Abstract
The production of major human heat shock protein Hsp70 (HSPA1A) in a eukaryotic expression system is needed for testing and possible medical applications. In this study, transgenic mice were produced containing wild-type human Hsp70 allele in the vector providing expression in the milk. The results indicated that human Hsp70 was readily expressed in the transgenic animals but did not apparently preserve its intact structure and, hence, it was not possible to purify the protein using conventional isolation techniques. It was suggested that the protein underwent glycosylation in the process of expression, and this quite common modification for proteins expressed in the milk complicated its isolation. To check this possibility, we mutated all presumptive sites of glycosylation and tested the properties of the resulting modified Hsp70 expressed in E. coli. The investigation demonstrated that the modified protein exhibited all beneficial properties of the wild-type Hsp70 and was even superior to the latter for a few parameters. Based on these results, a transgenic mouse strain was obtained which expressed the modified Hsp70 in milk and which was easy to isolate using ATP columns. Therefore, the developed construct can be explored in various bioreactors for reliable manufacture of high quality, uniform, and reproducible human Hsp70 for possible medical applications including neurodegenerative diseases and cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat shock protein 70 (Hsp70) proteins and their co-chaperones have been studied in various prokaryotic and eukaryotic organisms chiefly because of their participation in protein folding under normal and stress conditions and because of their apparent role in aging and various pathologies, such as neurodegeneration and cancer (Fleshner and Johnson 2005; Calderwood et al. 2007; Kim et al. 2013; Evgen’ev et al. 2014).
Hsp70, in humans encoded by the HSPA1A gene, is a key component of the machinery protecting the cell from various stress conditions (Nollen et al. 2000; Calderwood et al. 2007; Hartl et al. 2011; Radons 2016). Briefly, Hsp70 binds partially unfolded or misfolded proteins and either assists in their refolding or directs them to a safe disposal (Mayer 2010; Duncan et al. 2015). Hsp70 may also have additional functions, including acting as cytokine-like molecules (Asea et al. 2000; Asea 2008a; Calderwood et al. 2007; Multhoff and Hightower 2011; Ghosh et al. 2015; Radons 2016). Thus, in many types of cells such as neuronal and inflammatory cells subjected to stress, Hsp70 is released and signals to cells via several surface proteins including scavenger and Toll-like receptors (TLR2 and 4) (Asea 2008b; Ghosh et al. 2015).
The activity of endogenous Hsp70 appears insufficient in many pathological states, notably in various neurodegenerative disorders (Guzhova and Margulis 2006; Pratt et al. 2015). Hsp70 production also declines in the course of aging (Calderwood et al. 2009), with likely negative implications for the ability of the aging organism to maintain itself.
Accordingly, substantial efforts have been directed to the development of methods to stimulate the expression of Hsp70 in contexts where it is insufficient. Administration of exogenously produced Hsp70 protein usually produced in E. coli has also emerged as a potentially productive approach (Hansen et al. 2006; Ekimova et al. 2010; Shevtsov et al. 2014a, b).
Normally, Hsp70 is a cytoplasmic protein. However, it may come out from the cell via unusual mechanisms, different from regular secretory process (Multhoff and Hightower 1996; De Maio 2014; Fleshner and Johnson 2005). Frequently, Hsp70 comes out from the cell under stress condition and/or in transformed cells (Srivastava 2002; Voos 2009; Multhoff and Hightower 2011). The utility of exogenously produced or artificially induced Hsp70 has been demonstrated in several model systems (Kustanova et al. 2006; Ekimova et al. 2010; Hoshino et al. 2011; Kakimura et al. 2002; Bobkova et al. 2014, 2015), and there is considerable interest in exploring it further with the objective of deriving benefits to human health. The field of potential applications of Hsp70 preparations extends from oncology to the therapy of neurodegenerative pathologies. One of promising examples is the application of human recombinant Hsp70 in the therapy of brain tumors; in this study, it was shown that the post-surgery administration of Hsp70 is at least safe and impacts to a greater survival rate (Shevtsov et al. 2014a). Another possible application of Hsp70 preparations is a therapy of ischemic injuries exemplified by a recent study of Shevtsov and coauthors (Shevtsov et al. 2014c). Certainly, among applications of the exogenous Hsp70, the neurodegenerative disorders form a big group of targets such as described in the work of Evgen’ev’s group (Bobkova et al. 2014, 2015).
It is thus desirable to develop ways of reliably manufacturing both prokaryotic and eukaryotic expression systems’ high quality, uniform, and reproducible human Hsp70 for testing and possible medical applications. Yet this is not straightforward, because although Hsp70 is an evolutionarily highly conserved molecular chaperone, it is known to be post-translationally modified when produced in eukaryotic expression systems. These modifications that may disturb its chaperone properties and complicate its isolation include methylation, phosphorylation, and ubiquitination and their physiological significance has never been fully elucidated (Nunes et al. 2015).
Since for many purposes an efficient production of Hsp70 is needed in various eukaryotic systems from yeast to animal milk or bloodstream, one must try to avoid the negative effects of possible deleterious modifications of the polypeptide such as glycosylation usually taking place in the milk and other eukaryotic expression systems (Mellquist et al. 1998; Drickamer and Taylor 2006).
It is of note that many active human proteins for medical applications are produced in the milk of transgenic animals (Li et al. 2013; Batista et al. 2014; Park et al. 2015).
