrial potential was observed under a fluorescence microscope (32). Additionally, ATP production was measured to determine mitochondrial function. The ATP measurements were performed using an ATP Bioluminescence Assay Kit. First, samples were washed with cold-PBS and cells were then lysed followed by centrifugation at 10,000×g for 2 min at 4 °C. The obtained supernatant was treated with the luciferase reagent provided in the ATP Bioluminescence Assay Kit. The ATP concentration was measured using a microplate luminometer (Gao et al. 2017).
Mitochondrial ROS detection and mPTP opening assay
The MitoSOX red mitochondrial superoxide indicator (Molecular Probes, USA) was used to detect mitochondrial reactive oxygen species (ROS) (Griffiths et al. 2017). As for the mPTP opening assay, tetramethylrhodamine ethyl ester fluorescence was used to observe opening of the mPTP according to previous studies. The fluorescence intensity of tetramethylrhodamine ethyl ester that was reduced by half of the initial fluorescence intensity was recorded (Liu et al. 2017b).
MTT assay, TUNEL detection, and caspase 9 activity
Cell viability was assessed through MTT assay and TUNEL assay. For viability assays, 96-well plates were used, and 104 cells were cultured per well. After washing three times at room temperature with cold-PBS, 50 μL of MTT were added to the samples. Then, the cells were incubated at 37 °C in the dark for approximately 2 h (Lin et al. 2017). Subsequently, 200 μL of DMSO were added to each well to solubilize the MTT. Then, the fluorescence intensity was recorded using a microplate luminometer. TUNEL staining was conducted using an In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) (Wang et al. 2017b). Samples were washed three times with cold-PBS. Samples were then incubated with TUNEL staining for approximately 30 min at room temperature. Subsequently, cellular nuclei were labeled with DAPI. Samples were then observed using an Olympus BX 50 microscope. Caspase 9 activity was measured using a commercial kit (Beyotime) as previously described (Hambright et al. 2017).
The small RNA interference assay and trypan blue staining
The siRNA against NR4A1 was used to knockdown its expression. The siRNA was purchased from Yangzhou Ruibo Biotech Co., Ltd. The primers for NR4A1 were as follows: the sense strand siRNA 5′-GAGCTACTCTCAGCAATUAGU-3′ and antisense strand siRNA 3′-TGCCATGAGTCATCGATGAUTAU-5′ were used (Sigala et al. 2017). The siRNA transfection was performed using Lipofectamine 2000 transfection reagent according to the manufacturer’s protocol (Iggena et al. 2017).
As for the trypan blue staining, 0.4% trypan blue was incubated with cells for 2 min at room temperature. Subsequently, the number of trypan blue-positive cells were counted and observed using an Olympus BX 50 microscope (Zhai et al. 2017).
Statistical analysis
The data in the present study were presented as the mean ± standard deviation (SD) of at least three independent experiments and were analyzed by one-way analysis of variance (ANOVA). The limit of statistical significance between the treated and control groups was P < 0.05.
Results
AS is developed in HFD mice with increased endothelial cell apoptosis
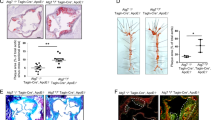
First, ApoE(−/−) mice with HFD were used to establish the AS model. Through H&E staining of the aortic sinus, we found that HFD-treated ApoE−/− mice developed more serious atherosclerotic lesions when compared to LFD-treated ApoE−/− mice (Fig. 1a–c). This result indicated that in ApoE−/− mice, AS developed because of HFD treatment. Subsequently, the aortic arch was isolated and tissue proteins were obtained to analyze the plaque composition. As shown in Fig. 1d–g, the aortic arch in HFD-treated ApoE−/− mice contained less CD31 but expressed more α-SMA and vimentin when compared to that in LFD-treated ApoE−/− mice. These data indicated that atherosclerotic lesions in HFD-treated mice contained more smooth muscle and fibrous tissue. However, the density of endothelial cells was reduced in HFD-treated mice during the plaque formation when compared to that in LFD-treated mice. Subsequently, we isolated CD31+ endothelial cells from HFD- and LFD-treated mice by flow cytometry. Western blot analysis for apoptotic proteins demonstrated that pro-apoptotic proteins were elevated in endothelial cells from HFD mice compared to proteins in LFD-treated mice (Fig. 1h–l). These results were similar to previous findings that high-fat damage caused endothelial apoptosis in a caspase-3 dependent manner (Chen et al. 2016b; Xue et al. 2017). In contrast, anti-apoptotic protein expression was reduced in HFD-treated mice when compared to that in LFD-treated mice, suggesting that HFD treatment increased endothelial cell apoptosis during the development of AS.
