Abstract
Heat shock proteins (HSP) are highly conserved across eukaryotic and prokaryotic species. These proteins play a role in response to cellular stressors, protecting cells from damage and facilitating recovery. In tumor cells, HSPs can have cytoprotective effects and interfere with apoptotic cascades. This study was performed to assess the prognostic and predictive values of the gene expression of HSP family members in canine osteosarcoma (OS) and their potential for targeted therapy. Gene expressions for HSP were assessed using quantitative PCR (qPCR) on 58 snap-frozen primary canine OS tumors and related to clinic-pathological parameters. A significant increased expression of HSP60 was found in relation to shorter overall survival and an osteoblastic phenotype. Therefore, the function of HSP60 was investigated in more detail. Immunohistochemical analysis revealed heterogeneous staining for HSP60 in tumors. The highest immunoreactivity was found in tumors of short surviving dogs. Next HSP expression was shown in a variety of canine and human OS cell lines by qPCR and Western blot. In two highly metastatic cell lines HSP60 expression was silenced using siRNA resulting in decreased cell proliferation and induction of apoptosis in both cell lines. It is concluded that overexpression of HSP60 is associated with a poor prognosis of OS and should be evaluated as a new target for therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary bone tumor, osteosarcoma (OS) is the most common type of bone tumor in large and giant dog breeds (Norrdin et al. 1989; Ru et al. 1998). Most often, dogs present with clinical signs of lameness or painful swelling of the affected bone. At an advanced stage, some dogs present with evidence of pathological fractures. However, almost 90 % of dogs have metastatic disease at the time of clinical presentation. Metastasis, predominantly to the lungs and with fewer incidences in other skeletal and soft tissue locations, is a major end-point for this disease in both humans and dogs (Morello et al. 2010). There are many causes and contributing factors for the development and pathogenesis of OS in dogs (Kirpensteijn et al. 2008; Levine et al. 2002; Misdorp and Hart 1979). Although improvements in diagnosis and therapy have been made over the past 20 years, the prognosis for dogs with OS is still grim. Elevated levels of several genetic markers and proteins have been found in human and canine OS (Selvarajah and Kirpensteijn 2010). However, very few of these markers have been independently validated in a large cohort of samples for their prognostic values, especially among dogs with OS.
Heat shock proteins (HSPs) are highly conserved proteins that are expressed in both prokaryotic and eukaryotic organisms. They are classified into separate families according to their molecular weight (in kilodaltons). In this paper, we will use this classification which can be converted to human nomenclature (Kampinga et al. 2009). Each family may consist of several molecules that show great resemblance in primary structure and perform similar functions in different sub-cellular compartments. In times of chemical or physical stress, these HSPs prevent protein misfolding and aggregation by refolding denatured proteins or by assisting in their degradation (Burel et al. 1992; Calderwood et al. 2006; Hartl and Hayer-Hartl 2002; Helmbrecht et al. 2000; Hendrick and Hartl 1993; Romanucci et al. 2008). In addition to their role during cellular stress, HSPs are also involved in tumor development, with roles in apoptotic pathways, cell cycle regulation and immune modulation.
HSP expression has been explored in various tumors and has been observed to have diagnostic, prognostic and therapeutic predictive values in both humans and dogs (Cappello et al. 2008; Faried et al. 2004; Kumaraguruparan et al. 2006; Romanucci et al. 2005). Heat shock proteins have long been examined in human OS, and some valuable insights have been made. A positive correlation has been found between the presence of anti-HSP70 serum antibodies and lung metastases in OS patients at the time of diagnosis, but no difference in anti-HSP70 antibodies could be observed between sera from patients and healthy controls. Anti-HSP60 antibodies are significantly increased in human patients with OS compared to a healthy control group. This increase in anti-HSP60 antibodies could be used as a potential diagnostic marker (Trieb et al. 2000b). Meanwhile, others have found no prognostic association for HSP60 expression in primary human OS tumors (Moon et al. 2010). In human OS, a correlation has been detected between the presence of anti-HSP90 antibodies and a better response to neo-adjuvant chemotherapy, whereas the absence of anti-HSP90 antibodies correlates with the occurrence of metastases (Trieb et al. 2000a). Additionally, HSP72 protein expression seems to be a predictive immunohistochemical marker for OS. HSP72-positive tumors show a significantly better response to chemotherapy than HSP72-negative tumors (Trieb et al. 1998). At the biopsy and surgical level, immunohistochemical classification has revealed a correlation between HSP27 overexpression and poorer prognosis. Therefore, HSP27 is a potential negative prognostic marker of OS (Moon et al. 2010; Uozaki et al. 2000). A cDNA microarray study on human OS cell lines observed an increased expression level of HSP90beta in OS cell lines compared to normal human osteoblasts, indicating that HSP90beta may play a role in the development or progression of OS (Wolf et al. 2000). To date, the value of HSP10, HSP22 and HSP40 expression has not been reported for human OS, although their implications in other types of cancer have been reported (Akyol et al. 2006; Cappello 2003; Cappello et al. 2003; 2005; Mitra et al. 2009; Sun et al. 2007).