It is thus desirable to control the extent of glycosylation of Hsp70, for example, by modifying the primary structure of the Hsp70 protein. However, any modification in the structure of a protein carries the risk of interfering with its function. Accordingly, the challenge is to achieve a useful control of glycosylation of the Hsp70 protein while ensuring that its beneficial functions are retained in full.
In the present study, we obtained human Hsp70 with mutated putative glycosylation sites and demonstrated that such a modified protein apparently preserved its native structure and characteristic beneficial activities after expression in bacterial cells (E. coli) and in the milk of transgenic mice.
Materials and methods
Site-directed mutagenesis
The original plasmid containing human HSPA1A gene coding Hsp70 is a kind gift of Dr. R. Morimoto from the Northwestern University. General genetic engineering procedures were performed essentially as described (Sambrook et al. 1989). Modified Hsp70 proteins were obtained by performing site-directed mutagenesis, exploring a DNA construct containing a cloned human HSP70 mRNA. Modified proteins include a polyhistidine-tag for ease of purification.
The starting point for the generation of the modified HSP70 proteins described here was the plasmid pBlueScriptSK(+) containing human HSPA1A cDNA insert. This plasmid encodes wild-type human Hsp70 protein as given in the following National Center for Biotechnology Information (NCBI) reference sequence: gi|194248072|ref|NP_005336.3| heat shock 70 kDa protein 1A/1B [Homo sapiens].
We used the method of site-directed mutagenesis described in Young and Dong (2003) with a few modifications concerning enzymes, i.e., use of Pfu DNA polymerase (SibEnzyme) and Taq DNA ligase (Picard et al. 1994) instead of T4 enzymes. The oligonucleotides were synthesized by a commercial provider. Primer sequences are provided in Supplementary Data and Materials, as well as details of mutagenesis and cloning. The generated Hsp70 coding sequences were re-cloned into pET-14b-derived plasmid to provide the N-terminal polyhistidine tag (MGSSHHHHHHSSGLVPRGSH), shown in Supplementary Figure S1.
Production and purification of the modified variants of Hsp70 from E. coli cells
Recombinant Hsp70 proteins, including modified ones, containing a polyhistidine (6xHis) affinity tag were produced in E. coli. We found that the modified Hsp70 proteins were produced in a soluble state even at high levels of expression, such as when produced in the E. coli strain BL21 (DE3). The proteins with 6xHis were purified by affinity chromatography using Ni-NTA resin (Qiagen) according to the manufacturer’s protocol, with modifications, as described. The details of purification are given in the Supplementary Materials. The presence of the polyhistidine-tag, however, did not affect the performance of the modified proteins we studied and, hence, in all experiments described below, the proteins, including the modified rhHsp70.128, were used with the polyhistidine-tag attached (Fig. S1).
Wild-type human Hsp70 (“wt Hsp70”) without His-tag and other modifications designated “WT” was isolated from E. coli transformed with a pMSHsp70 plasmid according to good manufacturing practice standards. Briefly, Hsp70 was purified by anion exchange chromatography using DEAE-Sepharose (GE Healthcare Life Sciences, Cleveland, OH, USA) followed by adenosine triphosphate (ATP)-affinity chromatography on ATP-agarose (Sigma-Aldrich, St. Louis, MO, USA) (Welch and Feramisco 1985). Endotoxin was depleted by polymyxin B-sepharose endotoxin-removing gel (Sigma-Aldrich). Electrophoresis and blotting of recombinant Hsps was performed as described in Magi and Liberatori (2004). The proteins were detected by exploring monoclonal antibodies SPA822 (EnzoLab) or polyclonal antibodies kindly provided by L. Sashchenko (Institute of Gene Biology, Russian Academy of Sciences, Moscow).
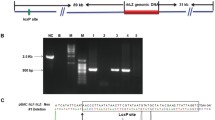
Constructs used in this study and obtaining transgenic animals
For injections, the human wild-type or mutated Hsp70 cDNA was cloned in pBC plasmid (Invitrogen) used to provide the production of the Hsp70 protein to the milk (Fig. S2), as described in Supplementary Materials. To obtain primary transgenic animals, DNA (5 ng/mL in 0.1 mmol/L EDTA and 10 mmol/L Tris (pH 7.4)) was injected into the male pronuclei of F1 fertilized ova of (CBA × C57BL/6) mice, and the surviving microinjected zygotes were implanted into pseudopregnant recipients. The ova were obtained by inducing superovulation (Zvezdova et al. 2010).
Animal breeding and milk production
Primary transgenic females were crossed with wild-type males, and the content of the recombinant Hsp70 in their milk was tested by Western blotting during the first pregnancy. The transgenic males were crossed with wild-type females, and the content of the human recombinant Hsp70 was evaluated in the milk of transgenic females obtained in these crosses. The procedures of mouse milking and the analysis of the milk are given in Supplementary Materials.
Various in vitro assays to monitor characteristics of recombinant forms of Hsp70 expressed in E. coli and in the transgenic mice
The assays were performed using human recombinant Hsp70 isolated from E. coli biomass using chromatography steps as described above; the preparation contained no less than 97 % Hsp70, and according to the E-TOXATE assay (Sigma-Aldrich, USA), the level of lipopolysaccharide in the final Hsp70 preparation was lower than 0.1 MU/ml (Shevtsov et al. 2014a). In several experiments, we also used LPS-free human recombinant Hsp70 expressed in Spodoptera cells and purified as described (Rozhkova et al. 2010).
