Abstract
Heat shock proteins (HSPs) play important roles in cellular stress resistance. Previous reports had already suggested that HSP27 played multiple roles in preventing doxorubicin-induced cardiotoxicity. Although HSP25 might have biological functions similar to its human homolog HSP27, the mechanism of HSP25 is still unclear in doxorubicin-induced cardiomyocyte apoptosis. To investigate HSP25 biological function on doxorubicin-induced apoptosis, flow cytometry was employed to analyze cell apoptosis in over-expressing HSP25 H9c2 cells in presence of doxorubicin. Unexpectedly, the H9c2 cells of over-expressing HSP25 have no protective effect on doxorubicin-induced apoptosis. Moreover, no detectable interactions were detected by coimmunoprecipitation between HSP25 and cytochrome c, and HSP25 over-expression failed in preventing cytochrome c release induced by doxorubicin. However, down-regulation of endogenous HSP25 by a specific small hairpin RNA aggravates apoptosis in H9c2 cells. Subsequent studies found that HSP25, but not HSP90, HSP70, and HSP20, interacted with SIRT1. Knockdown of HSP25 decreased the interaction between SIRT1 and p53, leading to increased p53 acetylation on K379, up-regulated pro-apoptotic Bax protein expression, induced cytochrome c release, and triggered caspase-3 and caspase-9 activation. These findings indicated a novel mechanism by which HSP25 regulated p53 acetylation through dissociation of SIRT1 from p53 in doxorubicin-induced H9c2 cell apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The highly conserved heat shock proteins (HSPs), which function mainly as molecular chaperones, allow cells to adapt to changes in their environment and survive in otherwise lethal conditions. Under conditions of cellular stress, they interact and stabilize target proteins to protect them from denaturation or, if damage has occurred, promote disaggregation and allow refolding back to an active conformation. HSPs were classified into families on the basis of their size, structure, and function: the HSP110, HSP90, HSP70, HSP60, and small HSP families. Small HSPs are molecular chaperones that play major roles in organismic development, stress responses, and diseases. One small HSP is a 25-kDa protein, termed HSP25 (a homologue of human HSP27), whose expression is correlated with increased survival in various stressful conditions. As a molecular chaperone, HSP25 directly influences on the mechanisms of caspase activation, modulation of oxidative stress, and regulation of the cytoskeleton (Clarke and Mearow 2013; Franke et al. 2003; Lee et al. 2005, 2012a; Mounier and Arrigo 2002). Recently, the novel roles of extracellular soluble HSP25, which is present in the blood, have been found to regulate angiogenic balance (Lee et al. 2012b) and antagonize doxorubicin-induced cardiotoxicity (Krishnamurthy et al. 2012). Undoubtedly, full elucidation of the role of HSP25 will have many potential benefits as therapies for several ailments.
Doxorubicin, an antibiotic of the anthracycline group, is used in chemotherapeutic protocols for the treatment of different kinds of cancer. It affects the nucleic acid synthesis, inhibiting DNA and RNA polymerase activity by binding two strands of DNA helix, interspersing among the pairs of adjacent nucleotides, with modification of chromosome structure. But, the precise mechanism of antitumor activity of this drug is still under investigation. Notably, doxorubicin and its other formulations were known to leading congestive heart failure because of irreversible structural changes in the myocardium among cancer patients. Previous studies explored that increased cardiomyocyte apoptosis plays an important role in doxorubicin-induced cardiotoxicity (Chua et al. 2006; Nakamura et al. 2000). Some published work originally described the protective role of human HSP27 against doxorubicin (Ciocca et al. 1992; Vargas-Roig et al. 1998), and subsequent studies also showed that HSP27 plays multiple roles in preventing doxorubicin-induced cardiotoxicity (Turakhia et al. 2007; Venkatakrishnan et al. 2006, 2008). We are thus led to a hypothesis that HSP25 might have the same function as its human homolog in preventing doxorubicin-induced myocardial cell apoptosis. As a test of this hypothesis, the effect of HSP25 over-expression on doxorubicin-induced rat cardiomyocyte-derived H9c2 cell apoptosis was evaluated. However, HSP25 over-expression showed unsuccessful protective effect on doxorubicin-induced H9c2 cell apoptosis, which was not the same as we expected. But, here, we define a novel interplay between HSP25 and Sirtuin 1 (SIRT1) and suggest a new mechanism by which HSP25 down-regulation enhanced p53 acetylation through dissociation of SIRT1 from p53.
Materials and methods
Cell culture
H9c2 cardiac myoblast cell line was obtained from the American Type Culture Collection (CRL-1446) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 15 % heat-inactivated fetal calf serum (FCS; Gibco) at 37 °C in a fully humidified incubator with 5 % CO2 and 95 % air. The medium was replaced twice a week, and cells were passaged every 4 days at a 1:3 ratio.