The atherosclerosis is developed in HFD mice. In the current study, ApoE−/− mice were treated with low-fat diet (LFD) and/or high-fat diet (HFD). a, b After 12 weeks, H&E staining was performed on aortic sinus and the results demonstrated a significant increase in aortic lesion size, shown by representative images (a) and by quantification (b). c The quantification of lipid content by Oil-red-O-staining on aortic sinus. d–g The proteins in aortic arch were isolated and analyzed the expression of CD31, α-SMA, and vimentin via western blotting. h–l The changes of pro-apoptotic proteins in aortic arch. *P < 0.05. n = 10/group
NR4A1 was activated by high-fat damage promoting endothelial cell apoptosis
To investigate the mechanism by which HFD induced endothelial cell apoptosis, we focused on NR4A1 expression. In vivo, we isolated CD31+ endothelial cells from the dissociated mouse aortic arch by flow cytometry. Through western blots analysis, we found that the expression of NR4A1 was significantly increased in endothelial cells isolated from HFD-treated mice (Fig. 2a, b). Further, aortic endothelial cells (ACEs) and oxidized low-density lipoprotein (ox-LDL) were used as an in vitro model to study the high-fat damage to endothelial cells in the setting of AS. TUNEL staining and MTT assays confirmed that ox-LDL significantly increased endothelial apoptosis (Fig. 2c–e). Following western blot analysis for NR4A1, we reconfirmed that NR4A1 is increased in response to high-fat injury, and these data were in accordance with the results obtained in animals (Fig. 2f, g).
NR4A1 was activated by high-fat damage promoting endothelial cell apoptosis. a, b The NR4A1 expression in mouse aortic arch with HFD treatment or LFD treatment. c The ox-LDL was used in vitro to mimic the high-fat damage and MTT assay was performed to evaluate the cellular viability. d, e TUNEL assay demonstrated that ox-LDL treatment significantly increased the number of apoptotic cells. f, g NR4A1 expression in vitro under ox-LDL treatment. Meanwhile, the siRNA against the NR4A1 was used and the western blotting was carried out to analyze the knockdown efficiency. h, i Trypan blue staining for the apoptotic cells. Knockdown of NR4A1 under ox-LDL treatment obviously reduced the number of trypan blue-positive cells. j–l The western blotting was conducted to measure the alterations of apoptotic cells with NR4A1 silencing under ox-LDL treatment. *P < 0.05 vs. Ctrl group; #P < 0.05 vs. ox-LDL + si-Ctrl group
To verify whether NR4A1 is involved in endothelial apoptosis, siRNA against NR4A1 expression was used. Blockade of NR4A1 via siRNA significantly reduced the high-fat-induced NR4A1 upregulation (Fig. 2f, g). Moreover, deletion of NR4A1 attenuated cellular apoptosis, which was determined using a trypan staining assay (Fig. 2h, i). Additionally, caspase 3 expression and its substrate PARP activation that was induced by high-fat damage were also blocked after NR4A1 inhibition (Fig. 2j–l). In summary, these data indicated both that NR4A1 was activated by high-fat damage and that higher NR4A1 expression promoted endothelial cell apoptosis.
High-fat induced endothelial death via mediating mitochondrial dysfunction
To demonstrate whether mitochondrial damage was responsible for the harmful effect of NR4A1 on high-fat-induced endothelial cell death, we assessed mitochondrial function. The mitochondrial membrane potential (MMP) allows mitochondria to generate ATP, which fuels a cell’s biological function. However, ox-LDL treatment significantly reduced the MMP whereas inhibition of NR4A1 reversed the MMP (Fig. 3a, b), as revealed by more red fluorescence and less green fluorescence.
NR4A1 promoted the mitochondrial dysfunction under high-fat damage. a, b The JC1 staining for the mitochondrial potential. c The opening rate of mPTP with NR4A1 knockdown or not. d, e The flow cytometry was used to analyze the mROS production. f The leakage of mitochondrial cyt-c into cytoplasm and nuclear via immunofluorescence assay. g–k Western blotting assay was carried out to analyze the changes of mitochondrial apoptotic proteins. *P < 0.05 vs. Ctrl group; #P < 0.05 vs. ox-LDL + si-Ctrl group
The reduction in MMP may have been derived from the opening of the mitochondrial permeability transition pore (mPTP). As shown in Fig. 2c, compared to the control group, ox-LDL induced more mPTP opening that was repressed by NR4A1 deficiency. In response to the mPTP opening and MMP reduction, mitochondria initiate classical mitochondrial apoptotic pathways. Mitochondrial apoptosis is defined as excessive ROS production and leakage of pro-apoptotic factors from mitochondria into cytoplasm. Through the analysis of mitochondrial ROS, we confirmed that ox-LDL overproduced more mROS, and this effect was inhibited by NR4A1 (Fig. 3d, e). Furthermore, immunofluorescence assays for the pro-apoptotic factor cyt-c demonstrated that ox-LDL promoted cyt-c liberation from mitochondria into cytoplasm and even diffusion into the nucleus (Fig. 3f). This tendency was reversed by NR4A1 deletion. Similarly, western blot results were also in accordance with the results of the immunofluorescence assay (Fig. 3g, h). Moreover, we also observed that ox-LDL upregulated mitochondrial pro-apoptotic proteins and downregulated anti-apoptotic factors, indicating the activation of mitochondrial apoptosis. In contrast, NR4A1 deletion corrected the imbalance between pro-apoptotic proteins and anti-apoptotic factors (Fig. 3g–k).