The expression of HSPs has also been reported in canine cancers, including mammary carcinomas, transmissible venereal tumors, intracutaneous cornifying epitheliomas, squamous cell carcinomas, and osteosarcomas (Chu et al. 2001; Romanucci et al. 2005; Romanucci et al. 2008; Romanucci et al. 2012). In a previous study on the cDNA microarray gene expression profiling of canine OS tissues, members of different heat shock protein (HSP) families, including HSP90alpha, HSPA9 (HSP70, Mortalin) and HSP60, were associated with poor survival (Selvarajah et al. 2009). In another study, HSP90 gene inhibition was performed in both canine and human OS cell lines and resulted in loss of cell viability, inhibition of cell proliferation and induction of apoptosis, demonstrating a value for targeting HSP90 in OS in vitro models (McCleese et al. 2009). Although these gene expression profiling studies have suggested important roles for HSPs in the prognosis of canine OS, these findings are preliminary and deserve further validation using independent samples and other molecular approaches. The present research, therefore, aimed to evaluate the mRNA expression of 8 different HSPs and their association with several clinicopathological parameters and survival in dogs with OS. Based on mRNA analyses, HSP60 expression was negatively correlated with survival. Hence, HSP60 was selected for gene silencing using RNAi in two metastatic canine OS in vitro cell line models.
Material and methods
Canine OS tissue specimens and clinicopathological data
Fifty-eight histology-confirmed, primary canine OS tissue samples were selected from the bone tumor bank. These dogs were presented to the Veterinary Teaching Hospital of Utrecht University, The Netherlands, between 1994 and 2007. No dogs had therapy prior to harvesting the tumor tissue. Dogs included in the study had amputation or primary tumor resection and were followed until death. During surgery, all tumor samples were harvested under sterile conditions, snap-frozen in liquid nitrogen and stored in sterile tubes at −70 °C. Diagnosis and histological grading of OS was determined by a board-certified veterinary pathologist according to a previously described histological grading system (Kirpensteijn et al. 2002). Clinical data and histological data were reviewed retrospectively.
Cell lines and culture conditions
Eight well-characterized canine OS cell lines (D17, COS31, HMPOS, POS, KOS-001, KOS-002, KOS-003, and KOS-004) and three human OS cell lines (SaoS2, MG63 and U2OS) were used in this study. The mycosensor quantitative PCR (qPCR) assay kit (Agilent Technologies, US) was used to ensure that all cell lines were negative for mycoplasma. Cells were maintained in a sub-confluent monolayer in Dulbecco’s Modified Essential Medium (DMEM, Invitrogen®, The Netherlands) supplemented with 10 % fetal bovine serum (FBS, PAA Laboratories GmbH, Austria) at 37 °C in a humidified atmosphere with 5 % CO2. The cells were trypsinized and passaged every 4 days.
RNA isolation and cDNA synthesis
RNA isolation and purification from the OS tumors were performed as previously described (Selvarajah et al. 2009) with the RNeasy® Mini kit (Qiagen, The Netherlands) according to the manufacturer’s protocol. Cells grown in culture under normal or experimental conditions were washed once with Hank’s Balance Salt Solution (PAA Laboratories GmbH, Austria), followed by cell lysis using the appropriate volume of RLT cell lysis buffer (Qiagen, The Netherlands) before extraction of RNA. In both the bone tumor and the cell line RNA isolation protocols, an additional DNA digestion step was included to assure genomic DNA removal. Total RNA was quantified using a NanoDrop® ND-1000 spectrophotometer (Isogen Life sciences, The Netherlands) and subsequently assessed for quality using a 2100 Bioanalyzer (Agilent Technologies, The Netherlands). Synthesis of cDNA was carried out with 0.5 μg total RNA of each sample in a 20-μl reaction volume using the iScript cDNA Synthesis Kit (Bio-Rad, The Netherlands) according to the manufacturer’s instructions. To ensure that DNA contamination did not occur during the process of RNA and cDNA synthesis, several reactions were also performed without the reverse transcriptase enzyme.