Cell transfection
The plasmid containing coding sequence of SIRT1 was a kind gift of Professor Fuyuki Ishikawa (Takata & Ishikawa, 2003). A full-length HSP25 complementary DNA (cDNA) inserts were fragmented with restriction endonucleases and directionally subcloned into the expression vector pcDNA3.1 (Invitrogen). The integrity of the DNA constructs was verified by sequencing. Transient transfections of H9c2 cells have been achieved using TurboFect™ in vitro Transfection Reagent (Fermentas), according to the manufacturer’s instructions. Briefly, H9c2 cells were trypsinized and plated in a total volume of 3 mL/plate of DMEM plus 10 % FCS to achieve 70–80 % confluence. The amount of DNA (4 μg/well) required to effectively transfect cells was diluted in serum-free DMEM and then mixed with TurboFect™ (8 μL/well) by pipet. After being incubated for 15–20 min at room temperature, the TurboFect™/DNA mixture was added carefully to the cells while gently agitating the plate to achieve even distribution of the complexes. The cells were incubated in an incubator at 37 °C with 5 % CO2 and 95 % air. The transgene expression was analyzed 24–48 h later.
An RNA interference (RNAi) kit for HSP25 gene (pGPU6/GFP/Neo-small hairpin RNA (shRNA)) was purchased from GenePharma Co. (Shanghai, China). The shRNA was designed using parameters based on previously published measurements (Goldbaum et al. 2009). pGPU6/GFP/Neo-shRNA for HSP25 (reverse primer: 5′-GAT CCA AAA AAG GAA CAG TCT GGA GCC AAG TCT CTT GAA CTT GGC TCC AGA CTG TTC C-3′; forward primer: 5′-CAC CGG AAC AGT CTG GAG CCA AGT TCA AGA GAC TTG GCT CCA GAC TGT TCC TTT TTT G-3′; target sequence: 5′-GGA ACA GTC TGG AGC CAA G-3′) was transfected into H9c2 cells using Lipofectamine (Invitrogen) according to the manufacturer’s instructions. Briefly, H9c2 cells were seeded onto a six-well plate at 4–6 × 105 cells/well and grew to 95 % confluence. The cells were washed twice with serum-free DMEM and then incubated with a mixture of DNA, Lipofectamine, and serum-free medium for 6 h when the medium was changed to the complete DMEM containing 10 % FCS. Forty-eight hours after transfection, the cells were harvested and the gene-silencing efficiency was detected.
Apoptosis assay by flow cytometry
The amount of apoptosis in the cardiomyocytes was determined using the annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (BD Biosciences, San Diego, CA) for the detection of apoptosis according to the manufacturer’s instructions. Cells in the initial stage of apoptosis were defined as annexin V (+)/propidium iodide (PI) (−), while late apoptotic cells were defined as annexin V (+)/PI (+). Finally, annexin V (+) PI (−) and annexin V (+) PI (+) cells were detected under flow cytometry, and the percentages in the total number of cells in each group were compared. Briefly, H9c2 cells transfected with pcDNA3.1-Neo or pcDNA3.1-HSP25-Myc and pGPU6/GFP/Neo-shRNA for HSP25 gene or for negative control were, respectively, treated with 1 μmol/L doxorubicin for 24 h and then washed in phosphate-buffered saline and stained with FITC-conjugated annexin V and PI for 15 min at room temperature. The percentage of positive cells determined over 10,000 events was analyzed by a FACSCalibur system (BD BioSciences, San Jose, CA, USA). The data were acquired and analyzed using Cell Quest program (Becton Dickinson).
Isolation of subcellular fractionation
Mitochondria and cytosolic fractions from cultured H9c2 cells were isolated with the Mitochondria Isolation Kit (PIERCE) according to the manufacturer’s instructions. Cells were lysed in lysis buffer A [20 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 2 mmol/L EDTA, and 1 % Triton X100 with protease and phosphatase inhibitors] for 20 min at 4 °C, and mitochondria were extracted in a Dounce homogenizer in mitochondrial buffer (1 mmol/L EDTA, 210 mmol/L mannitol, 70 mmol/L sucrose, and 5 mmol/L Tris-HCl, pH 7.5), followed by centrifugation at 1300×g for 10 min at 4 °C. The supernatant was further centrifuged at 17,000×g for 15 min at 4 °C to pellet the mitochondria, and the resulting supernatant was stored as the cytosolic fraction. The crude mitochondrial fraction was resuspended for washing and centrifuged at 17,000×g for 15 min at 4 °C. The pellets were collected as the mitochondrial fraction. Subcellular fractionation and Western blotting analysis were used to detect cytochrome c content in cytosol and mitochondria. The increase in the cytosol with a concomitant decrease in mitochondria is indicative of cytochrome c release from mitochondria. Voltage-dependent anion channel (VDAC), an outer mitochondrial membrane protein, is used as a mitochondrial marker.