NR4A1 activated Parkin-related mitophagy
Previous studies have suggested that Parkin-mediated mitophagy induces endothelial damage in response to cardiac ischemia reperfusion injury (Zhou et al. 2017d). In our study, we wanted to know whether Parkin-related mitophagy accounted for the mitochondrial damage that was induced by high-fat damage. In vitro, we found that ox-LDL treatment enhanced Parkin phosphorylation (Fig. 4a, b), which is an indicator of Parkin activity. Moreover, the mitophagy markers, including Atg5, mitochondrial LC3II (mito-LC3II), and Beclin1, were also increased in ox-LDL-treated cell (Fig. 4a–e). These data indicated that high-fat damage elevated mitophagy activity. Interestingly, loss of NR4A1 in vitro suppressed Parkin phosphorylation and repressed the upregulation of Atg5, mito-LC3II, and Beclin1 (Fig. 4a–e), suggesting that higher NR4A1 promotes mitophagy activation. Moreover, to gain more evidence for mitophagy activation, we co-stained mitochondria and lysosomes at the same time. Compared to the control group, ox-LDL enhanced the fusion of mitochondria and lysosomes (Fig. 4f, g). In contrast, loss of NR4A1 reversed the interaction between mitochondria and lysosomes, indicating mitophagy inhibition. Altogether, these data indicated that high-fat damage elevated mitophagy activity via NR4A1.
Parkin-related mitophagy induced mitochondrial energy disorder
To explain the role of mitophagy in mitochondrial damage, we assessed ATP production, which is a key feature of mitochondrial function. As shown in Fig. 5a–c, ox-LDL treatment repressed ATP production, the state 3 and state 4 respiratory rate. However, loss of NR4A1 reversed the mitochondrial respiratory function. Interestingly, application of FCCP, an inducer of mitophagy, disrupted ATP production and mitochondrial respiratory function despite deletion of NR4A1 (Fig. 5a–c). These data confirmed that NR4A1 damaged mitochondrial oxidative phosphorylation under high-fat injury via mitophagy.
NR4A1-activated mitophagy aggravated the mitochondrial energy dysfunction. a The ATP production with NR4A1 knockdown or with mitophagy activation via FCCP. b, c The state 3 and state 4 mitochondrial respiratory function with NR4A1 knockdown and mitophagy activation. d–h The alteration of mitochondrial respiratory complex via western blotting assay. *P < 0.05 vs. Ctrl group; #P < 0.05 vs. ox-LDL + si-Ctrl group; @P < 0.05 vs. ox-LDL + si-NR4A1 group
Furthermore, mitochondrial energy metabolism is functionally dependent on the activity of the mitochondrial electron respiratory complex (ETC). However, ox-LDL reduced the expression of the ETC, and this tendency was reversed by NR4A1 inhibition (Fig. 5d–h). Interestingly, reactivation of mitophagy via FCCP reduced the ETC expression despite deletion of NR4A1. Thus, these data collectively indicate that NR4A1 upregulation is associated with mitophagy activation that consumes mitochondria, leading to ETC downregulation, ATP undersupply, and cellular death.
NR4A1 activated Parkin via CaMKII pathways
Finally, we wanted to know how NR4A1 modulated Parkin-related mitophagy activity under high-fat injury conditions. CaMKII is considered the upstream initiator of Parkin activation. Accordingly, we first explored whether NR4A1 activated Parkin via CaMKII. As shown in Fig. 6a, b, compared to the control group, ox-LDL treatment increased the CaMKII activity as evidenced by more phosphorylated CaMKII. However, NR4A1 deletion reduced the ox-LDL-induced CaMKII activation. In contrast, loss of NR4A1 reduced the level of p-CaMKII. These data reveal that NR4A1 is the upstream activator of CaMKII.
NR4A1 regulated the Parkin activity via CaMKII pathway. a–c Western blotting assay about the CaMKII and Parkin. KN93, the inhibitor of CaMKII was used as the negative control group which reduced the expression of CaMKII and Parkin. d, e The mitophagy activity with NR4A1 knockdown or CaMKII inhibition. f The caspase 9 activity with NR4A1 knockdown and CaMKII inhibition. *P < 0.05 vs. Ctrl group; #P < 0.05 vs. ox-LDL group
To determine whether CaMKII is involved in activating Parkin-mediated mitophagy, the inhibitor of CaMKII, KN-93, was used. After inhibition of CaMKII with KN-93 under ox-LDL treatment conditions, CaMKII activity was blocked, and Parkin expression was also inhibited (Fig. 6a–c), which is similar to the results obtained in the NR4A1 knockdown group. These data indicate that CaMKII is involved in Parkin regulation.
