Abstract
Recent findings suggest that hypoxia of the tumor microenvironment contributes to immune escape from natural killer (NK) cell-mediated cytotoxicity. Heat shock protein 70 (Hsp70) and the stress-regulated major histocompatibility class I chain-related protein A and B (MICA/B) both serve as ligands for activated NK cells when expressed on the cell surface of tumor cells. Herein, we studied the effects of hypoxia and hypoxia-inducible factor-1α (HIF-1α) on the membrane expression of these NK cell ligands in H1339 with high and MDA-MB-231 tumor cells with low basal HIF-1α levels and its consequences on NK cell-mediated cytotoxicity. We could show that a hypoxia-induced decrease in the membrane expression of MICA/B and Hsp70 on H1339 and MDA-MB-231 cells, respectively, is associated with a reduced sensitivity to NK cell-mediated lysis. A knockdown of HIF-1α revealed that the decreased surface expression of MICA/B under hypoxia is dependent on HIF-1α in H1339 cells with high basal HIF-1α levels. Hypoxia and HIF-1α did not affect the MICA/B expression in MDA-MB-231 cells but reduced the Hsp70 membrane expression which in turn also impaired NK cell recognition. Furthermore, we could show that the hypoxia-induced decrease in membrane Hsp70 is independent of HIF-1α in MDA-MB-231. Our data indicate that hypoxia-induced downregulation of both NK cell ligands MICA/B and Hsp70 impairs NK cell-mediated cytotoxicity, whereby only MICA/B appears to be regulated by HIF-1α.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoxia is a fundamental characteristic of locally advanced solid tumors that contributes to malignant progression by increasing the metastatic potential as well as the resistance to radio- and chemotherapy. The adaptation of tumor cells to hypoxia is primarily mediated by the hypoxia-inducible factor-1α (HIF-1α). Under normoxic conditions, HIF-1α is ubiquitinated and rapidly degraded by the proteasome. Following hypoxia, HIF-1α accumulates in the cytosol, translocates into the nucleus, and dimerizes with the subunit HIF-1ß to form the HIF-1 complex. The HIF-1 complex binds to the hypoxia-responsive element (HRE) in the promoter region and thus induces the transcription of oxygen-regulated genes which are involved in the adaptation of cells to hypoxic conditions. Furthermore, the HIF-1 complex is known to promote angiogenesis, invasion, and metastatic dissemination of tumor cells.
Recent findings suggest that hypoxia in the tumor microenvironment also can inhibit antitumor immune responses and thus enhances tumor immune escape (Lee et al. 2010). Natural killer (NK) cells which belong to the innate immune system provide the first line of defense against viral and bacterial infections and cancer. However, the efficacy of NK cell-based immunotherapies in solid tumors is still limited. Therefore, a better understanding of the mechanisms that can cause tumor immune escape from NK cells is of major importance.
The major histocompatibility class I chain-related proteins A and B (MICA/B) which are frequently expressed on the surface of tumor cells serve as potent ligands for NK cells. The interaction of cell surface-bound MICA/B with the activating NK cell receptor natural killer group 2 member D (NKG2D) results in tumor recognition, activation of NK cells, and tumor cell lysis. Therefore, a downregulation of MICA/B on the membrane of tumor cells by either shedding after proteolytic cleavage by metalloproteinases, release in exosomes, or recycling to internal compartments can impair recognition by NK cells (Fernandez-Messina et al. 2012). A relationship between a hypoxia-induced accumulation of HIF-1α, an increased expression of the metalloproteinase ADAM10, a decreased MICA membrane expression, and an increased resistance to lysis mediated by peripheral blood lymphocytes has been reported (Barsoum et al. 2011).
Besides MICA/B, membrane-bound heat shock protein 70 (Hsp70) also serves as a tumor-specific recognition structure for NK cells (Multhoff et al. 1995). Incubation of NK cells with the Hsp70-derived peptide TKD plus low-dose IL-2 results in an increased cell surface density of the receptors CD94, CD56, and NKG2D concomitant with an enhanced cytotoxic activity against Hsp70 membrane-positive tumor cells (Multhoff et al. 2001; Gastpar et al. 2005). Furthermore, an elevated Hsp70 cell surface density on tumor cells was associated with an increased lysis mediated by TKD/IL-2 activated NK cells.