Protein preparation and Western blot
Cultured cardiomyocytes were extracted in radioimmunoprecipitation assay (RIPA) lysis buffer [10 mmol/L Tris-Cl, 1 mmol/L EDTA, 0.5 mmol/L EGTA, 140 mmol/L NaCl, 1 % (v/v) Triton X-100, 0.1 % Na-deoxycholate, 0.1 % sodium dodecyl sulfate, and 1 mmol/L PMSF], and cell lysates were cleared by a brief centrifugation (12,000g for 10 min). All buffers contained a cocktail of protease inhibitors (Roche). Concentrations of proteins in the supernatant were determined by the BCA assay. Total protein (10–50 μg/lane) was electrophoresed and separated on 10 or 15 % SDS-polyacrylamide gel and transferred to a PVDF membrane (Millipore), which was soaked in 5 % bovine serum albumin (BSA) in Tris-buffered saline Tween (TBST, pH 7.6). The membrane was incubated overnight with primary respective specific antibodies on a rotating platform at 4 °C. Subsequently, the membrane was rinsed in TBST (pH 7.6) and incubated with horseradish peroxidase-conjugated IgG antibodies diluted in TBST (1:2000) for 2 h on a rotating platform at 4 °C. Bands were visualized using an HRP developer, and background-subtracted signals were quantified on a laser densitometer (Bio-Rad). Relative protein loading was determined by immunoblotting with glycolytic glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies to ensure equal loading of lysates. The following primary antibodies were used: SIRT1 (Millipore, 07-131), cytochrome c (Santa Cruz Biotechnology, sc-7159), HSP25 (Stressgen, SPA-801), K379Ac-p53 (Cell Signaling Technology, 2570), GAPDH (Cell Signaling Technology, 2118), Flag-Tag (Cell Signaling Technology, 2368) and Myc-Tag (Cell Signaling Technology, 2276).
Coimmunoprecipitation
Cells were harvested and rapidly extracted in RIPA lysis buffer. Protein (500 μg) was precleared with Protein G Plus/Protein A Agarose Suspension (Calbiochem, IP05) and then incubated with 4 μg respective specific antibodies at 4 °C overnight. After the incubation, the reaction mixture was gently rocked by adding 100 μL Protein G Plus/Protein A Agarose Suspension at 4 °C for 4 h. The agarose beads were collected by centrifugation and washed three times with RIPA lysis buffer, boiled for 5 min in 2× SDS sample buffer, and frozen until used for Western blot.
Assay of caspase-3 and caspase-9 activities
The enzymatic activities of the caspases were determined by a colorimetric assay kit (R&D Systems, BF3100 and BF10100) according to our previously published measurements (Feng et al. 2012). Briefly, cells (1 × 106) were lysed with 50 μL of cell lysis buffer for 10 min. After microcentrifugation (12,000g, 1 min, 4 °C), the protein concentration in the supernatant was determined by the Bradford method (DingGuo Biotechnology). Supernatants containing equal amounts of protein are used for assay of caspase-3 and caspase-9 activities. After 2 h of incubation at 37 °C, the absorbance at 405 nm was measured by a microtiter plate reader. Fold increases in caspase-3 or caspase-9 activities over their controls were determined.
Statistical analysis
Data were collected from repeated experiments and are presented as mean ± SD. One-way ANOVA and Student’s t test were used for statistical analysis. Differences were considered to be significant at P < 0.05.
Results
HSP25 over-expression showed unsuccessful protective effect on doxorubicin- induced H9c2 cell apoptosis
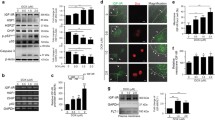
Forty-eight hours after pcDNA3.1-HSP25-Myc transfection, HSP25 expressions were analyzed by Western blot using HSP25 antibody. Exogenetic HSP25 expressed a relatively higher level (Fig. 1a, b). To evaluate the role of HSP25 in doxorubicin-induced apoptosis, H9c2 cells were transfected with pcDNA3.1-HSP25-Myc or neo vector control after 48 h, and then, the cell apoptosis was induced by 1 μmol/L doxorubicin for 24 h. Cell apoptosis was analyzed by flow cytometry. The result showed that initial stage of cell apoptosis (R2), late apoptotic cells (R1), and total apoptotic cells (R1 + R2) have no significant decrease in HSP25 over-expression cell groups (R1 15.9 ± 4.6 %, R2 14.1 ± 2.8 %, R1 + R2 30.0 ± 5.5 %) compared with neo vector control cell group (R1 15.1 ± 3.9 %, R2 15.3 ± 2.5 %, R1 + R2 30.4 ± 6.2 %; P > 0.05, Fig. 1c, d).