To gain additional evidence for the role of CaMKII in mitophagy, mitochondria and lysosome co-staining was performed. Inhibition of CaMKII under ox-LDL treatment conditions inhibited the fusion of mitochondria and lysosomes (Fig. 6d, e), which is similar to the results observed in the NR4A1 knockdown group. This finding confirmed that CaMKII activation accounted for the NR4A1-triggered mitophagy in response to high-fat injury. At last, to determine whether CaMKII is also involved in endothelial apoptosis, caspase 9 activity was measured. Compared to the control group, ox-LDL treatment elevated caspase 9 activity (Fig. 6f), which was inhibited by NR4A1 knockdown or CaMKII inhibition. Altogether, our data indicate that mitophagy is signaled by NR4A1 via CaMKII, which contributes to endothelial apoptosis following high-fat damage.
Discussion
Cumulative evidence has identified endothelial damage as an early characteristic of AS because endothelial cell dysfunction and injury occur before the onset of structural alterations to the vessel wall (Chatterjee et al. 2014). The consequence of endothelial damage in response to high-fat injury is accumulation of lipid in the intima, leading to proliferation of smooth muscle cells and activation of fibroblasts (Foteinos et al. 2005). Therefore, strategies to protect endothelial cells from high-fat damage are vital to retard the formation of atherosclerotic plaques and development of AS. Herein, through experiments in animals and cultured cells, we demonstrated that NR4A1 is the pathogenic agent of endothelial apoptosis in response to high-fat exposure. Upregulated NR4A1 activated CaMKII pathways, which triggered Parkin phosphorylation and mitophagy activation. Excessive Parkin-mediated mitophagy overtly consumed mitochondrial mass, leading to ETC downregulation, ATP undersupply, and endothelial mitochondrial apoptosis. To the best of our knowledge, this is the first study to explain the role of NR4A1 in endothelial damage via regulation of mitophagy.
NR4A1 is a member of the Nur nuclear receptor family of intracellular transcription factors. NR4A1 has the ability to regulate the cell cycle, inflammation, proliferation, tumorigenesis, and apoptosis (McMorrow and Murphy 2011; Zhao and Bruemmer 2010). Several researchers have reported that NR4A1 inhibition can activate the glycolytic pathways and thus promote cellular survival in acute promyelocytic leukemia cells (Corrocher et al. 2017; Yu et al. 2017). Additionally, NR4A1 has been found to modulate mitochondrial apoptosis via p38 and Bcl2 (Liu et al. 2017a). Furthermore, NR4A1 can affect calcium homeostasis in cardiomyocytes and can also affect adverse cardiac remodeling (Medzikovic et al. 2015). In the current study, we illustrated that NR4A1 is a novel negative regulator of endothelial cell viability in response to high-fat damage (Xu et al. 2017). Loss of NR4A1 enhances the resistance of endothelial cells to ox-LDL-mediated mitochondrial apoptosis. This finding provides insights into the role of NR4A1 in the cardiovascular system. Meanwhile, our data also indicate that NR4A1 is a potential target for protecting endothelial cell function during the development of AS.
We found that NR4A1 evoked endothelial cell apoptosis by increasing mitophagy activity. Upregulated NR4A1 enhanced CaMKII activity, and the latter facilitated phosphorylated activation of Parkin, which thereby delivered mitochondria to lysosomes. These data established the upstream regulatory signaling for Parkin-mediated mitophagy in the context of high-fat damage (Das et al. 2017; Liu et al. 2017b). Notably, previous studies have also confirmed the promotive effects of NR4A1 on autophagy and/or mitophagy. In response to an inflammatory response, NR4A1 can be ubiquitinated and can promote mitophagy activation via tumor necrosis factor receptor-associated factor 2 (TRAF2) (Hu et al. 2017a). NR4A1 has also been shown to signal autophagy via IGFBP-3, contributing to airway inflammation and fibrosis (Yin et al. 2017). In fatty liver disease, NR4A1 is also activated and contributes to mitochondrial dysfunction via modulation of mitophagy and mitochondrial fission. These findings all suggest that NR4A1 may be the upstream activator of mitophagy.
Although several researchers have argued that mitophagy can block mitochondrial apoptosis and promote cellular survival via timely removal of bad or unhealthy mitochondria (Zhou et al. 2017b, 2018a), our data demonstrate that excessive mitophagy is harmful for cell viability. Excessive mitophagy consumed mitochondrial mass, leading to mitochondrial respiratory complex downregulation and energy disorder. Notably, a previous study has also identified that Parkin-mediated mitophagy is involved in cardiac microvascular endothelial cell apoptosis during ischemia reperfusion injury (Zhou et al. 2017d). In liver cancer, activation of mitophagy has been reported to impair cancer mobility and migration by preventing mitochondrial ATP production (Shi et al. 2018). Together, these studies indicate that excessive mitophagy may be fatal to cellular homeostasis (Lee et al. 2017; Oanh et al. 2017). Accordingly, strategies to correct moderate mitophagy to prevent mitochondrial loss could be vital for cell viability. However, more evidence is needed both in vivo and in clinical practice to support our conclusions.