Substrate-binding activity
Chaperonic activity of recombinant Hsp70 proteins was assessed by a modified immunoenzyme method (Cheetham et al. 1994; Lazarev et al. 2011). Briefly, irreversibly denatured protein, carboxymethylated lactalbumin, was applied onto wells of a 96-well microtiter plate. After washing off the unbound protein, the tested samples of Hsp70 in various concentrations were added to the wells, followed by the addition of polyclonal antibodies RSIII, Institute of Cytology (Lazarev et al. 2016). RSIII antibodies recognize Hsp70 bound to denatured protein and thus permit quantification of the extent of binding. The anti-rabbit antibody conjugated with peroxidase (Jackson ImmunoResearch Inc., USA) was employed to visualize the complex.
Refolding capacity assay
To estimate the refolding activity of Hsp70 preparations, we employed a protocol established by Cassel and coauthors with some modifications (Cassel et al. 2012). According to this protocol, firefly luciferase (Sigma-Aldrich, USA) inactivated with 8 M urea was mixed with the extract of heat-stressed K-562 eryrthroblastoma cells (total protein concentration 500 ug/ml in solution containing 125 HEPES-KOH pH7.5, 5 mM MgCl2 and 2 mM ATP). After a 100-fold dilution with the above solution, pure recombinant Hsp70 (final concentration 0.5 uM), creatine phosphate, and creatine phosphokinase were added for 60 min followed by the Bright-Glo solution of luciferase substrate (Promega, USA) and the luminescence intensity was measured with the aid of a Charity multifunctional reader (Probanauchpribor, Russia).
The ability to displace endogenous Hsp70 from cells
Functional exogenous Hsp70, when administered to cells, has the ability to displace endogenous Hsp70 to the cell surface. The ability of various preparations of human Hsp70 to displace endogenous Hsp70 was assayed by a previously described method (Mikhaylova et al. 2016). Briefly, rat glioblastoma C6 cells were incubated with Hsp70 in complete DMEM medium in wells of a 96-well microtiter plate. After incubation, the cells were washed to remove residual Hsp70 and stained using antibodies cmHsp70.1, which is selectively recognizing the TKD-peptide of intracellular Hsp70 displaced to the cell surface (Stangl et al. 2011; Shevtsov et al. 2014b). Staining intensity (absorbance) measured with a plate reader is proportional to the amount of the displaced endogenous Hsp70.
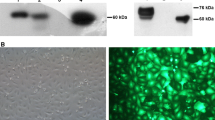
The ability of exogenous Hsp70 to pull its endogenous analog out from the cells was tested also in experiments when each sample of recombinant Hsp70 was conjugated with Alexa Fluor 555 (red; Invitrogen, USA) and added in a concentration of 50 μg/ml to human erythroblastoma K-562 cells (50 μg/ml). After an 18-h incubation, the localization of the labeled protein and of total Hsp70 stained with polyclonal antibodies RSIII (green) in non-permeabilized cells was studied using confocal microscopy as described elsewhere (Shevtsov et al. 2014b). Endogenous stained Hsp70 appeared at the cell surface as bright green spots, and the number of such cells increased with time.
The ability to stimulate natural killer activity toward cancer cells in vitro
Non-activated splenocytes, used as effectors, were isolated from C3H mice using a standard protocol. According to the data of flow cytometry, the fraction of natural killer cells comprised 2–7 % of the total cell amount. Human erythroblastoma K-562 cells, serving as the target population, were treated with 50 μg/mL Hsp70 protein preparations for 18 h. Effector and target cells were mixed 50:1 or 100:1 and placed into wells of a 96-well microtiter plate. Cell death was assessed using the CytoTox 96 system (Promega), which measures the activity of lactate dehydrogenase released from dead cells (Shevtsov et al. 2014a, b).
The ability to decrease endotoxin-induced ROS production
Our previous experiments showed that administration of recombinant human Hsp70 before LPS addition had a protective effect and significantly decreased ROS induction (Kustanova et al. 2006; Rozhkova et al. 2010). In the experiments performed here human neutrophils were preconditioned with different preparations of recombinant Hsp70 essentially as described (Rozhkova et al. 2010). To monitor the effect of LPS and rHsp70 addition on ROS production in neutrophils, LPS was added 5 min after the addition of rHsp70, and the cells were stimulated by 1 μM formyl-methionine-leucine-phenylalanine (fMLP) in the presence of 35 μM luminol 25 min later. Chemiluminescence (CL) was measured at the 10-min mark in a 1.250 LKB luminometer (Sweden).
Statistical analysis
The data are generally reported as mean ± SE. One- or two-tailed unpaired Student’s t tests were used to evaluate the differences between the control and treatment groups; differences were considered to be statistically significant when P < 0.05.
Results and discussion
Design of modified human Hsp70 proteins and their generation by site-directed mutagenesis
Glycosylation of HSP70 may be a problem when manufacturing Hsp70 in eukaryotic cells, particularly when the producing cells are secreting Hsp70 and this process may potentially complicate the outcome of administering exogenous Hsp70 to patients and induce an autoimmune response (Fleshner and Johnson 2005).