Effects of HSP25 over-expression on doxorubicin (DOX)-induced H9c2 cell apoptosis were measured by flow cytometry. Western blot analyses of HSP25 expression were performed after pcDNA3.1-HSP25-Myc transient transfection (left panel) or after treated transfected cells by DOX for 24 h (right panel) (a). Densitometric analysis of Western blots for HSP25 bands normalized to GAPDH after H9c2 cell transfection by pcDNA3.1-HSP25-Myc or after treated transfected cells by DOX for 24 h. *P < 0.05 vs neo vector control group (b). After the DOX treatment period of 24 h, cells were stained with annexin V-FITC and PI followed by analysis in the flow cytometry (c). Cells in the initial stage of apoptosis were defined as annexin V (+)/PI (−), while late apoptotic cells were defined as annexin V (+)/PI (+). The percentages of annexin V (+) PI (+) cells were shown as R1, and the percentages of annexin V (+) PI (−) cells were shown as R2 (d). Finally, R1, R2, and R1 plus R2 in each group were compared. Data shown are representative of three separated experiments
HSP25 down-regulation via shRNA aggravated doxorubicin-induced H9c2 cell apoptosis
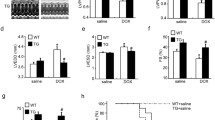
Forty-eight hours after shRNA-HSP25 expressing plasmid transfection in H9c2 cells, Western blot was employed to validate gene-silencing efficiency of HSP25 in control shRNA and HSP25-shRNA groups (Fig. 2a, b). Cell apoptosis induced by 1 μmol/L doxorubicin for 24 h was analyzed using flow cytometry. Our data demonstrated that initial stage of cell apoptosis (R2), late apoptotic cells (R1), and total apoptotic cells (R1 + R2), as identified by annexin V and PI staining, were significantly increased in HSP25-shRNA group (R1 16.5 ± 4.7 %, R2 21.9 ± 6.4 %, R1 + R2 38.4 ± 8.7 %) compared with control shRNA group (R1 13.2 ± 4.1 %, R2 14.9 ± 5.5 %, R1 + R2 28.0 ± 8.1 %; *P < 0.05, Fig. 2c, d).
Effects of HSP25 down-regulation on doxorubicin (DOX)-induced H9c2 cell apoptosis were measured by cytometric analysis. Western blot analyses of HSP25 expression were performed after HSP25-specific shRNA transient transfection (left panel) or after treated transfected cells by DOX for 24 h (right panel) (a). Densitometric analysis of Western blots for HSP25 bands normalized to GAPDH after H9c2 cell transfection by HSP25-specific shRNA or after treated transfected cells by DOX for 24 h. *P < 0.05 vs control shRNA group (b). After the DOX treatment period of 24 h, cells were stained with annexin V-FITC and PI followed by analysis in the flow cytometer (c). Cells in the initial stage of apoptosis were defined as annexin V (+)/PI (−), while late apoptotic cells were defined as annexin V (+)/PI (+). The percentages of annexin V (+) PI (+) cells were shown as R1, and the percentages of annexin V (+) PI (−) cells were shown as R2 (d). Finally, R1, R2, and R1 plus R2 in each group were compared. *P < 0.05, Data shown are representative of three separated experiments
No interaction between HSP25 and cytochrome c could be detected
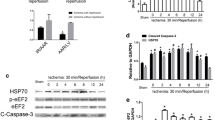
Considering the possibility that HSP25 has biological functions similar to HSP27 in binding to cytochrome c during cell apoptosis as previously reported (Bruey et al. 2000; Paul et al. 2002), the interaction between HSP25 and cytochrome c was detected by coimmunoprecipitation in H9c2 cells. However, as shown in Fig. 3a, there were no detectable interactions between HSP25 and cytochrome c in H9c2 cells with or without doxorubicin treatment.