In the present study, we illustrated the important role of NR4A1 in endothelial cell apoptosis under high-fat injury conditions in vivo and in vitro. NR4A1 amplified apoptotic signaling to endothelial cells by targeting the mitochondria. In this process, mitophagy was the indispensable element in mitochondrial damage and contributed to cellular apoptosis. NR4A1 increased mitophagy to augment mitochondrial apoptosis and energy disorder, which evoked more cellular damage. These findings suggest that inhibition of NR4A1 and suppression of mitophagy activity might be a practical and efficient adjuvant for AS treatment.
References
Abdou HS, Villeneuve G, Tremblay JJ (2013) The calcium signaling pathway regulates leydig cell steroidogenesis through a transcriptional cascade involving the nuclear receptor NR4A1 and the steroidogenic acute regulatory protein. Endocrinology 154:511–520. https://doi.org/10.1210/en.2012-1767
Bravo-San Pedro JM, Kroemer G, Galluzzi L (2017) Autophagy and mitophagy in cardiovascular disease. Circ Res 120:1812–1824. https://doi.org/10.1161/CIRCRESAHA.117.311082
Caja S, Enriquez JA (2017) Mitochondria in endothelial cells: sensors and integrators of environmental cues. Redox Biol 12:821–827. https://doi.org/10.1016/j.redox.2017.04.021
Camare C, Pucelle M, Negre-Salvayre A, Salvayre R (2017) Angiogenesis in the atherosclerotic plaque. Redox Biol 12:18–34. https://doi.org/10.1016/j.redox.2017.01.007
Chatterjee S, Bedja D, Mishra S, Amuzie C, Avolio A, Kass DA, Berkowitz D, Renehan M (2014) Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in apolipoprotein E−/− mice and rabbits fed a high-fat and -cholesterol diet. Circulation 129:2403–2413. https://doi.org/10.1161/CIRCULATIONAHA.113.007559
Chattopadhyay R, Raghavan S, Rao GN (2017) Resolvin D1 via prevention of ROS-mediated SHP2 inactivation protects endothelial adherens junction integrity and barrier function. Redox Biol 12:438–455. https://doi.org/10.1016/j.redox.2017.02.023
Chen HH, Chen YT, Yang CC, Chen KH, Sung PH, Chiang HJ, Chen CH, Chua S, Chung SY, Chen YL, Huang TH, Kao GS, Chen SY, Lee MS, Yip HK (2016a) Melatonin pretreatment enhances the therapeutic effects of exogenous mitochondria against hepatic ischemia-reperfusion injury in rats through suppression of mitochondrial permeability transition. J Pineal Res 61:52–68. https://doi.org/10.1111/jpi.12326
Chen X, Pang S, Lin J, Xia J, Wang Y (2016b) Allicin prevents oxidized low-density lipoprotein-induced endothelial cell injury by inhibiting apoptosis and oxidative stress pathway. BMC Complement Altern Med 16:133. https://doi.org/10.1186/s12906-016-1126-9
Chen J, Wang YX, Dong MQ, Zhang B, Luo Y, Niu W, Li ZC (2017) Reoxygenation reverses hypoxic pulmonary arterial remodeling by inducing smooth muscle cell apoptosis via reactive oxygen species-mediated mitochondrial dysfunction. J Am Heart Assoc 6:e005602. https://doi.org/10.1161/JAHA.117.005602
Corrocher FA, Bueno de Paiva L, ASS D, Ferro KP, Silveira LR, de Lima TI, Olalla Saad ST, Lazarini M (2017) Reduced expression of NR4A1 activates glycolytic pathway in acute promyelocytic leukemia cells. Leuk Lymphoma:1–4. https://doi.org/10.1080/10428194.2017.1387900
Das N, Mandala A, Naaz S, Giri S, Jain M, Bandyopadhyay D, Reiter RJ, Roy SS (2017) Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J Pineal Res 62. https://doi.org/10.1111/jpi.12404
Foteinos G, Afzal AR, Mandal K, Jahangiri M, Xu Q (2005) Anti-heat shock protein 60 autoantibodies induce atherosclerosis in apolipoprotein E-deficient mice via endothelial damage. Circulation 112:1206–1213. https://doi.org/10.1161/CIRCULATIONAHA.105.547414
Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang Z, Li Z, Feng X, Hao J, Zhang K, Xiao B, Chen M, Huang W, Xiong S, Wu X, Deng W (2017) Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-kappaB/iNOS signaling pathways. J Pineal Res 62. https://doi.org/10.1111/jpi.12380
Griffiths HR, Gao D, Pararasa C (2017) Redox regulation in metabolic programming and inflammation. Redox Biol 12:50–57. https://doi.org/10.1016/j.redox.2017.01.023