N-glycosylation consists of the attachment of glycoside moieties to the amide nitrogen of asparagine and glutamine (Asn, or N in single-letter code, Gln or Q) residues of a protein. N-glycosylation sites on proteins typically have the following sequence: an asparagine (N) or glutamine (Q), followed by any amino acid other than a proline, followed by a threonine (T) or serine (S) (Mellquist et al. 1998; Drickamer and Taylor 2006).
We have focused on the following potential N-glycosylation sites listed here in the N-terminus to C-terminus order with their amino acid context in single-letter code, with the relevant asparagine residue in parentheses: QG(N)RTPSY, YF(N)DSQRQ, DL(N)KSINP, KR(N)STIPT, and IL(N)VTATD. We reviewed the regions corresponding to the putative glycosylation sites described above in the published 3D structures of homologous proteins and fragments of human Hsp70 (Protein Data Bank records 2QWL, 1CKR, 3CQX, 2E8A, 2E88). The majority of the putative glycosylation sites were found to have likely importance for the function of Hsp70. For example, the first (from the N-terminus) putative glycosylation site of those described above, namely QG(N)RTPSY, overlaps with the nucleotide binding part of active site of Hsp70.
Accordingly, both insight and experimentation are required to achieve the desired outcome with respect to post-translational modifications while retaining the function of the protein intact.
With respect to the site QGNRTPSY, which is the first (from the N-terminus) putative glycosylation site of Hsp70, we have initially hypothesized that mutating the threonine (T) residue to alanine (A) may be useful. Such variant, containing QGNRAPSY was constructed and expressed in E. coli. However, our analysis of the 3D structure of the active site and the sequences of natural prokaryotic homologues of Hsp70, such as the E. coli dnaK protein, suggested that it may be better to maintain the hydroxyl (–OH) group of the threonine residue intact and disrupt the potential N-glycosylation site by changing the asparagine (N) to aspartate (D) residue and, hence, making the active site similar to that of orthological bacterial Hsp70 (dnaK). This second variant containing QGDRTPSY site was constructed and expressed in E. coli. Similar considerations were applied to selecting the means of disrupting the other potential N-glycosylation sites. The choice for rhHsp70 variant for expression in eukaryotes was made after in vitro refolding experiments where this variant demonstrated more promising activity. A preferred modified version of human recombinant Hsp70 protein is designated rhHsp70.128 (for “recombinant human HSP70 protein, version 128”) and, hence, in this text, references to the modified Hsp70 refer to the rhHsp70.128. For expression in E. coli, we also used the nonmodified variant carrying 6his designated rhHsp70.135 (Fig. S1).
Therefore, rhHsp70.128 principally differs from the wild-type protein (rhHsp70.135) in the five putative N-glycosylation sites; in the first of which an asparagine (N) is replaced with an aspartate (D) and in the others, a threonine (T) or serine (S) moiety is replaced with an alanine (A), as follows: QGDRTTPSY, YFNDAQRQA, DLNKAINPD, KRNSAIPTK, and ILNVAATDK (Fig. S1). All obtained variants of the constructs were expressed in E. coli and produced proteins with normal ATP-binding activity (data not shown). The rhHsp70 proteins isolated from E. coli harboring the plasmids have expected molecular weight (Fig. S3).
Modified HSP70 expressed in E. coli has intact chaperone activities
Binding of denatured protein and chaperonic activity
Chaperone activity of the preparations of recombinant Hsp70 was assessed as described in M&M using carboxymethylated lactalbumin as substrate protein (Lazarev et al. 2011).
Figure 1a shows the results of binding for various concentrations of Hsp70 variants, relative to the activity of a reference preparation of wild-type human Hsp70 designated “wt.” All three Hsp70 samples were shown to recognize and bind denatured lactalbumin with similar efficacy in this assay.
Chaperone activities of various Hsp70 samples. a Substrate-binding activity of Hsp70 samples applied in different concentrations. b Chaperone refolding activity measured with the aid of the luc-assay. “WT”: a highly pure recombinant wild-type human Hsp70 without any modifications; “135”: rhHsp70.135, a wild-type Hsp70 protein with 6xHis tag; “128”: modified rhHsp70.128 with 6xHis tag. (See Fig. S1). All proteins were expressed in E. coli and purified as described in M&M
Another assay employed to estimate chaperone activity of the Hsp70 preparations was a refolding test whose principle is to measure the activity of luciferase after its denaturation and recovery with the help of Hsp70 preparation. The data show that all three samples tested were almost equally active in recovering of luciferase activity (Fig. 1b), while both tagged proteins even slightly exceeded wild-type Hsp70 by this parameter.
The data of both above assays demonstrate that the key chaperone features of Hsp70, ability to recognize a denatured polypeptide and to convert previously denatured enzyme into active form of the modified Hsp70 with mutated potential glycosylation sites are similar to those of wild-type human recombinant Hsp70 expressed in E. coli employed as the reference preparation.
Modified Hsp70 protein retains the ability to displace endogenous Hsp70 from cells
It is known that fully functional exogenous Hsp70, when administered to cells, has the ability to displace endogenous Hsp70 to the cell surface and to make by this, the tumor or virally infected cells, more sensitive to cytotoxic lymphocytes (Shevtsov et al. 2014b). In our experiments, rat glioblastoma C6 cells were incubated with various samples of Hsp70 in complete DMEM medium in wells of a 96-well microtiter plate. After incubation, the cells were stained using antibodies cmHsp70.1, which known to react selectively with intracellular Hsp70 displaced to the cell surface (Shevtsov et al. 2014b). Staining intensity (absorbance) measured with a plate reader is proportional to the amount of the displaced endogenous Hsp70. Exogenous Hsp70 was fully washed out after its reaction with cells as the special measurements have shown.