Quantitative real-time PCR assays
Primers for the target genes were designed using Primer3 version 0.4.0 software and M-fold version 3.2 (http://mfold.bioinfo.rpi.edu). The optimal annealing temperature (Ta) of all primers was determined by a qPCR gradient protocol, using the iQ SYBR green SuperMix (Bio-Rad, The Netherlands) according to the manufacturers’ protocol. To confirm the specificity of the primers, the amplification product of each primer pair was cycle-sequenced using the MJ MiniCycler™ (BioRad, The Netherlands) and the 3130 XI Genetic Analyzer (Applied Biosystems, California, USA), followed by a nucleotide BLAST-search. Primers for the reference genes were designed and sequenced for specificity as described above. The geNorm algorithm analysis was performed to determine the number and type of reference genes appropriate for normalization of gene expression data.
Independent assays were performed for the bone tumors and cell lines. The standard dilution was prepared from a pool of cDNA (a mix from all samples) within an experimental setup. The standard concentration for qPCR analyses was generated using a fourfold dilution series. Each sample cDNA was diluted threefold with RNAse-free water, and aliquots of 2-μl diluted cDNA were further subjected for qPCR reactions. All qPCR reactions were performed in duplicate using the Bio-Rad MyiQ™ Single Color Real-Time PCR Detection System. A 2-step qPCR protocol was performed for the target genes HSP27, -40, -60 and 90beta as well as for the reference genes GAPDH, GUSB, HNRNPH, RPS19 and RPS5. The thermal cycling conditions were 95 °C for 3 min followed by 45 cycles of 95 °C for 10 s and Ta °C for 30 s. A three-step qPCR protocol was performed for the target genes HSP10, -22, -70, and -90alpha as well as for the reference genes HPRT and RPL8. The thermal cycling conditions for this protocol were 95 °C for 3 min, 45 cycles of 95 °C for 10 s, Ta °C for 30 s and 72 °C for 30 s. The annealing temperature was customized for each target and reference gene using a gradient qPCR assay prior to sample analyses.
Immunohistochemistry
Thirty six formalin-fixed, paraffin-embedded canine OS tissue sections from which also frozen specimen for qPCR were available were stained for anti-HSP60 (working dilution 0.2 μg/μl) (LK-1, mouse monoclonal, Stressgen, Canada). In addition, four human OS and canine normal liver (positive control for HSP60) tissue sections were also included. A negative control immunostaining was performed for all tissue samples by replacing the primary antibody with isotype-specific mouse serum IgG, using the identical concentration as the primary antibody. Tissue sections were deparaffined in xylene, rehydrated in decreasing alcohol dilutions and rinsed in PBS-Tween 0.1 % (PBST) for 10 min (2 cycles of 5 min). Antigen retrieval was performed by heating the sections in 10 mM sodium citrate buffer at pH6 for 1 h at 72 °C and cooling down for an additional 30 min. The slides were then rinsed twice in PBS and incubated in 10 % normal goat serum and 1 % bovine serum albumin in PBST (DAKO, The Netherlands) for 30 min at room temperature to reduce non-specific binding. Incubation with the primary antibody was performed overnight in a humidified chamber at 4 °C. After rinsing with PBST, the sections were incubated with 0.35 % hydrogen peroxide in PBS for 15 min to block endogenous peroxidase activity and rinsed with PBST before the sections were incubated with the secondary HRP-conjugated antibody (1:10,000, goat anti-mouse, DAKO, The Netherlands) for 30 min at room temperature. Afterwards, the sections were rinsed in PBS and incubated with DAB-chromogen mix (Sigma, The Netherlands) for 5 min, followed by a counterstaining with hematoxylin (Vector laboratories, Burlingame, Canada). Finally, the sections were rinsed in running water, dehydrated in increasing alcohol dilutions and mounted with Vecta Mount (Vector laboratories).