Hambright WS, Fonseca RS, Chen L, Na R, Ran Q (2017) Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12:8–17. https://doi.org/10.1016/j.redox.2017.01.021
Hamilton JA, Hasturk H, Kantarci A, Serhan CN, Van Dyke T (2017) Atherosclerosis, periodontal disease, and treatment with resolvins. Curr Atheroscler Rep 19:57. https://doi.org/10.1007/s11883-017-0696-4
Han L, Wang H, Li L, Li X, Ge J, Reiter RJ, Wang Q (2017) Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J Pineal Res 63. https://doi.org/10.1111/jpi.12431
Hu M, Luo Q, Alitongbieke G, Chong S, Xu C, Xie L, Chen X, Zhang D, Zhou Y, Wang Z, Ye X, Cai L, Zhang F, Chen H, Jiang F, Fang H, Yang S, Liu J, Diaz-Meco MT, Su Y, Zhou H, Moscat J, Lin X, Zhang XK (2017a) Celastrol-induced Nur77 interaction with TRAF2 alleviates inflammation by promoting mitochondrial ubiquitination and autophagy. Mol Cell 66(141–153):e146. https://doi.org/10.1016/j.molcel.2017.03.008
Hu SY, Zhang Y, Zhu PJ, Zhou H, Chen YD (2017b) Liraglutide directly protects cardiomyocytes against reperfusion injury possibly via modulation of intracellular calcium homeostasis. J Geriatr Cardiol 14:57–66. https://doi.org/10.11909/j.issn.1671-5411.2017.01.008
Iggena D, Winter Y, Steiner B (2017) Melatonin restores hippocampal neural precursor cell proliferation and prevents cognitive deficits induced by jet lag simulation in adult mice. J Pineal Res 62. https://doi.org/10.1111/jpi.12397
Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma S, Zhu H, Ren J, Zhou H (2018) DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol 14:576–587. https://doi.org/10.1016/j.redox.2017.11.004
Kalyanaraman B (2017) Teaching the basics of cancer metabolism: developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol 12:833–842. https://doi.org/10.1016/j.redox.2017.04.018
Kozlov AV, Lancaster JR Jr, Meszaros AT, Weidinger A (2017) Mitochondria-meditated pathways of organ failure upon inflammation. Redox Biol 13:170–181. https://doi.org/10.1016/j.redox.2017.05.017
Lee K, Back K (2017) Overexpression of rice serotonin N-acetyltransferase 1 in transgenic rice plants confers resistance to cadmium and senescence and increases grain yield. J Pineal Res 62. https://doi.org/10.1111/jpi.12392
Lee HJ, Jung YH, Choi GE, Ko SH, Lee SJ, Lee SH, Han HJ (2017) BNIP3 induction by hypoxia stimulates FASN-dependent free fatty acid production enhancing therapeutic potential of umbilical cord blood-derived human mesenchymal stem cells. Redox Biol 13:426–443. https://doi.org/10.1016/j.redox.2017.07.004
Lin S, Hoffmann K, Gao C, Petrulionis M, Herr I, Schemmer P (2017) Melatonin promotes sorafenib-induced apoptosis through synergistic activation of JNK/c-jun pathway in human hepatocellular carcinoma. J Pineal Res 62. https://doi.org/10.1111/jpi.12398
Liu J, Wang GH, Duan YH, Dai Y, Bao Y, Hu M, Zhou YQ, Li M, Jiang F, Zhou H, Yao XS, Zhang XK (2017a) Modulation of the Nur77-Bcl-2 apoptotic pathway by p38alpha MAPK. Oncotarget 8:69731–69745. https://doi.org/10.18632/oncotarget.19227
Liu Z, Gan L, Luo D, Sun C (2017b) Melatonin promotes circadian rhythm-induced proliferation through Clock/histone deacetylase 3/c-Myc interaction in mouse adipose tissue. J Pineal Res 62. https://doi.org/10.1111/jpi.12383
McMorrow JP, Murphy EP (2011) Inflammation: a role for NR4A orphan nuclear receptors? Biochem Soc Trans 39:688–693. https://doi.org/10.1042/BST0390688
Medzikovic L, Schumacher CA, Verkerk AO, van Deel ED, Wolswinkel R, van der Made I, Bleeker N, Cakici D, van den Hoogenhof MMG, Meggouh F, Creemers EE, Remme CA, Baartscheer A, de Winter RJ, de Vries CJM, Arkenbout EK, de Waard V (2015) Orphan nuclear receptor Nur77 affects cardiomyocyte calcium homeostasis and adverse cardiac remodelling. Sci Rep 5:15404. https://doi.org/10.1038/srep15404
Min AK, Bae KH, Jung YA, Choi YK, Kim MJ, Kim JH, Jeon JH, Kim JG, Lee IK, Park KG (2014) Orphan nuclear receptor Nur77 mediates fasting-induced hepatic fibroblast growth factor 21 expression. Endocrinology 155:2924–2931. https://doi.org/10.1210/en.2013-1758
Oanh NTK, Park YY, Cho H (2017) Mitochondria elongation is mediated through SIRT1-mediated MFN1 stabilization. Cell Signal 38:67–75. https://doi.org/10.1016/j.cellsig.2017.06.019