HSP25 has not been found to interact with cytochrome c and failed in preventing cytochrome c release. The coimmunoprecipitation assay was performed in triplicate and showed no direct interaction between HSP25 with cytochrome c (a). The effect of HSP25 over-expression on doxorubicin (DOX)-induced H9c2 cell cytochrome c release from the mitochondria into the cytosol was validated by Western blot. A band of cytochrome c in the cytosolic fraction and a corresponding decrease in the mitochondrial fraction were observed (b). Quantitative analysis of cytochrome c release from the mitochondria into the cytosol (c). IP immunoprecipitation, WB Western blot, DOX doxorubicin, Mito mitochondrial, Cyto cytosolic. Data shown are representative of three separated experiments
HSP25 over-expression failed in preventing cytochrome c release induced by doxorubicin
After the doxorubicin treatment, cytosolic and mitochondrial cytochrome c were measured by Western blot in HSP25 over-expression or neo vector control H9c2 cells. To ensure that there was no cross-contamination between the cytosolic and mitochondrial fractions caused by the isolation procedure, VDAC in each fraction was also measured. Doxorubicin significantly increased the cytosolic cytochrome c, with a concomitant decrease in the mitochondria, as shown in Fig. 3b. Quantitative data showed that the cytosolic cytochrome c induced by doxorubicin treatment was increased to 52 ± 8 % of the total cellular concentration in the neo vector control cell group, whereas HSP25 over-expression did not significantly suppress this effect (P > 0.05, Fig. 3c).
An interaction between SIRT1 and HSP25 appeared to be important for SIRT1 function
We found that over-expressing HSP25 cells did not result in perceivable change to resist the cell apoptosis induced by doxorubicin treatment, which coincided with the release of cytochrome c into the cytosol. But, down-regulation of HSP25 enhanced the cell apoptosis induced by doxorubicin treatment. Therefore, we presume that HSP25 may play a major role as molecular chaperone in affecting some important protein during cell apoptosis. After coimmunoprecipitation analysis, a novel and intriguing interaction between HSP25 and SIRT1 was discovered (Fig. 4a, b). Furthermore, members of other HSP families, such as HSP90, HSP70, and HSP20, coincidently have no detected interaction with SIRT1 (Fig. 4c).
A novel interaction between HSP25 and SIRT1 was discovered. Interaction of endogenous SIRT1 and HSP25 was confirmed by coimmunoprecipitation assay (a). Coimmunoprecipitation studies of cell lysates from H9c2 cells transfected with pcDNA3-SIRT1-Flag and pcDNA3.1-HSP25-Myc demonstrate that SIRT1-Flag can interact with HSP25-Myc (b). HSP25 interacts, but HSP90, HSP70, and HSP20 do not interact, with SIRT1 in H9c2 cells (c). IP immunoprecipitation, WB Western blot. Data shown are representative of three separated experiments
HSP25 down-regulation enhanced p53 acetylation on K379 via weakened SIRT1-p53 interaction
We also investigated the effect of HSP25 down-regulation on SIRT1-mediated p53 deacetylation in response to doxorubicin in H9c2 cells. The interaction between SIRT1 and p53 was analyzed by coimmunoprecipitation after transfection with HSP25-specific shRNA or control shRNA. Our results showed that down-regulation of HSP25 weakened the interaction between SIRT1 and p53 (Fig. 5a) and increased the level of acetylated K379 in p53 (Fig. 5b, c, *P < 0.05). These data suggested that the effect of SIRT1 on p53 deacetylation was mediated by HSP25. To determine whether HSP25 down-regulation contributed to doxorubicin-induced H9c2 cell apoptosis, Bax, cytosolic cytochrome c, and activation of caspase-3 and caspase-9 were analyzed after transfection with HSP25-specific shRNA or control shRNA. The results showed that HSP25 down-regulation could markedly up-regulate the expression of the pro-apoptotic Bax protein (Fig. 5b, d), significantly increase the cytosolic cytochrome c (Fig. 5e, f), and obviously enhance doxorubicin-induced activation of caspase-3 and caspase-9 (Fig. 5g, h) (*P < 0.05).