Herein, we investigated the influence of hypoxia and HIF-1α on NK cell-mediated immune responses against human tumor cell lines that differ drastically in their basal HIF-1α levels.
Material and methods
Reagents
Cells and cell culture
The human lung cancer cell line, H1339, was cultured in RPMI 1640 supplemented with 10 % heat-inactivated FCS, 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 1 mM sodium pyruvate. The human breast cancer cell line, MDA-MB-231, was cultivated in the same medium supplemented with 10 mM HEPES. Cells were routinely checked for mycoplasma contamination. The authenticity of the cell lines was tested by the DSMZ (German Collection of Microorganisms and Cell Cultures).
shRNA transfection
The FG-12 RNAi delivery system was used to deliver short hairpin RNA (shRNA) against HIF-1α, as previously described (Li et al. 2007; Woodley et al. 2009). As a control, shRNA against β-galactosidase (lacZ) was used. In brief, HEK293T cells were transfected by using a calcium phosphate transfection kit (Invitrogen) with a plasmid containing the HIF-1α or lacZ shRNA and with two plasmids encoding the lentiviral packaging and envelope genes. The virus-containing supernatant of transfected HEK293T cells was mixed with polybrene and used to infect H1339 and MDA-MB-231 tumor cells.
Hypoxia
Cells were incubated for 24 h under hypoxic conditions ([O2] = 0.66 %) at 37 °C. Hypoxic conditions were achieved as described previously (Schilling et al. 2009).
Western blot analysis and ELISA
Cells were lysed in TBST buffer as described previously (Schilling et al. 2007). The protein content in the cell lysates was determined using the BCA™ Protein Assay Kit (Pierce). On immunoblots, proteins were detected with antibodies against HIF-1α (R&D Systems), CA IX (Lifespan Biosciences), Hsp70 (Enzo Life Sciences), and β-actin (Sigma-Aldrich). Quantification of immunoblots was performed with ImageJ. MICA and MICB concentrations in the cell lysates were measured by ELISA (R&D Systems), and the concentrations were calculated relative to the total protein content of each sample.
HRE luciferase assay
Cells were transfected with a HRE reporter plasmid that contains a HIF-responsive firefly luciferase construct (Qiagen). One day after transfection, cells were exposed to hypoxia. After 24 h, the luciferase activity was measured using the Dual Glo Luciferase assay system (Promega). A constitutive Renilla luciferase construct served as an internal control for normalizing transfection efficiencies, cell viability, and cell numbers.
Flow cytometry
Tumor cells were incubated with the allophycocyanin (APC)-conjugated Hsp70 monoclonal antibody, cmHsp70.1 (multimmune GmbH), the APC-conjugated MICA and MICB antibodies (R&D Systems), the PE-conjugated ADAM10 antibody (R&D Systems), or the corresponding isotype-matched control antibodies for 30 min at 4 °C. Cells were stained with propidium iodide (PI) and analyzed on a FACSCalibur flow cytometer (BD Biosciences). Only viable (PI negative) cells were gated to measure the membrane expression of Hsp70, MICA/B and ADAM10. The percentage of positive cells and mean fluorescence intensity (MFI) were adjusted to the respective isotype control.
NK cell isolation and europium assay
NK cell-mediated cytotoxicity was measured in a standard 4-h europium assay. NK cells were generated by a CD3/CD19 depletion of PBMCs from two healthy human volunteers using a magnetic separation method (Miltenyi Biotec). The purity of NK cells was determined by flow cytometry with antibodies against CD19 (BD), CD3 (BD), and CD56 (BD). NK cells were stimulated with 100 IU/ml IL-2 (Novartis) and 2 μg/ml Hsp70 peptide TKD (ECM Biosciences) for 4 days. The NK cell stimulation was proven by flow cytometry with antibodies against CD94 (BD), CD16 (BD), CD56 (BD), NKG2D (R&D Systems), and CD69 (BD) or the corresponding isotype-matched control antibodies.
Tumor cells were exposed to hypoxic or normoxic conditions for 24 h. In brief, BATDA-labeled (Perkin Elmer) tumor cells were co-incubated with NK cells at different ratios in a V-bottom 96-well plate in 200 μl medium. After a 4 h co-incubation period at 37 °C, 25 μl of supernatants were transferred into ELISA plates containing 200 μl Europium (Perkin Elmer). The time-resolved fluorescence was measured using Victor X4 plate reader (Perkin Elmer).