Figure 2a shows the relative displacement ability (measured in arbitrary units of absorbance as described in M&M). In the assay, rhHsp70.128 is equivalent to rhHSP70.135 and the reference wild-type Hsp70 (wt). Thus, three Hsp70 protein samples demonstrated equal ability to displace their endogenous analog to a cell surface.
The displacement of endogenous Hsp70. a Results of in vitro experiments exploring rat glioblastoma C6 cells incubated with various samples of Hsp70 and antibodies cmHsp70.1, which react selectively with intracellular Hsp70 displaced to the cell surface Two concentrations of probes were used (50 and 125 μg/ml); b Results of confocal microscopy studies with erythroblastoma cells (К-562) incubated with recombinant Alexa-555-labeled (Invitrogen; red) Hsp70 probes for 18 h and stained without permeabilization with polyclonal antibodies (RSIII) which recognize Hsp70 (green color). (Shevtsov et al. 2014b). All samples of Hsp70 but not BSA (control) efficiently displace endogenous Hsp70 to a cell surface (green dots). WT-reference preparation of Hsp70; 128-modified Hsp70 with mutated glycosylation sites and His-tag; 135 wild-type Hsp70 with His-tag
Another independent series of experiments exploring confocal microscopy was performed to corroborate the ability of modified recombinant Hsp70 to displace endogenous HSP70 from the cells (Fig. 2b). In these experiments, erythroblastoma cells (К-562) were incubated with recombinant Alexa-555-labeled (Invitrogen; red) Hsp70 probes for 18 h and stained without permeabilization with polyclonal antibodies (RSIII) which recognize Hsp70 (green color). Eighteen hours after the beginning of incubation with the tested probes of exo-Hsp70, cells exhibited powerful and separate staining of green and red colors, indicating that the chaperone pumping machinery functioned in the majority of the cell population. Notably, the green spots did not co-localize with the red staining of exo-Hsp70. Bovine serum albumin (BSA) conjugated with Alexa-555 served as a control (Fig. 2b).
It is evident that modified rhHSP70.128 as well as wild-type probe (wt) entered the cells and displaced the endogenous Hsp70 (green dots). rhHsp70.135 for some reason in our experiments exhibited a high level of aggregation but was also able to displace the endogenous Hsp70. However, the part of the aggregates was not able to enter the cells and they stuck to the cell surface and seen as big yellow spots (Fig. 2b). The control protein (BSA) was not able to enter the cells in these experiments.
Modified HSP70 protein stimulates natural killer cytotoxicity
The data presented above (Fig. 2) indicate that Hsp70 added to the culture of cancer cells is able to displace its cellular analog onto the cell surface. These data prompted us to check whether this exposition on the outer cell membrane can stimulate the cytotoxic activity of lymphocytes in the way demonstrated earlier (Shevtsov et al. 2014a, b). To check this, we employed non-activated splenocytes which were added to K-562 erythroblasts after the latter were incubated with various samples of Hsp70 (see M&M).
Figure 3 depicts the percentage of target cells killed for various preparations of human Hsp70. In this assay, rhHsp70.128 demonstrated superior activity compared to the reference preparation of human Hsp70 (wt) and rhHsp70.135 probe suggesting that it can be successfully applicable in the immunotherapeutical techniques.
Effect of Hsp70 preparations on the sensitivity of K-562 cells to the action of CTL. wt: a highly pure recombinant wild-type human Hsp70 without any modifications; 135: rhHsp70.135, a wild-type Hsp70 protein with 6xHis tag; 128: modified rhHsp70.128 with 6xHis tag. Standard assay for cytotoxic lymphocytes (CTL) was employed in the study; C3H mouse splenocytes used as effector cells were taken in 50:1 and 100:1 ratio to K-562 target cells. The value of control cytotoxicity (without Hsp70) was subtracted from the data of all three groups
Modified Hsp70 efficiently decreased LPS-induced ROS level
It is well-known that bacterial pathogens interact with target myeloid cells via special receptors. Increased ROS concentration represents one of the first indications of endotoxemia (Victor et al. 2005). We showed that different variants of exogenous Hsp70 exhibit a protective effect when applied 5 min before LPS administration by monitoring ROS levels. Importantly, modified protein (rhHsp70.128) provided maximal inhibiting effect on the ROS level (Fig. 4). The results obtained are in general in agreement with the results of our previous studies on LPS-induced activation of human neutrophils and other studies implicating Hsp70 as a powerful anti-oxidant (Fleshner and Johnson 2005; Rozhkova et al. 2010).