The weakening SIRT1-p53 interaction induced by HSP25 down-regulation enhanced p53 K379 acetylation, Bax protein expression, cytochrome c release, and the activation of caspase-3 and caspase-9. Interaction between SIRT1 and p53 was analyzed by coimmunoprecipitation after transfection with HSP25-specific shRNA or control shRNA (a). Western blot analyses of acetylated lysine (K) 379 in p53 and Bax protein level were performed after HSP25-specific shRNA transient transfection (b). Densitometric analysis of Western blots for p53 K379 and Bax bands normalized to GAPDH after transfection by HSP25-specific shRNA or control shRNA (c, d). After being treated by 1 μmol/L doxorubicin for 24 h, cytosolic and mitochondrial cytochrome c distributions in HSP25 down-regulation cells or control group cells were analyzed by Western blot (e, f). And, the cell lysates from the HSP25 down-regulation cells or control group cells were also assayed for in vitro caspase-3 and caspase-9 activities (g, h). IP immunoprecipitation, WB Western blot, DOX doxorubicin, Mito mitochondrial, Cyto cytosolic. *P < 0.05. Data shown are representative of three separated experiments
Discussion
It was already known that HSPs constitute a group of chaperone proteins which help to maintain stability of client proteins and/or target the degradation of unfolded proteins when cells are exposed to heat shock or other kinds of stress. Similar to other members of the HSP family, HSP27 was initially characterized in response to heat shock as a molecular chaperone to aid in refolding of nonnative proteins. It forms complexes with such proteins, thus preventing their non-specific aggregation and allowing them to be subsequently restored to their native structure. Subsequent to its identification as a molecular chaperone, HSP27 has been shown to have other functions, including roles in cell proliferation, differentiation, and death. In unstressed cells, constitutively expressed HSP27 is generally low levels and essential for maintaining cell homeostasis. During the stress response, an increase in the level of HSP27 expression is preceded by a phosphorylation-induced reorganization of the multimeric status of the protein and correlated with the ability to prevent cell apoptosis (Concannon et al. 2003). Previous reports had already suggested an interaction of HSP27 with components of the apoptotic cascade and an antiapoptotic function. HSP27 binds DAXX during Fas-FasL-mediated apoptosis and prevented the subsequent binding of Ask1 by DAXX (Charette and Landry 2000). HSP27 was involved in protection from programmed cell death by inhibition of caspase-dependent apoptosis (Calderwood et al. 2006). Enhanced HSP27 expression also inhibited conformational Bax activation, oligomerization, and translocation to mitochondria, having significantly improved cell survival after stress (Havasi et al. 2008). The interaction between HSP27 and Akt was necessary for activation of Akt, leading to an attenuated rate of constitutive neutrophil apoptosis (Rane et al. 2003). Additionally, HSP27 bound to cytochrome c released from the mitochondria to the cytosol and inhibited apoptotic pathways triggered by cytochrome c-mediated activation of caspases (Bruey et al. 2000; Franke et al. 2003).
These previous investigations led us to hypothesize that HSP25 has the same function as HSP27 in preventing cell apoptosis. We therefore asked whether changes of HSP25 gene expression by using transient transfection are sufficient to reveal anti-apoptosis functions of HSP25. However, an unexpected observation was that HSP25 over-expression showed unsuccessful protective effect on doxorubicin-induced H9c2 cell apoptosis, and HSP25 over-expression failed in preventing cytochrome c release induced by doxorubicin. In spite of a previous study reporting that an enhancement of the extracellular HSP25 level in circulation can have beneficial consequences for the heart in resisting doxorubicin-induced cardiotoxicity via a mechanism involving the antagonism of Toll-like receptor 2, but we should also realized that HSP25-enriched blood plasma pretreatment have not significantly increased the intracellular HSP25 protein level in cardiomyocytes (Krishnamurthy et al. 2012). These results suggested that cardioprotection against doxorubicin induced by HSP25-enriched blood plasma transfusion could not be due to a high intracellular level of HSP25. In our present study, we also found that there was no obvious difference in cell apoptosis induced by doxorubicin between HSP25 over-expression and basal expression groups. Furthermore, our intriguing finding is that HSP25 down-regulation aggravated doxorubicin-induced H9c2 cell apoptosis. These data indicated that HSP25 was involved in protection from cell apoptosis by interaction with a key undiscovered regulator of apoptosis. To further investigate the role of HSP25 in H9c2 cell apoptosis, we extended our study and confirmed that HSP25, but not HSP90, HSP70, and HSP20, interacts with SIRT1 in H9c2 cells.
SIRT1, a mammalian homologue of the Saccharomyces cerevisiae chromatin silent information regulator Sir2, was implicated in various cellular functions, ranging from gene silencing, the control of the cell cycle, and apoptosis to energy homeostasis (Yamamoto et al. 2007). Most importantly, SIRT1 could deacetylate p53 and attenuate its ability to transactivate its downstream target genes, such as p21 for cell cycle arrest and Bax for apoptosis (Luo et al. 2001; Vaziri et al. 2001). As the p53 protein has several acetylation sites, whose hyperacetylation stabilizes and activates itself to trigger the apoptosis (Chen et al. 2009; Kim et al. 2013; Rokudai et al. 2013), it plays an important role in doxorubicin-induced cardiomyocyte apoptosis (Ma et al. 2013; Zhang et al. 2011). Our published work also showed that resveratrol, one of the numerous polyphenolic compounds found in several vegetal sources, attenuated doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Therefore, SIRT1 plays an important role in DOX-induced cardiomyocyte apoptosis via deacetylating p53 and attenuating its ability to transactivate its downstream target genes (Zhang et al. 2011).