Quantitative real-time PCR
RNA was isolated with the RNeasy Mini Kit (Qiagen), and reverse transcription of RNA was performed with the QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s instructions. Quantitative real-time (qRT)-PCR was performed in a LightCycler 480 (Roche) by using the QuantiTect SYBR Green PCR Kit (Qiagen). The primers used for qRT-PCR were as follows: ACTB-F: GACGACATGGAGAAAATCTG, ACTB-R: ATGATCTGGGTCATCTTCTC; MICA-F: ATATCTAGAATCCGGCGTAG, MICA-R: TGATATTCCGGGGATAGAAG; and MICB-F: ATGCTGCAAAGTGTTAGTAG, MICB-R: TCCAATGGAATGTTGAGTTG. Each sample was measured in triplicate, and the mean Ct was calculated. Relative expression was calculated using the ΔΔCt method. The mRNA expression of ß-actin was used as internal control.
Statistics
Statistical analysis was performed using SPSS 18.0.2 software (IBM). The Student’s t test was used to evaluate significant differences (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Results
Differential expression and activity of HIF-1α in H1339 and MDA-MB-231 human tumor cells
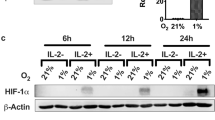
In this study, two human tumor cell lines were used that differ significantly in their basal HIF-1α expression. H1339 cells express very high amounts of HIF-1α and the HIF target gene carbonic anhydrase IX (CA IX) under normoxia and hypoxia (Schilling et al. 2012a, b), whereas MDA-MB-231 cells exhibit a low expression of HIF-1α and CA IX under normoxia and hypoxia (Fig. 1). The transcriptional activity of HIF-1α in both tumor cell types was determined in a HRE luciferase reporter assay. As expected, the HIF activity was 10-fold higher in H1339 cells compared to MDA-MB-231 cells under normoxic conditions (Fig. 2). Under hypoxia, the HIF activity was significantly upregulated in both tumor cell lines.
HIF-1α activity in H1339 and MDA-MB-231 cells. Luciferase activity of H1339 and MDA-MB-231 cells transfected with a reporter plasmid that contains a HIF responsive firefly luciferase construct and incubated for 24 h under normoxic (N) or hypoxic (H) conditions. Graphs represent mean values ± SEM of at least 3 independent experiments. Significant differences between normoxia and hypoxia are indicated (*p ≤ 0.05; **p ≤ 0.01)
Hypoxia reduces the sensitivity of both tumor cell lines against NK cell-mediated lysis
In order to investigate whether hypoxia and HIF-1α contribute to tumor immune escape, HIF-1α was knocked down by a lentiviral infection. As a control shRNA against ß-galactosidase (lacZ), a gene which is not present in human cells was used. The successful knockdown of HIF-1α in H1339 and MDA-MB-231 cells is illustrated in Fig. 3.
Control and HIF-1α knockdown tumor cells were exposed either to normoxic or hypoxic condition for 24 h and subsequently used as target cells in a standard cytotoxicity assay. NK cells purified from two different healthy human volunteers (effector cells) were incubated with low-dose IL-2 (100 IU/ml) and the Hsp70-derived peptide TKD (2 μg/ml) for 4 days to stimulate their cytotoxic activity against MICA/B and Hsp70 on tumor cells (Krause et al. 2004; Multhoff et al. 2001; Gross et al. 2003; Stangl et al. 2008). Following stimulation, the expression density of the cell surface markers CD94, CD56, NKG2D, CD16, and CD69 was increased on the NK cells of the two different donors (Table 1).
Upon hypoxia, the lysis of H1339 and MDA-MB-231 tumor cells (control) by activated NK cells was significantly reduced (Fig. 4a, b, left panel). However, the hypoxia-induced reduction in NK cell-mediated cytotoxicity could be reversed in H1339 cells when HIF-1α was knocked down (Fig. 4a, right). In contrast, in MDA-MB-231 cells, a HIF-1α knockdown did not affect the hypoxia-induced reduction in NK cell-mediated cytotoxicity (Fig. 4b, right). These data indicate that hypoxia protects H1339 and MDA-MB-231 tumor cells from NK cell-mediated lysis, but only in H1339 tumor cells that this effect is dependent on HIF-1α.