A semi-quantitative immunohistochemistry assessment was performed by two assessors who evaluated the slides and scored them based on their consensus agreement of the observed expression. Scoring for HSP60 protein expression was performed using a four-scale grading method (0: no positive cells; 1: <10 % positive cells; 2: <10–50 % of positive cells; and 3: >50 % of positive cells). Due to the low number of tissue sections used for this assessment, the four-scale grading was reduced to a two-scale grading: low % of positive cells (grades 0 and 1) and high % of positive cells (grades 2 and 3). Immunopositivity was also assessed for localization (cytoplasmic or nuclear) and for intensity (high or low).
Protein isolation and western blot analyses
Cells grown under normal culture or experimental conditions in 24-well plates were washed once with cold Hank’s Balance Salt Solution (HBSS) and incubated with RIPA buffer containing 1 % Igepal, 0.6 mM phenylmethylsulfonylfluoride, 15 μg/ml aprotinin and 1 mM sodium orthovanadate (Sigma-Aldrich Chemie BV, The Netherlands) for 30 min on ice. Lysates were collected and homogenized at 10,000 rpm at 4 °C, and the supernatant was collected for protein determination using the Bradford-based assay with DC Protein Assay Reagents (Bio-Rad, The Netherlands). Protein acquired from the eight canine and three human OS cell lines was further diluted in PBS to 1 μg/μl. The protein samples were diluted 1:1 with 2× Laemmli sample buffer containing 15 mg/ml DTT (Sigma, The Netherlands). Afterwards the samples were heated for 2 min at 95 °C to denature the proteins. Aliquots of protein were applied to an 8 % SDS-polyacrylamide gel, and SDS-PAGE was carried out for approximately 1.5 h at 100 V. The proteins were blotted for 1 h at 100 V on a Hybond-C Membrane (Amersham, UK). Subsequently, 60 min blocking of the membrane was performed using 4 % ECL blocking powder (Amersham, UK, ECL™ Advance Western Blotting Detection Kit) diluted in 50 mM Tris buffered (pH 7.6) saline containing 0.1 % (v/v) Tween20 (0.1%TBST). The membrane was incubated overnight at 4 °C with primary antibody (HSP60, LK-1, 1:100 in 0.1%TBST, 2 ng/μl), followed by 15 min (3 cycles of 5 min) of washing in 0.1 % TBST. Next, the membrane was incubated for 60 min with HRP-conjugated secondary antibody (1:20,000, goat anti-mouse, DAKO) and subsequently washed for 15 min (3 cycles of 5 min) in 0.3 % TBST. After adding the ECL™ Advance Western Blotting Detection Kit (Amersham, UK), the appropriate band corresponding to the expected molecular weight was detected using the Chemi-Doc imager (Bio-Rad, The Netherlands) and Quantity One Analysis software (Bio-Rad, The Netherlands). The blots were stripped using the Restore™ Western Blot stripping buffer (Thermo Scientific, IL, USA) and the same protocol was applied to incubate the antibody for beta-actin (for loading control). Blot images were processed in Image J 1.43u (National Institute of Health, USA), band intensity was semi-quantified, and the expression of HSP60 was normalized against beta-actin expression.
siRNA design and synthesis
Canine sequence-specific HSP60 (synonym: HSPD1) (GenBank: XM_536016) siRNA was designed on the website http://www.dharmacon.com/designcenter/designcenterpage.aspx (Dharmacon RNAi Technologies, ThermoScientific, USA). Universal mock siRNA was used as the negative control for siRNA experiments. The designed siRNA sequences were blasted against the canine genome database to ensure there was no cross-silencing of non-target genes. The sequence of the canine-specific HSP60 siRNA duplex is as follows: sense—GUGAAUACGAAAAGGAAAAUU and antisense—UUUUCCUUUUCGUAUUCACUU. Cell transfections were optimized using siGLO (Dharmacon, Colorado USA) in 24-well plates (Primaria, BD, The Netherlands) using the transfection reagent DharmaFECT1 (Dharmacon, Colorado, USA). A concentration curve of 0–100 nM siGLO was tested together with a DharmaFECT1 range of 0.5 μl to 2.5 μl per well at different cell densities for several incubation periods ranging from 24 to 96 h. The transfection efficiencies were assessed by determining the ratio between the fluorescent (transfected) cells and the non-fluorescent (untransfected) cells. The highest number of cells transfected with high cell viability was observed for transfections performed on 40,000 cells/well seeded in a 24-well plate with 50 nM siGLO and 2 μl DharmaFECT1 transfection reagent.