Cardiac cell line H9c2, a clonal heart muscle cell line derived from embryonic rat hearts that retains many cardiomyocyte phenotypes, could be used as a model system to evaluate various characteristics of cardiomyocytes, including investigations on myocardial protection (Jin et al. 2013; Lin et al. 2013; Wang et al. 2013). In the present study, we also selected the H9c2 cell line to explore a relationship between SIRT1 and HSP25. To demonstrate that SIRT1 interacts with HSP25 in H9c2 cells, the ability to coimmunoprecipitate endogenous SIRT1 and HSP25 as well as transiently expressed epitope-tagged versions of the proteins was examined. Immunoprecipitation of whole-cell extracts from H9c2 cells with anti-HSP25 or anti-SIRT1 antibodies followed by Western blot analysis with anti-SIRT1 or anti-HSP25 antibody, respectively, demonstrated that the endogenous SIRT1 and HSP25 proteins interact under physiological conditions. Consistent with this observation, FLAG-tagged SIRT1 and Myc-tagged HSP25 were also coimmunoprecipitated from transfected H9c2 cells. Our further studies of the mechanism whereby the HSP25 down-regulation augmented doxorubicin-induced H9c2 cell apoptosis have shown that HSP25 down-regulation decreased the interaction between SIRT1 and p53, leading to increased p53 acetylation on K379, up-regulated pro-apoptotic Bax protein expression, induced cytochrome c released from mitochondria, and triggered caspase-3 and caspase-9 activation. Therefore, in H9c2 cells, we consider that SIRT1 may potentially be a key regulator of doxorubicin-induced cardiomyocyte apoptosis. HSP25 mediates the interaction between SIRT1 and p53, and importantly, this interaction increased deacetylation of p53 and consequently decreased cell apoptosis.
Abbreviations
- shRNA:
-
Small hairpin RNA
- cDNA:
-
Complementary DNA
- RNAi:
-
RNA interference
- FITC:
-
Fluorescein isothiocyanate
- HSPs:
-
Heat shock proteins
- HSP25:
-
Heat shock protein 25
- HSP27:
-
Heat shock protein 27
- SIRT1:
-
Sirtuin 1
- DOX:
-
Doxorubicin
- GAPDH:
-
Glycolytic glyceraldehyde-3-phosphate dehydrogenase
- VDAC:
-
Voltage-dependent anion channel
- TBST:
-
Tris-buffered saline Tween
- BSA:
-
Bovine serum albumin
- FCS:
-
Fetal calf serum
- DMEM:
-
Dulbecco’s modified Eagle’s medium
References
Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C (2000) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2:645–652
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31:164–172
Charette SJ, Landry J (2000) The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann N Y Acad Sci 926:126–131
Chen D, Pacal M, Wenzel P, Knoepfler PS, Leone G, Bremner R (2009) Division and apoptosis of E2f-deficient retinal progenitors. Nature 462:925–929
Chua CC, Liu X, Gao J, Hamdy RC, Chua BH (2006) Multiple actions of pifithrin-alpha on doxorubicin-induced apoptosis in rat myoblastic H9c2 cells. Am J Physiol Heart Circ Physiol 290:H2606–2613
Ciocca DR, Fuqua SA, Lock-Lim S, Toft DO, Welch WJ, McGuire WL (1992) Response of human breast cancer cells to heat shock and chemotherapeutic drugs. Cancer Res 52:3648–3654
Clarke JP, Mearow KM (2013) Cell stress promotes the association of phosphorylated HspB1 with F-actin. PLoS One 8:e68978
Concannon CG, Gorman AM, Samali A (2003) On the role of Hsp27 in regulating apoptosis. Apoptosis 8:61–70
Feng Y, Zhang C, Luo Q, Wei X, Jiang B, Zhu H, Zhang L, Jiang L, Liu M, Xiao X (2012) A novel WD-repeat protein, WDR26, inhibits apoptosis of cardiomyocytes induced by oxidative stress. Free Radic Res 46:777–784
Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C (2003) PI3K/Akt and apoptosis: size matters. Oncogene 22:8983–8998
Goldbaum O, Riedel M, Stahnke T, Richter-Landsberg C (2009) The small heat shock protein HSP25 protects astrocytes against stress induced by proteasomal inhibition. Glia 57:1566–1577
Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, Schwartz JH, Borkan SC (2008) Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem 283:12305–12313
Jin HJ, Xie XL, Ye JM, Li CG (2013) TanshinoneIIA and cryptotanshinone protect against hypoxia-induced mitochondrial apoptosis in H9c2 cells. PLoS One 8:e51720
Kim JH, Yoon EK, Chung HJ, Park SY, Hong KM, Lee CH, Lee YS, Choi K, Yang Y, Kim K, Kim IH (2013) p53 acetylation enhances Taxol-induced apoptosis in human cancer cells. Apoptosis 18:110–120