Hypoxia reduces lysis of H1339 and MDA-MB-231 tumor cells by activated NK cells. Tumor cells transfected with lacZ (control) or HIF-1α shRNA were cultivated under normoxic (N) or hypoxic (H) conditions for 24 h. NK cells were stimulated for 4 days with IL-2 (100 U/ml) and TKD (2 μg/ml). The cytotoxicity of unstimulated (unstim) and stimulated NK cells (IL-2 + TKD) was measured after a 4-h co-incubation with H1339 (a) or MDA-MB-231 (b) tumor cells transfected with lacZ (control, left) or HIF-1α (right) shRNA by europium assay. The relative cytotoxicity of NK cells is shown. The cytotoxicity of stimulated NK cells against normoxic tumor cells at a ratio of 5:1 was set to 1. Graphs represent mean values ± SEM of at least three independent experiments. Significant differences between normoxia and hypoxia are indicated (*p ≤ 0.05; **p ≤ 0.01)
Hypoxia and HIF-1α differentially affect the expression of NK cell ligands MICA/B and Hsp70 in H1339 and MDA-MB-231 tumor cells
To explain the hypoxia-induced reduction in NK cell-mediated lysis, we investigated the expression of the NK cell ligands MICA/B and Hsp70 in normoxic and hypoxic tumor cells. In order to investigate the impact of HIF-1α, control and HIF-1α knockdown tumor cells were analyzed. The cell surface expression of MICA/B and Hsp70 was measured by flow cytometry using APC-conjugated antibodies against MICA/B (R&D Systems) and the Hsp70 monoclonal antibody, cmHsp70.1 (Stangl et al. 2011).
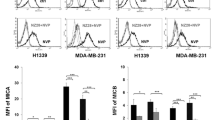
As shown in Fig. 5a, b, hypoxia significantly downregulated the membrane expression of MICA/B (mean fluorescence intensity and percentage positive cells) on H1339 cells. Since HIF-1α knockdown abrogated the hypoxia-induced reduction of membrane MICA/B, we speculate that HIF-1α is involved in downregulating the membrane expression of MICA/B. In contrast to MICA/B, the Hsp70 membrane status remained unaltered after hypoxia and HIF-1α knockdown in H1339 tumor cells (Fig. 5a, b). Other NK cell ligands such as ULBP1, 2, and 3 were nearly not detectable on H1339 cells (below 10 % positive cells) under normoxic and hypoxic conditions.
Effect of hypoxia and HIF-1α on MICA, MICB and Hsp70 membrane expression in H1339 and MDA-MB-231 cells. H1339 (a, b) and MDA-MB-231 (c, d) cells transfected with lacZ (control) or HIF-1α shRNA were exposed to normoxic (N) or hypoxic (H) conditions for 24 h. The MICA, MICB, and Hsp70 membrane expression was determined by flow cytometry. a, c. Representative flow cytometric analysis (FACS) showing MICA, MICB, and Hsp70 surface staining. The black solid lines represent MICA, MICB, or Hsp70 staining of normoxic cells; the gray solid lines represent MICA, MICB, or Hsp70 staining of hypoxic cells. Dotted lines represent the respective isotype controls. b, d. The percentage of positive cells (left) and the mean fluorescence intensity (MFI, right) are shown. Graphs represent mean values ± SEM of at least three independent experiments. Significant differences between normoxia and hypoxia and between control and HIF-1α knockdown cells are indicated (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001)
Interestingly, neither hypoxia nor a HIF-1α knockdown did affect the MICA/B membrane expression in MDA-MB-231 cells (Fig. 5c, d). However, the Hsp70 expression (mean fluorescence intensity and percentage positive cells) was significantly reduced upon hypoxia in control and HIF-1α knockdown cells (Fig. 5c, d). Therefore, we assume that the hypoxia-induced reduction of membrane Hsp70 in MDA-MB-231 cells is independent of HIF-1α. With respect to the differences in the expression pattern of NK cell ligands on H1339 and MDA-MB-231 cells, we assume that the reduced sensitivity of tumor cells against NK cell-mediated lysis under hypoxia is due to a decreased membrane expression of MICA/B in H1339 and a reduced Hsp70 expression in MDA-MB-231 cells.