Okamoto T, Suzuki K (2017) The role of gap junction-mediated endothelial cell-cell interaction in the crosstalk between inflammation and blood coagulation. Int J Mol Sci 18. https://doi.org/10.3390/ijms18112254
Pawlak A, Strzadala L, Kalas W (2015) Non-genomic effects of the NR4A1/Nur77/TR3/NGFIB orphan nuclear receptor. Steroids 95:1–6. https://doi.org/10.1016/j.steroids.2014.12.020
Ranhotra HS (2015) The NR4A orphan nuclear receptors: mediators in metabolism and diseases. J Recept Signal Transduct Res 35:184–188. https://doi.org/10.3109/10799893.2014.948555
Sato A, Arimura Y, Manago Y, Nishikawa K, Aoki K, Wada E, Suzuki Y, Osaka H, Setsuie R, Sakurai M, Amano T, Aoki S, Wada K, Noda M (2006) Parkin potentiates ATP-induced currents due to activation of P2X receptors in PC12 cells. J Cell Physiol 209:172–182. https://doi.org/10.1002/jcp.20719
Shi C, Cai Y, Li Y, Li Y, Hu N, Ma S, Hu S, Zhu P, Wang W, Zhou H (2018) Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol 14:59–71. https://doi.org/10.1016/j.redox.2017.08.013
Sigala F, Efentakis P, Karageorgiadi D, Filis K, Zampas P, Iliodromitis EK, Zografos G, Papapetropoulos A, Andreadou I (2017) Reciprocal regulation of eNOS, H2S and CO-synthesizing enzymes in human atheroma: correlation with plaque stability and effects of simvastatin. Redox Biol 12:70–81. https://doi.org/10.1016/j.redox.2017.02.006
Tallman KA, Kim HH, Korade Z, Genaro-Mattos TC, Wages PA, Liu W, Porter NA (2017) Probes for protein adduction in cholesterol biosynthesis disorders: Alkynyl lanosterol as a viable sterol precursor. Redox Biol 12:182–190. https://doi.org/10.1016/j.redox.2017.02.013
Vendrov AE, Stevenson MD, Alahari S, Pan H, Wickline SA, Madamanchi NR, Runge MS (2017) Attenuated superoxide dismutase 2 activity induces atherosclerotic plaque instability during aging in hyperlipidemic mice. J Am Heart Assoc 6:e006775. https://doi.org/10.1161/JAHA.117.006775
Wang Z, Ni L, Wang J, Lu C, Ren M, Han W, Liu C (2016) The protective effect of melatonin on smoke-induced vascular injury in rats and humans: a randomized controlled trial. J Pineal Res 60:217–227. https://doi.org/10.1111/jpi.12305
Wang L, Feng C, Zheng X, Guo Y, Zhou F, Shan D, Liu X, Kong J (2017a) Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J Pineal Res 63. https://doi.org/10.1111/jpi.12429
Wang N, Liu H, Li X, Zhang Q, Chen M, Jin Y, Deng X (2017b) Activities of MSCs derived from transgenic mice seeded on ADM scaffolds in wound healing and assessment by advanced optical techniques. Cell Physiol Biochem 42:623–639. https://doi.org/10.1159/000477872
Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, Wang J, Qin Y, Liu Y, Tang C, He L, Greka A, Zhou Z, Liu F, Dong Z, Sun L (2017) The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol 11:297–311. https://doi.org/10.1016/j.redox.2016.12.022
Xu J, Wu Y, Lu G, Xie S, Ma Z, Chen Z, Shen HM, Xia D (2017) Importance of ROS-mediated autophagy in determining apoptotic cell death induced by physapubescin B. Redox Biol 12:198–207. https://doi.org/10.1016/j.redox.2017.02.017
Xue Z, Yuan W, Li J, Zhou H, Xu L, Weng J, Li X, Zhang X, Wang Z, Yan J (2017) Cyclophilin A mediates the ox-LDL-induced activation and apoptosis of macrophages via autophagy. Int J Cardiol 230:142–148. https://doi.org/10.1016/j.ijcard.2016.12.042
Yin H, Zhang S, Sun Y, Li S, Ning Y, Dong Y, Shang Y, Bai C (2017) MicroRNA-34/449 targets IGFBP-3 and attenuates airway remodeling by suppressing Nur77-mediated autophagy. Cell Death Dis 8:e2998. https://doi.org/10.1038/cddis.2017.357
Yu S, Wang X, Geng P, Tang X, Xiang L, Lu X, Li J, Ruan Z, Chen J, Xie G, Wang Z, Ou J, Peng Y, Luo X, Zhang X, Dong Y, Pang X, Miao H, Chen H, Liang H (2017) Melatonin regulates PARP1 to control the senescence-associated secretory phenotype (SASP) in human fetal lung fibroblast cells. J Pineal Res 63. https://doi.org/10.1111/jpi.12405
Zhai M, Li B, Duan W, Jing L, Zhang B, Zhang M, Yu L, Liu Z, Yu B, Ren K, Gao E, Yang Y, Liang H, Jin Z, Yu S (2017) Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res 63. https://doi.org/10.1111/jpi.12419