siRNA in vitro transfections and RNA isolation
Two canine OS cell lines, HMPOS and KOS-003, were selected for the siRNA-based functional assays. Cells were cultured in antibiotic-free media, and passages from 5 to 12 were used for the transfection protocols and cell culture experiments. Transfections were performed in 24-well plates (Primaria,BD The Netherlands) or 96-well plates (Cell Bind, Corning The Netherlands), depending on the type of cell-based experiment that was performed. Cells were seeded at a density of either 40,000 or 6,400 cells/well for the 24-wells or 96-wells plate setup. Transfections were carried out according to the siGLO-optimized conditions described above. Briefly, cells grown in antibiotic-free media were seeded in culture medium with FBS and allowed to attach over 12 h. Next, 70 % confluent cells were washed once with HBSS and replaced with media without FBS. The cells were transfected with 50 nM HSP60 siRNA or mock (non-targeting siRNA) and 2 μl DharmaFECT1 and diluted in RNase- and serum-free DMEM according to the manufacturer’s instructions for 12 h at 37 °C. Next, the transfection media was removed and replaced with complete DMEM media with 10 % FBS, and the cells were further incubated for 24 to 96 h. The same protocol was employed for the 96-well plate transfections with a ratio of 1:5 compared to the 24-well plate set ups. Cells were lysed for RNA from 24-well plates at several time points between 18 and 84 h post-transfection. Cell lysis was performed by adding 250 μl RLT buffer into each well, and total RNA was isolated using the RNeasy mini kit according to the manufacturer’s protocol (Qiagen, The Netherlands). Synthesis of cDNA was performed using 0.5 μg total RNA in a 20 μl reaction volume using the iScript™ cDNA synthesis kit (Bio-Rad, The Netherlands).
MTT cell proliferation assay
Cell proliferation was determined by means of the colorimetric 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay (MTT, Sigma, The Netherlands). Briefly, cells seeded in 24-well plates were transfected according to experimental setup, and cell viability was determined by incubating 100 μl of MTT solution (5 mg/ml) in each well with 500 μl media at several time points (24, 48, 72, and 96 h). After 2 h of incubation, the culture media was removed, and 200 μl of dimethylsulfoxide was added to each well. The dissolved blue formazan crystals were transferred to a 96-well plate, and absorbance was measured at 595 nm with a reference reading at 650 nm. The background absorbance intensity was subtracted, and cell viability was calculated as a percentage of absorbance of the non-treated cells. The measurements are represented as the average and standard deviation of two independent experiments that were performed in triplicate.
DNA content measurement
Total DNA content was measured at several time points after siRNA transfections using the PicoGreen® dsDNA Quantification Reagent (Molecular Probes, Inc., USA). Briefly, the cells were washed once with HBSS and freeze–thawed at −70 °C thrice for a duration of 1 h per cycle. The DNA standard curve was prepared according to the manufacturer’s protocol, and DNA content measurements were carried out in 96-well flat-bottomed plates (Greiner). The fluorescence signal, measured as relative fluorescence units, was quantified at 485 nm excitation and 535 nm emission.
Tritium-thymidine incorporation assay
Cells were seeded at a density of 40,000 cells/well and siRNA transfection protocols were performed in 24-well Multiwell™ Primaria plates (Falcon®, USA) according to the protocol described above. The assay was performed on cells at 72 h post-transfection with the siRNA protocol. To each well, 0.1 μCi/mL tritium-thymidine (GE Healthcare, UK) was added and incubated for 4.5 h. After 4.5 h, the culture medium was removed, and the cells were washed with HBSS prior to adding 0.1 mL of 0.2 % SDS. The cells were further incubated for 15 min, and then 400 μl RNAse-free water was added. Three milliliters of scintillation fluid was added to 400 μl cell lysate, and tritium-thymidine incorporation was measured by a liquid scintillation analyzer (TRI-CARB 2900TR, Packard, USA). DNA content from 6 independent wells for each cell line was measured in parallel with the tritium-thymidine incorporation assay. Experiments were performed in six-well plates in triplicate and repeated twice. Differences in tritium-thymidine incorporation after normalization for DNA content (siRNA-treated/mock cells) were calculated using the student’s t test and p < 0.05 was considered significant.