To explain the differences in the MICA/B membrane expression upon hypoxic exposure and HIF-1α knockdown in H1339 cells, we measured the expression of the metalloproteinase ADAM10 which has been described to play a role in the hypoxia-induced shedding of MICA. Neither hypoxia nor a HIF-1α knockdown had any significant influence on the ADAM10 expression (Fig. 6a) and the release of MICA/B in H1339 cells (data not shown). The intracellular MICA/B expression (Fig. 6b) was slightly but not significantly reduced by hypoxia in H1339 control cells. MICA/B mRNA levels (Fig. 6c) were not influenced by HIF-1α knockdown but were significantly reduced by hypoxia in control cells. Since the MICA/B mRNA expression levels (Fig. 6c) reflect the MICA/B membrane expression (Fig. 5c, d), we assume that the HIF-1α-dependent hypoxia-mediated downregulation of mMICA/B is due to reduced MICA/B transcription.
Effect of hypoxia and HIF-1α on ADAM10 membrane expression, intracellular MICA/B, and MICA/B mRNA expression in H1339 cells. H1339 cells transfected with lacZ (control) or HIF-1α shRNA were exposed to normoxic (N) or hypoxic (H) conditions for 24 h. a The ADAM10 membrane expression was determined by flow cytometry. The mean fluorescence intensity (MFI) is shown. Graphs represent mean values ± SEM of three independent experiments. b The intracellular levels of MICA (icMICA, left) and MICB (icMICB, right) of H1339 cells transfected with lacZ (control) or HIF-1α shRNA were determined by ELISA. The concentrations in the cell lysates were calculated relative to the total protein content of each sample. Graphs represent mean values ± SEM of four independent experiments. c The mRNA expression of MICA (left) and MICB (right) of H1339 cells transfected with lacZ (control) or HIF-1α shRNA was quantified by qRT-PCR. The mRNA levels were normalized to the housekeeping gene ß-actin. Graphs represent mean values ± SEM of three independent experiments performed in triplicate. Significant differences between normoxia and hypoxia and between control and HIF-1α knockdown cells are indicated (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001)
In order to exclude that the hypoxia-induced reduction of membrane Hsp70 in MDA-MB-231 cells is due to a decreased Hsp70 synthesis or an increased secretion of Hsp70, we determined the intracellular and secreted levels of Hsp70. Neither intracellular Hsp70 (Fig. 7) nor secreted Hsp70 levels (data not shown) were significantly affected by hypoxia or HIF-1α knockdown. These data suggest that the hypoxia-induced reduction in membrane Hsp70 is due to a yet unidentified inhibition of transport to the membrane or an increased internalization rather than to changes in intracellular or extracellular Hsp70 levels.
Effect of hypoxia and HIF-1α on intracellular Hsp70 expression in MDA-MB-231 cells. Representative immunoblot and quantification of the expression of Hsp70 and ß-actin in MDA-MB-231 cells transfected with lacZ (control) or HIF-1α shRNA and incubated for 24 h under normoxic (N) or hypoxic (H) conditions. Graphs represent mean values ± SEM of three independent experiments
Discussion
Hypoxia has been described to induce tumor immune escape (Barsoum et al. 2011; Yamada et al. 2012; Barsoum et al. 2014; Noman et al. 2012). Herein, we show that hypoxia reduces the sensitivity of different human tumor cells to NK cell-mediated lysis by a differential downregulation of the NK cell ligands such as MICA/B and Hsp70.
In H1339 cells, a HIF-1α knockdown abrogated the hypoxia-induced downregulation of the MICA/B membrane expression and reduced the sensitivity against NK cell-mediated lysis. This is in accordance with data showing that HIF-1α is necessary for the hypoxia-induced reduction of the MICA membrane expression on malignantly transformed cells (Barsoum et al. 2011; Yamada et al. 2012). However, in nontransformed human cells, MICA/B membrane expression was found to be upregulated by a hypoxia/reoxygenation-induced HIF-1α overexpression (Luo et al. 2010; Wei et al. 2010).