Effect of Hsp70 preparations on ROS production in neutrophils. LPS (20 ng/mL) was added after 5 min, and fMLP (1 μM) was added 25 min later. C Chemiluminescence of control not treated cells; 128 modified Hsp70; 135 wild-type Hsp70; Hsp human Hsp70 expressed and isolated from Spodoptera cells. The results are expressed as the percentage of the control. Means ± standard errors of six experiments are shown (P < 0.001)
Expression of wild-type and mutant human HSP70 in transgenic mice
The expression of human Hsp70 in the milk of two strains of transgenic mice was investigated by immunoblotting. Figure 5 depicts the results of this analysis. Electrophoretic mobility shift in the case of wt Hsp70 is evident (lanes 2 and 3) apparently due to posttranslational modification. On the other hand, modified Hsp70 (corresponding to rhHsp.128) expressed in the milk of the second mouse strain (lanes 5 and 6) exhibits a molecular weight similar to the referenced wt protein expressed in E. coli (lane 1). Interestingly, we were not able to detect wt Hsp70 in the milk of transgenic mice using commercial monoclonal antibodies (SPA822, EnzoLab) and the protein in the first transgenic strain was detected only by exploring polyclonal antibodies described in the M&M indicating that the intactness of Hsp70 was disturbed, apparently due to glycosylation. Furthermore, we failed to purify wt Hsp70 from the milk using conventional methods DEAE-ATP-columns applied for isolation of Hsp70 expressed in E. coli (data not shown).
Milk from transgenic and control mice was analyzed in denaturing 10 % SDS gel with subsequent blotting and staining using rabbit polyclonal antibody to hHsp70. 1 rhHsp70 from E. coli without histidine tag, 0.1 μg; 2, 3 0.1 μl of whole milk of transgenic mice expressing wt hHSP70; 4 0.1 μl of control whole milk (non-transgenic mouse); 5, 6 0.1 μl of milk of transgenic mice, expressing modified Hsp70 (equivalent of rhHSP70.128)
The concentration of Hsp70 in the milk of the transgenic mice determined by comparison with referenced protein of known concentration varies from 1 to 2 mg/ml depending on the animal.
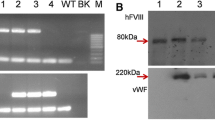
However, in the second series of experiments with the mouse strain expressing modified Hsp70 (equivalent of hrHsp70.128) in the milk, we demonstrated that mutant Hsp70, unlike non-modified Hsp70, can be efficiently isolated using ATP columns and reacted to commercial antibodies (Fig. 6).
Purification of mutant Hsp70 from the milk using ATP-column. a 1 milk; 2 milk flow through fraction; 3 resin after Hsp70 elution; 4 eluted Hsp70; (1–4 milk from transgenic mice containing Hsp70-containing construct); 5 milk; 6 milk flow-through fraction; 7 resin after elution; 8 eluted Hsp70 (5-8 milk from control non-transgenic mice); 9 molecular weight standard. b Western blotting of the gel depicted in a with monoclonal SPA822 bodies recognizing human Hsp70
Furthermore, the performed analysis using various in vitro assays demonstrated that the modified protein containing mutated glycosylation sites after expression in the milk apparently preserves major chaperone characters and does not differ from analogous protein expressed in E. coli. Figure 7 depicts the results of a comparative analysis of the properties of Hsp70 expressed in the milk of transgenic animals compared to those of wt Hsp70 probe such as binding of denatured proteins and elevating CTL-activity (Fig. 7a, b). The data of the assay show that wt and milk Hsp70 samples are almost identical in their binding of denatured protein while the sample isolated from the milk contains a slightly smaller amount of active chaperone (Fig. 7a).
Chaperone activities of various Hsp70 samples expressed in E. coli and in the milk of transgenic mice. a Substrate-binding and b the effect of Hsp70 preparations on the sensitivity of K-562 cells to the action of CTL (see legends in Fig. 3)
On the other hand, Hsp70 protein isolated from the milk exceeded wild-type protein in terms of cytotoxic activity stimulation which is a very good result for Hsp70-mediated immunostimulatory action (Fig. 7b).
Generally speaking, secretory production is technically favorable over cytoplasmic due to ease of purification. Scientific applications of our constructs may include expression of modified Hsp70 in the bloodstream or tissue environment using transgenesis techniques or viral vector tranfection.
Conclusions
In this study, we obtained a modified version of human HSP70 with mutated presumptive glycosylation sites, that in addition to having the desired property of a decreased propensity for glycosylation, also possesses equal or even superior beneficial activities compared to the wild-type protein. These properties include substrate binding and refolding activity, the ability to pull out endogenous Hsp70 from the cells, the ability to stimulate cytotoxic activity toward tumor cells, and the ability to efficiently reduce LPS-induced ROS levels in human neutrophils. The construct comprising the modified version of human Hsp70 was used to develop transgenic mice that expressed this protein in the milk, and the resultant protein was easily purified and as expected exhibited major chaperone activities. The expression of functionally active modified human HSP70 in the mammary glands of transgenic mice constitutes an important step toward a low-cost and efficient production of this protein drug with full biological activity in the mammary gland bioreactor for its potential clinical utility.