Zhang Y, Zhou H, Wu W, Shi C, Hu S, Yin T, Ma Q, Han T, Zhang Y, Tian F, Chen Y (2016) Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic Biol Med 95:278–292. https://doi.org/10.1016/j.freeradbiomed.2016.03.035
Zhao Y, Bruemmer D (2010) NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler Thromb Vasc Biol 30:1535–1541. https://doi.org/10.1161/ATVBAHA.109.191163
Zhao W, Feng H, Sun W, Liu K, Lu JJ, Chen X (2017) Tert-butyl hydroperoxide (t-BHP) induced apoptosis and necroptosis in endothelial cells: roles of NOX4 and mitochondrion. Redox Biol 11:524–534. https://doi.org/10.1016/j.redox.2016.12.036
Zhou H, Yang J, Xin T, Li D, Guo J, Hu S, Zhou S, Zhang T, Zhang Y, Han T, Chen Y (2014) Exendin-4 protects adipose-derived mesenchymal stem cells from apoptosis induced by hydrogen peroxide through the PI3K/Akt-Sfrp2 pathways. Free Radic Biol Med 77:363–375. https://doi.org/10.1016/j.freeradbiomed.2014.09.033
Zhou H, Li D, Shi C, Xin T, Yang J, Zhou Y, Hu S, Tian F, Wang J, Chen Y (2015a) Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci Rep 5:12898. https://doi.org/10.1038/srep12898
Zhou H et al (2015b) Exendin-4 enhances the migration of adipose-derived stem cells to neonatal rat ventricular cardiomyocyte-derived conditioned medium via the phosphoinositide 3-kinase/Akt-stromal cell-derived factor-1alpha/CXC chemokine receptor 4 pathway. Mol Med Rep 11:4063–4072. https://doi.org/10.3892/mmr.2015.3243
Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P, Ma Q, Tian F, Chen Y (2017a) Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc 6:e005328. https://doi.org/10.1161/JAHA.116.005328
Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S, Zhang Y, Han T, Ren J, Cao F, Chen Y (2017b) Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J Pineal Res 63. https://doi.org/10.1111/jpi.12438
Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J (2017c) Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol 15:335–346. https://doi.org/10.1016/j.redox.2017.12.019
Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q, Jin Q, Cao F, Tian F, Chen Y (2017d) Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res 63:e12413. https://doi.org/10.1111/jpi.12413
Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, Hu S, Ren J, Cao F, Chen Y (2017e) Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol 13:498–507. https://doi.org/10.1016/j.redox.2017.07.007
Zhou W, Yu L, Fan J, Wan B, Jiang T, Yin J, Huang Y, Li Q, Yin G, Hu Z (2017f) Endogenous parathyroid hormone promotes fracture healing by increasing expression of BMPR2 through cAMP/PKA/CREB pathway in mice. Cell Physiol Biochem 42:551–563. https://doi.org/10.1159/000477605
Zhou H, Du W, Li Y, Shi C, Hu N, Ma S, Wang W, Ren J (2018a) Effects of melatonin on fatty liver disease: the role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J Pineal Res 64. https://doi.org/10.1111/jpi.12450
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y (2018b) Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J Pineal Res. https://doi.org/10.1111/jpi.12471
Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D, Zhou H, Chen Y (2017) Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca2+]c/VDAC-[Ca2+]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones 23:101–113. https://doi.org/10.1007/s12192-017-0827-4
Funding
This study was supported by grants from National Natural Science Foundation of China (Number 81501195). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
PL, YZB, and JW were involved in conception and design, performance of experiments, data analysis and interpretation, and manuscript writing; XZ, LYT, and TT were involved in data analysis and interpretation; JR, YA, and JW were involved in conception and design, data analysis and interpretation, financial support, and final approval of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have declared that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Li, P., Bai, Y., Zhao, X. et al. NR4A1 contributes to high-fat associated endothelial dysfunction by promoting CaMKII-Parkin-mitophagy pathways. Cell Stress and Chaperones 23, 749–761 (2018). https://doi.org/10.1007/s12192-018-0886-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-018-0886-1