The mechanism of how hypoxia and HIF-1α affect the MICA/B membrane expression is still a matter of debate. The metalloproteinase ADAM10 which is upregulated under hypoxia via accumulation of HIF-1α was assumed to be responsible for a reduced MICA membrane expression; however, in this study, the amount of secreted MICA was not investigated (Barsoum et al. 2011). Whereas some reports indicate that the downregulation of MICA membrane expression by hypoxia is associated with an increase in soluble MICA, others show a downregulation of membrane MICA under hypoxia without an increase in soluble MICA (Siemens et al. 2008; Yamada et al. 2012). In accordance with the latter, we found no significant changes in the expression of the metalloproteinase ADAM10 and in the amount of released MICA/B upon hypoxia and/or HIF-1α knockdown in H1339 cells.
Agüera-Gonzalez et al. have shown that the MICB surface expression rapidly decreases when protein synthesis is inhibited. This group suggests that the presence of MICB on the cell surface requires novel protein synthesis (Aguera-Gonzalez et al. 2009). In line with these data, we show a concomitant downregulation of MICA/B mRNA and membrane expression under hypoxia in H1339 cells which depends on HIF-1α. To our knowledge, no direct effect of HIF-1α on MICA/B transcription has been described. However, HIF-1α knockdown has been demonstrated to increase the levels of SP1 (specificity protein 1) (Culver et al. 2011) which constitutes an essential transcription factor for MICA/B (González et al. 2008). Therefore, we speculate that HIF-1α activation by hypoxia might reduce SP1 levels and subsequently MICA/B mRNA expression in H1339 cells leading to the reduced MICA/B membrane expression.
In the tumor cell line MDA-MB-231, the membrane expression of MICA/B remained unaltered but the membrane expression of Hsp70 was downregulated upon hypoxia independently on HIF-1α. Despite a reduced Hsp70 membrane expression, we did not observe any significant changes in intra- or extracellular Hsp70 levels in MDA-MB-231 cells. The influence of hypoxia on intracellular Hsp70 levels is still controversially discussed in the literature. Whereas Baek et al. observed an increase in Hsp70 expression after hypoxic exposure in RIF cells (Baek et al. 2001), another group showed differential effects on the Hsp70 expression in 18 different melanoma cell lines (Shipp et al. 2012).
Recently, hypoxia has been demonstrated to disrupt the forward vesicular trafficking of the channel protein Kv1.3 to the plasma membrane by downregulating AP1, a protein responsible for vesicle formation (Chimote et al. 2012). Hypoxia has also been demonstrated to regulate endocytosis by modulating Rab11 and rabaptin-5 and thereby alters the expression of surface proteins (e.g., integrins, receptor tyrosine kinases) (Yoon et al. 2005; Wang et al. 2009). The downregulation of rabaptin-5 has been shown to be mediated by HIF. In contrast, Park et al. (2012) showed recently that hypoxia reduces the cell surface expression of the glutamate receptor through an HIF-1-independent mechanism. In line with this study, we demonstrated that HIF-1α knockdown did not affect the hypoxia-induced reduction of Hsp70 membrane expression in MDA-MB-231 cells. A nonclassical pathway involving the endolysosomal system has been implicated in Hsp70 trafficking (Juhasz et al. 2013; Mambula and Calderwood 2006; Gastpar et al. 2005). Therefore, we hypothesize that hypoxia might reduce the Hsp70 membrane expression by directly modulating proteins involved in the regulation of the endolysosomal system (e.g., Rab proteins) leading either to a reduced transport to the membrane or an increased internalization.
In summary, our data show that hypoxia impairs the lysis of tumor cells by NK cells. Strikingly, a differential regulation of the NK cell ligands Hsp70 and MICA/B by hypoxia and HIF-1α was found to be responsible for the downregulated lysis in different human tumor cell lines.