References
Asea A (2008a) Hsp70: a chaperokine. Novartis Found Symp 291:173–179
Asea A (2008b) Heat shock proteins and toll-like receptors. Handb Exp Pharmacol 183:111–127
Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6:435–442
Batista RI, Melo CH, Souza-Fabjan JM, Teixeira DI, Melo LM, Freitas VJ (2014) Phenotypic features of first-generation transgenic goats for human granulocyte-colony stimulation factor production in milk. Biotechnol Lett 36:2155–2162
Bobkova NV, Garbuz DG, Nesterova I, Medvinskaya N, Samokhin A, Alexandrova I, Yashin V, Karpov V, Kukharsky MS, Ninkina NN, Smirnov AA, Nudler E, Evgen’ev M (2014) Therapeutic effect of exogenous hsp70 in mouse models of Alzheimer’s disease. J Alzheimers Dis 38:425–435
Bobkova NV, Evgen’ev M, Garbuz DG, Kulikov AM, Morozov A, Samokhin A, Velmeshev D, Medvinskaya N, Nesterova I, Pollock A, Nudler E (2015) Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. Proc Natl Acad Sci U S A 112:16006–16011
Calderwood SK, Mambula SS, Gray PJ Jr, Theriault JR (2007) Extracellular heat shock proteins in cell signaling. FEBS Lett 581:3689–3694
Calderwood SK, Murshid A, Prince T (2009) The shock of aging: molecular chaperones and the heat shock response in longevity and aging. Gerontology 55:550–558
Cassel JA, Ilyin S, McDonnell ME, Reitz AB (2012) Novel inhibitors of heat shock protein Hsp70-mediated luciferase refolding that bind to DNA. J Bioorg Med Chem 20:3609–3614
Cheetham ME, Jackson AP, Anderton BH (1994) Regulation of 70-kDa heat-shock-protein ATPase activity and substrate binding by human DNAJ-like proteins, HSJ1a and HSJ1b. Eur J Biochem 226:99–107
De Maio A (2014) Extracellular Hsp70: export and function. Curr Protein Pept Sci 15:225–231
Drickamer K, Taylor ME (2006) Introduction to glycobiology, 2nd edn. Oxford University Press, USA
Duncan EJ, Cheetham ME, Chapple JP, van der Spuy J (2015) The role of HSP70 and its co-chaperones in protein misfolding, aggregation and disease. Subcell Biochem 78:243–273
Ekimova IV, Nitsinskaya LE, Romanova IV, Pastukhov YF, Margulis BA, Guzhova IV (2010) Exogenous protein Hsp70/Hsc70 can penetrate into brain structures and attenuate the severity of chemically-induced seizures. J Neurochem 4:1035–1044
Evgen’ev MB, Garbuz D, Zatsepina O (2014) Heat shock proteins and whole body adaptation to extreme environments. Springer, Netherlands, p 212
Fleshner M, Johnson JD (2005) Endogenous extra-cellular heat shock protein 72: releasing signal(s) and function. Int J Hyperth 21:457–471
Ghosh AK, Sinha D, Mukherjee S, Biswas R, Biswas T (2015) LPS stimulates and Hsp70 down-regulates TLR4 to orchestrate differential cytokine response of culture-differentiated innate memory CD8(+) T cells. Cytokine 73:44–52
Guzhova I, Margulis B (2006) Hsp70 chaperone as a survival factor in cell pathology. Int Rev Cytol 254:101–149
Hansen JE, Sohn W, Kim C, Chang SS, Huang NC, Santos DG, Chan G, Weisbart RH, Nishimura RN (2006) Antibody-mediated Hsp70 protein therapy. Brain Res 1088:187–196
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475:324–332
Hoshino T, Murao N, Namba T, Takehara M, Adachi H, Katsuno M, Sobue G, Matsushima T, Suzuki T, Mizushima T (2011) Suppression of Alzheimer’s disease-related phenotypes by expression of heat shock protein 70 in mice. J Neurosci 31:5225–5234
Kakimura J, Kitamura Y, Takata K, Umeki M, Suzuki S, Shibagaki K, Taniguchi T, Nomura Y, Gebicke-Haerter PJ, Smith MA, Perry G, Shimohama S (2002) Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. FASEB J 16:601–603
Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU (2013) Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 82:323–355
Kustanova GA, Murashev AN, Karpov VL, Margulis BA, Guzhova IV, Prokhorenko IR, Grachev SV, Evgen’ev MB (2006) Exogenous heat shock protein 70 mediates sepsis manifestations and decreases the mortality rate in rats. Cell Stress Chaperones 11:276–286
Lazarev VF, Onokhin KV, Antimonova OI, Polonik SG, Guzhova IV, Margulis BA (2011) Kinetics of chaperone activity of proteins Hsp70 and Hdj1 in human leukemia u-937 cells after preconditioning with thermal shock or compound u-133. Biochemistry (Mosc) 76:590–595
Lazarev VF, Nikotina AD, Mikhaylova ER, Nudler E, Polonik SG, Guzhova IV, Margulis BA (2016) Hsp70 chaperone rescues C6 rat glioblastoma cells from oxidative stress by sequestration of aggregating GAPDH. Biochem Biophys Res Commun 470:766–771
Li H, Liu Q, Cui K, Liu J, Ren Y, Shi D (2013) Expression of biologically active human interferon alpha 2b in the milk of transgenic mice. Transgenic Res 22:169–178
Magi S, Liberatori S (2004) Methods in molecular biology. In: Burns R (ed) Immunochemical protocols, vol 295, 3rd edn. Humana Press Inc, Totowa, pp 227–253
Mayer MP (2010) Gymnastics of molecular chaperones. Mol Cell 39:321–331
Mellquist JL, Kasturi L, Spitalnik SL, Shakin-Eshleman SH (1998) The amino acid following an asn-X-Ser/Thr sequon is an important determinant of N-linked core glycosylation efficiency. Biochemistry 37:6833–6837