Krishnamurthy K, Kanagasabai R, Druhan LJ, Ilangovan G (2012) Heat shock protein 25-enriched plasma transfusion preconditions the heart against doxorubicin-induced dilated cardiomyopathy in mice. J Pharmacol Exp Ther 341:829–839
Lee YJ, Lee DH, Cho CK, Bae S, Jhon GJ, Lee SJ, Soh JW, Lee YS (2005) HSP25 inhibits protein kinase C delta-mediated cell death through direct interaction. J Biol Chem 280:18108–18119
Lee HJ, Kwon HC, Chung HY, Lee YJ, Lee YS (2012a) Recovery from radiation-induced bone marrow damage by HSP25 through Tie2 signaling. Int J Radiat Oncol Biol Phys 84:e85–93
Lee YJ, Lee HJ, Choi SH, Jin YB, An HJ, Kang JH, Yoon SS, Lee YS (2012b) Soluble HSPB1 regulates VEGF-mediated angiogenesis through their direct interaction. Angiogenesis 15:229–242
Lin HH, Chen YH, Chiang MT, Huang PL, Chau LY (2013) Activator protein-2alpha mediates carbon monoxide-induced stromal cell-derived factor-1alpha expression and vascularization in ischemic heart. Arterioscler Thromb Vasc Biol 33:785–794
Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W (2001) Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107:137–148
Ma J, Wang Y, Zheng D, Wei M, Xu H, Peng T (2013) Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways. Cardiovasc Res 97:77–87
Mounier N, Arrigo AP (2002) Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones 7:167–176
Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E (2000) Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: in vivo study. Circulation 102:572–578
Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP (2002) Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol 22:816–834
Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB (2003) Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem 278:27828–27835
Rokudai S, Laptenko O, Arnal SM, Taya Y, Kitabayashi I, Prives C (2013) MOZ increases p53 acetylation and premature senescence through its complex formation with PML. Proc Natl Acad Sci U S A 110:3895–3900
Takata T, Ishikawa F (2003) Human Sir2-related protein SIRT1 associates with the bHLH repressors HES1 and HEY2 and is involved in HES1- and HEY2-mediated transcriptional repression. Biochem Biophys Res Commun 301:250–257
Turakhia S, Venkatakrishnan CD, Dunsmore K, Wong H, Kuppusamy P, Zweier JL, Ilangovan G (2007) Doxorubicin-induced cardiotoxicity: direct correlation of cardiac fibroblast and H9c2 cell survival and aconitase activity with heat shock protein 27. Am J Physiol Heart Circ Physiol 293:H3111–3121
Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR (1998) Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer J 79:468–475
Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107:149–159
Venkatakrishnan CD, Tewari AK, Moldovan L, Cardounel AJ, Zweier JL, Kuppusamy P, Ilangovan G (2006) Heat shock protects cardiac cells from doxorubicin-induced toxicity by activating p38 MAPK and phosphorylation of small heat shock protein 27. Am J Physiol Heart Circ Physiol 291:H2680–2691
Venkatakrishnan CD, Dunsmore K, Wong H, Roy S, Sen CK, Wani A, Zweier JL, Ilangovan G (2008) HSP27 regulates p53 transcriptional activity in doxorubicin-treated fibroblasts and cardiac H9c2 cells: p21 upregulation and G2/M phase cell cycle arrest. Am J Physiol Heart Circ Physiol 294:H1736–1744
Wang K, Lei J, Zou J, Xiao H, Chen A, Liu X, Liu Y, Jiang L, Xiao Z, Xiao X (2013) Mipu1, a novel direct target gene, is involved in hypoxia inducible factor 1-mediated cytoprotection. PLoS One 8:e82827
Yamamoto H, Schoonjans K, Auwerx J (2007) Sirtuin functions in health and disease. Mol Endocrinol 21:1745–1755
Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo Q, Liu M, Chen G, Xiao X (2011) Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc Res 90:538–545
Acknowledgments
This work was supported by grants from the Major National Basic Research Program of China (no. 2007CB512007), the National Natural Science Foundation of China (81100106; 81100212; 81300113), A Project Supported by Scientific Research Fund of Hunan Provincial Education Department (11C1094; 11C1095), and the National College Students’ Innovation and Entrepreneurship Training Program (201210555011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, C., Qu, S., Wei, X. et al. HSP25 down-regulation enhanced p53 acetylation by dissociation of SIRT1 from p53 in doxorubicin-induced H9c2 cell apoptosis. Cell Stress and Chaperones 21, 251–260 (2016). https://doi.org/10.1007/s12192-015-0655-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-015-0655-3