References
Aguera-Gonzalez S, Boutet P, Reyburn HT, Vales-Gomez M (2009) Brief residence at the plasma membrane of the MHC class I-related chain B is due to clathrin-mediated cholesterol-dependent endocytosis and shedding. J Immunol 182(8):4800–4808. doi:10.4049/jimmunol.0800713
Baek SH, Lee UY, Park EM, Han MY, Lee YS, Park YM (2001) Role of protein kinase Cd in transmitting hypoxia signal to HSF and HIF-1. J Cell Physiol 188(2):223–235. doi:10.1002/jcp.1117
Barsoum IB, Hamilton TK, Li X, Cotechini T, Miles EA, Siemens DR, Graham CH (2011) Hypoxia induces escape from innate immunity in cancer cells via increased expression of ADAM10: role of nitric oxide. Cancer Res 71(24):7433–7441
Barsoum IB, Smallwood CA, Siemens DR, Graham CH (2014) A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 74(3):665–674. doi:10.1158/0008-5472.CAN-13-0992
Chimote AA, Kuras Z, Conforti L (2012) Disruption of kv1.3 channel forward vesicular trafficking by hypoxia in human T lymphocytes. J Biol Chem 287(3):2055–2067. doi:10.1074/jbc.M111.274209
Culver C, Melvin A, Mudie S, Rocha S (2011) HIF-1alpha depletion results in SP1-mediated cell cycle disruption and alters the cellular response to chemotherapeutic drugs. Cell Cycle 10(8):1249–1260
Fernandez-Messina L, Reyburn HT, Vales-Gomez M (2012) Human NKG2D-ligands: cell biology strategies to ensure immune recognition. Front Immunol 3:299. doi:10.3389/fimmu.2012.00299
Gastpar R, Gehrmann M, Bausero M, Asea A, Gross C, Schroeder J, Multhoff G (2005) Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 65(12):5238–5247. doi:10.1158/0008-5472.CAN-04-3804
González S, López-Soto A, Suarez-Alvarez B, López-Vázquez A, López-Larrea C (2008) NKG2D ligands: key targets of the immune response. Trends Immunol 29(8):397–403. doi:10.1016/j.it.2008.04.007
Gross C, Schmidt-Wolf I, Nagaraj S, Gastpar R, Ellwart J, Kunz-Schughart L, Multhoff G (2003) Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones 8(4):348–360
Juhasz K, Thuenauer R, Spachinger A, Duda E, Horvath I, Vigh L, Sonnleitner A, Balogi Z (2013) Lysosomal rerouting of Hsp70 trafficking as a potential immune activating tool for targeting melanoma. Curr Pharm Des 19(3):430–440
Krause S, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, Pfister K, Multhoff G (2004) Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase i trial. Clin Cancer Res Off J Am Assoc Cancer Res 10(11):3699–3707. doi:10.1158/1078-0432.CCR-03-0683
Lee CT, Mace T, Repasky EA (2010) Hypoxia-driven immunosuppression: a new reason to use thermal therapy in the treatment of cancer? Int J Hyperth Off J Eur Soc Hyperth Oncol N Am Hyperth Group 26(3):232–246. doi:10.3109/02656731003601745
Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, Chinn C, Chen M, Woodley DT (2007) Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. EMBO J 26(5):1221–1233
Luo L, Lu J, Wei L, Long D, Guo JY, Shan J, Li FS, Lu PY, Li PY, Feng L (2010) The role of HIF-1 in up-regulating MICA expression on human renal proximal tubular epithelial cells during hypoxia/reoxygenation. BMC Cell Biol 11:91
Mambula S, Calderwood S (2006) Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol (Baltimore, Md : 1950) 177(11):7849–7857
Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD (1995) A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer 61(2):272–279
Multhoff G, Pfister K, Gehrmann M, Hantschel M, Gross C, Hafner M, Hiddemann W (2001) A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones 6(4):337–344
Noman MZ, Buart S, Romero P, Ketari S, Janji B, Mari B, Mami-Chouaib F, Chouaib S (2012) Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res 72(18):4629–4641. doi:10.1158/0008-5472.CAN-12-1383
Park EC, Ghose P, Shao Z, Ye Q, Kang L, Xu XZ, Powell-Coffman JA, Rongo C (2012) Hypoxia regulates glutamate receptor trafficking through an HIF-independent mechanism. EMBO J 31(6):1379–1393. doi:10.1038/emboj.2011.499