Mikhaylova ER, Lazarev VF, Nikotina AD, Margulis BA, Guzhova IV (2016) Glyceraldehyde 3-phosphate dehydrogenase augments the intercellular transmission and toxicity of polyglutamine aggregates in a cell model of Huntington disease. J Neurochem 136:1052–1063
Multhoff G, Hightower LE (1996) Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones 1:167–176
Multhoff G, Hightower LE (2011) Distinguishing integral and receptor-bound heat shock protein 70 (Hsp70) on the cell surface by Hsp70-specific antibodies. Cell Stress Chaperones 16:251–255
Nollen EA, Brunsting JF, Song J, Kampinga HH, Morimoto RI (2000) Bag1 functions in vivo as a negative regulator of Hsp70 chaperone activity. Mol Cell Biol 20:1083–1088
Nunes JM, Mayer-Hartl M, Hartl FU, Müller DJ (2015) Action of the Hsp70 chaperone system observed with single proteins. Nat Commun 6:6307
Park CW, Kang MH, Min KS (2015) Secretion of human protein C in mouse milk. Int J Mol Sci 16:4904–4917
Picard V, Ersdal-Badju E, Lu A, Bock SC (1994) A rapid and efficient one-tube PCR-based mutagenesis technique using Pfu DNA polymerase. Nucleic Acids Res 22:2587–2591
Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP (2015) Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu Rev Pharmacol Toxicol 55:353–371
Radons J (2016) The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones 21:379–404
Rozhkova E, Yurinskaya M, Zatsepina O, Garbuz D, Murashev A, Ostrov V, Margulis B, Evgenev M, Vinokurov M (2010) Exogenous mammalian extracellular HSP70 reduces endotoxin manifestations at the cellular and organism levels. Ann N Y Acad Sci 1197:94–107
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. 2nd Edition, Cold Spring Harbor
Shevtsov MA, Komarova EY, Meshalkina DA, Bychkova NV, Aksenov ND, Abkin SV, Margulis BA, Guzhova IV (2014a) Effective immunotherapy of rat glioblastoma with prolonged intratumoral delivery of exogenous heat shock protein Hsp70. Oncotarget 5:3101–3114
Shevtsov MA, Pozdnyakov AV, Mikhrina AL, Yakovleva LY, Nikolaev BP, Dobrodumov AV, Komarova EY, Meshalkina DA, Ischenko AM, Pitkin E, Guzhova IV, Margulis BA (2014b) Exogenously delivered heat shock protein 70 displaces its endogenous analogue and sensitizes cancer cells to lymphocytes-mediated cytotoxicity. Int J Cancer 135:2118–2128
Shevtsov MA, Nikolaev BP, Yakovleva LY, Dobrodumov AV, Dayneko AS, Shmonin AA, Vlasov TD, Melnikova EV, Vilisov AD, Guzhova IV, Ischenko AM, Mikhrina AL, Galibin OV, Yakovenko IV, Margulis BA (2014c) Neurotherapeutic activity of the recombinant heat shock protein Hsp70 in a model of focal cerebral ischemia in rats. Drug Des Devel Ther 8:639–650
Srivastava P (2002) Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol 20:395–425
Stangl S, Gehrmann M, Riegger J, Kuhs K, Riederer I, Sievert W, Hube K, Mocikat R, Dressel R, Kremmer E, Pockley AG, Friedrich L, Vigh L, Skerra A, Multhoff G (2011) Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc Natl Acad Sci U S A 108:733–738
Victor VM, Rocha M, Esplugues JV, De la Fuente M (2005) Role of free radicals in sepsis: antioxidant therapy. Curr Pharm Des 11:3141–3158
Voos W (2009) Mitochondrial protein homeostasis: the cooperative roles of chaperones and proteases. Res Microbiol 160:718–725
Welch WJ, Feramisco JR (1985) Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol 5:1229–1237
Young L, Dong Q (2003) TAMS technology for simple and efficient in vitro site-directed mutagenesis and mutant screening. Nucleic Acids Res 31:e11
Zvezdova ES, Silaeva YY, Vagida MS, Maryukhnich EV, Deikin AV, Ermolkevich TG, Kadulin SG, Sadchikova ER, Goldman IL, Kazansky DB (2010) Generation of transgenic animals expressing the a and b chains of the autoreactive T-cell receptor. Mol Biol 44:277–286
Acknowledgments
This work was supported by the Russian Science Foundation grant (No. 14-50-00060); specifically, all experiments dealing with development of transgenic mouse lines were done using this grant. The experiments exploring in vitro assays were done using a grant of the Ministry of Education and Science of the Russian Federation to M.E. (agreement No. 14.Z50.31.0014). We are grateful to Drs. M. Vinokurov and M. Yurinskaya for their studies of Hsp70 effect on ROS in neutrophils and to Dr. L. Astakhova for her participation in the displacement experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
David G. Garbuz and Nataliya V. Soshnikova contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Supplementary materials and methods. (DOC 558 kb)
Rights and permissions
About this article
Cite this article
Gurskiy, Y.G., Garbuz, D.G., Soshnikova, N.V. et al. The development of modified human Hsp70 (HSPA1A) and its production in the milk of transgenic mice. Cell Stress and Chaperones 21, 1055–1064 (2016). https://doi.org/10.1007/s12192-016-0729-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-016-0729-x