Apoptosis assay
Cells transfected with siRNA for 24, 48 and 72 h in opaque 96-well plates (CellBind, Corning) were incubated with 20 μl/well viability/cytotoxicity reagent (ApoTox-Glo™ Triplex assay kit, Promega Benelux, The Netherlands) for 2 h. The fluorescence measurement was performed using the Infinite 200, Tecan (Belgium) software according to the manufacturer’s recommendations. Next, 100 μl Caspase-Glo 3/7 reagent was added to measure apoptosis and incubated for 1 h before measuring the luminescence (Centro, Berthold, Belgium) according to the manufacturer’s instructions.
Statistical analyses
Differences in gene expression between groups of tumors with regard to clinical or pathological parameters were analyzed using REST2008 software. Univariate and multivariate survival analyses (Cox’s proportional hazard model) were performed to determine the prognostic value of the various HSPs. Kaplan–Meier survival curves were drawn to show differences in survival between groups. The corresponding hazard ratio (HR) and 95 % confidence interval (CI) were reported, with statistical significance defined as p < 0.05. SPSS16 software (IBM Company, Chicago, USA) was used for the statistical analyses. Statistical significance was defined as p < 0.05.
Results
Retrospective analysis of clinical and histological data
The 58 dogs included for qPCR analyses varied in clinicopathological parameters (Table 1). The median disease-free interval (DFI) was 111 days, and the median survival time (ST) was 161 days. ST was defined as days from initial diagnosis until death, and DFI was defined as days from initial diagnosis until the observed occurrence of metastasis or the recurrence of the tumor. The samples represent several large to medium-sized breeds and were collected from the predilection sites, including the proximal humerus, distal femur, and distal radius. Multiple chemotherapeutic protocols were employed across the dogs, i.e., single treatment with lobaplatin or carboplatin and combination therapies with carboplatin/doxorubicin or carboplatin/cisplatin. When performing group comparison analyses, the histological subtypes were divided into purely osteoblastic tumors and mixed tumors that were purely chondroblastic, fibroblastic, or telangiectatic or a combination of these subtypes. Most of the tumors were of high histological grade (Grade 3) and therefore considered highly malignant. As there were fewer tumors with medium or low histological grades, these tumors were combined to address a lower malignant class of OS for further statistical analyses. Dogs that were diagnosed with OS but did not undergo amputation or primary tumor resection due to the presence of metastatic disease or owner refusal of further therapy were only included for the assessment of gene expression correlation with few clinicopathological parameters and were excluded from survival analysis. Dogs that died for reasons other than tumor burden or metastatic disease were censored for survival analysis. Dogs that were lost to follow-up were censored as well, but the ST and DFI were recorded until the time they were lost to follow-up.
Univariate analysis of the variables (age, pre-surgical serum alkaline phosphatase levels, tumor histology grade, initiation of postoperative chemotherapy and tumor histology subtype) was performed. There was a positive impact of postoperative chemotherapy on survival; dogs without postoperative chemotherapy had a higher risk (HR 1.844, p = 0.048) of death than dogs who did receive postoperative chemotherapy. Because postoperative chemotherapy has a recognized influence on survival time, univariate analyses were repeated upon stratification for postoperative chemotherapy. Clinicopathological parameters including elevated pre-surgical serum alkaline phosphatase (AP) levels (p = 0.043) and histological-osteoblastic subtype (p = 0.005) were significantly associated with poorer survival on univariate analysis upon stratification for postoperative chemotherapy (Table 2). Multivariate analysis, stratified for postoperative chemotherapy, showed statistical significance for tumor subtype (HR = 0.341; 95 % CI = 0.169–0.688, p = 0.003). Therefore, the histology subtype was an independent prognosticator for the present dataset of canine OS.