Schilling D, Bayer C, Geurts-Moespot A, Sweep FC, Pruschy M, Mengele K, Sprague LD, Molls M (2007) Induction of plasminogen activator inhibitor type-1 (PAI-1) by hypoxia and irradiation in human head and neck carcinoma cell lines. BMC Cancer 7:143
Schilling D, Gehrmann M, Steinem C, De MA, Pockley AG, Abend M, Molls M, Multhoff G (2009) Binding of heat shock protein 70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells. FASEB J 23(8):2467–2477
Schilling D, Bayer C, Emmerich K, Molls M, Vaupel P, Huber RM, Multhoff G (2012a) Basal HIF-1a expression levels are not predictive for radiosensitivity of human cancer cell lines. Strahlenther Onkol. doi:10.1007/s00066-011-0051-6
Schilling D, Bayer C, Li W, Molls M, Vaupel P, Multhoff G (2012b) Radiosensitization of normoxic and hypoxic H1339 lung tumor cells by heat shock protein 90 inhibition is independent of hypoxia inducible factor-1a. PLoS One 7(2):e31110
Shipp C, Derhovanessian E, Pawelec G (2012) Effect of culture at low oxygen tension on the expression of heat shock proteins in a panel of melanoma cell lines. PLoS One 7(6):e37475. doi:10.1371/journal.pone.0037475
Siemens DR, Hu N, Sheikhi AK, Chung E, Frederiksen LJ, Pross H, Graham CH (2008) Hypoxia increases tumor cell shedding of MHC class I chain-related molecule: role of nitric oxide. Cancer Res 68(12):4746–4753
Stangl S, Gross C, Pockley AG, Asea AA, Multhoff G (2008) Influence of Hsp70 and HLA-E on the killing of leukemic blasts by cytokine/Hsp70 peptide-activated human natural killer (NK) cells. Cell Stress Chaperones 13(2):221–230. doi:10.1007/s12192-007-0008-y
Stangl S, Gehrmann M, Riegger J, Kuhs K, Riederer I, Sievert W, Hube K, Mocikat R, Dressel R, Kremmer E, Pockley AG, Friedrich L, Vigh L, Skerra A, Multhoff G (2011) Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc Natl Acad Sci USA 108(2):733–738
Wang Y, Roche O, Yan MS, Finak G, Evans AJ, Metcalf JL, Hast BE, Hanna SC, Wondergem B, Furge KA, Irwin MS, Kim WY, Teh BT, Grinstein S, Park M, Marsden PA, Ohh M (2009) Regulation of endocytosis via the oxygen-sensing pathway. Nat Med 15(3):319–324. doi:10.1038/nm.1922
Wei L, Lu J, Feng L, Long D, Shan J, Li S, Li Y (2010) HIF-1alpha accumulation upregulates MICA and MICB expression on human cardiomyocytes and enhances NK cell cytotoxicity during hypoxia-reoxygenation. Life Sci 87(3–4):111–119
Woodley DT, Fan J, Cheng CF, Li Y, Chen M, Bu G, Li W (2009) Participation of the lipoprotein receptor LRP1 in hypoxia-HSP90alpha autocrine signaling to promote keratinocyte migration. J Cell Sci 122(Pt 10):1495–1498
Yamada N, Yamanegi K, Ohyama H, Hata M, Nakasho K, Futani H, Okamura H, Terada N (2012) Hypoxia downregulates the expression of cell surface MICA without increasing soluble MICA in osteosarcoma cells in a HIF-1alpha-dependent manner. Int J Oncol 41(6):2005–2012. doi:10.3892/ijo.2012.1630
Yoon SO, Shin S, Mercurio AM (2005) Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the alpha6beta4 integrin. Cancer Res 65(7):2761–2769. doi:10.1158/0008-5472.CAN-04-4122
Acknowledgments
The authors want to thank Andrea Mair and Jessica Pelzel for excellent technical assistance and Eva and Thomas Kriehuber for purification of plasmids.
This work was supported by the Wilhelm Sander-Stiftung (2012.078.1), EU-CELLEUROPE (315963), BMBF (Strahlenkompetenz, 02NUK007E; 02NUK031B; 02NUK038A; Innovative Therapies, 01GU0823; NSCLC, 16GW0030; m4 - Leading Edge Cluster, 16EX1021C), and the DFG Cluster of Excellence: Munich Centre for Advanced Photonics MAP. The research leading to these results has received funding from the Deutsche Forschungsgemeinschaft (DFG) under Grant Agreement Nos. SFB 824/B4, INST 95/980-1 FUGG, and INST 411/37-1 FUGG irradiation devices.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schilling, D., Tetzlaff, F., Konrad, S. et al. A hypoxia-induced decrease of either MICA/B or Hsp70 on the membrane of tumor cells mediates immune escape from NK cells. Cell Stress and Chaperones 20, 139–147 (2015). https://doi.org/10.1007/s12192-014-0532-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-014-0532-5