Clinical and pathological association of the mRNA expression of various HSPs
A geNorm algorithm approach was first used to test for the optimal number of reference genes to be included in the expression analysis; a combination of five reference genes (RPS5, RPL8, RPS19, HNRNPH, and GAPDH) in the present dataset, resulted in the optimal number of reference genes for normalization. Quantitative real-time PCR primers and their corresponding annealing temperatures are provided in Table 3. Associations of the relative gene expression of HSPs with the various clinicopathological parameters are presented (Table 4). HSP60 and HSP10 were upregulated in osteoblastic tumors compared to mixed subtype tumors. HSP90beta was downregulated in older dogs (>5 years of age) and HSP40 was downregulated in highly necrotic (>50 %) tumors compared to low necrotic (<50 %) tumors. Additionally, HSP22 was augmented in appendicular tumors of dogs that did not respond well to postoperative chemotherapy and that lived less than 6 months compared to those dogs that survived longer than 6 months. This overexpression remained significant even upon inclusion of the seven dogs with OSs in axial locations (n = 58, HR = 1.694, p = 0.024). No significant differences in HSP gene expression were observed with regard to histological grade. HSP27, -70 and -90alpha did not display significant associations with any of the clinicopathological parameters assessed.
Prognostic value for heat shock protein mRNA expression in tumors
HSP10, -22, -27, -40, -70, -90alpha and -90beta mRNA expression (continuous data) did not show significant associations with survival time on univariate Cox regression analysis. However, a significant negative association between HSP60 and survival time was noted. Stratification for postoperative chemotherapy did not result in a significant change to the other HSPs, while the HSP60 association retained its significance (Table 5). However, when all parameters with a p value < 0.15 were analyzed by multivariate methods (backward stepwise LR), HSP60 did not retain its significance, and only tumor histology subtype was a strong predictor for survival among the 58 dogs with OS.
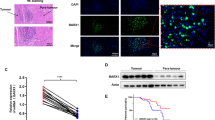
HSP60 protein expression and localization in tumors and cell lines
The panel of HSP mRNA expression was observed in all of the eight canine OS cell lines (Fig. 1). Because HSP60 appeared to be significantly associated with poor survival-based on tumor mRNA expression data, this protein was further selected for immunohistochemical analysis. A western blot analysis was carried out using total protein lysate isolated from eight canine OSs and three human OS cell lines. This analysis was performed to demonstrate the cross-species reactivity and specificity of the antibody used for subsequent immunohistochemistry and to quantify expression across the cell lines. A band corresponding to 60 kDa was detected by western blot in all OS cell lines (Fig. 2a) with, in the cases of KOS-001 and -004, an additional band due to potential post-translational modifications. Immunohistochemistry for HSP60 was performed on 32 of the histological sections available from the tumors used for the mRNA expression analysis. HSP60 immunoreactivity was detected as granular cytoplasmic staining in the canine OS tissues, with higher expression and intensity found within the multinucleated giant cells and less expression in tumor osteoblasts (Fig. 2b). A heterogeneous immune staining was observed within the tumor. Survival analyses revealed a trend for HSP60 protein expression comparable to the results of mRNA expression; medium/high HSP60 protein expression was detected for the 32 tumor sections. The comparison of tumors with positive staining based on the aforementioned classification of the 32 tumors did not reach significance for either survival (log rank score = 1.857, p = 0.173) or the disease-free interval (log rank score = 1.928, p = 0.165) (Fig. 3). However, a trend toward poor prognosis for tumors expressing high levels of HSP60 was demonstrated on Kaplan–Meier survival analysis, with a more than 1.8-fold higher risk for death. These tests revealed no significant difference in data distribution between the two groups (high vs. low HSP60 expression) with respect to sex, neuter status, postoperative chemotherapy, body weight, histological grade and subtype (Table 6).
Heat shock protein mRNA expression in canine osteosarcoma cell lines. Target gene mRNA expression was calculated based on the standard line calculated efficiencies (MyIQ software) and normalized with reference genes (geometric mean of GAPDH and RPS19). The normalized expression from three serial RNA samples of cells grown under a sub-confluent monolayer in normal culture conditions was averaged, and the data are presented with ± SD
Immunohistochemistry and western blot analyses for HSP60 in canine and human OS. a HSP60 protein expression was normalized to loading control beta-actin expression. Variable HSP60 expression levels were detected for eleven OS cell lines (3 human cell lines, U2OS, MG63, and SaOS2, and 8 canine cell lines). All cell lines demonstrated a main band at 60 kDa for the HSP60 antibody LK-1; b Immunohistochemistry of HSP60 in primary canine and human OS: (a) human OS with high expression in osteoblasts and osteoclasts; (b) high expression in canine OS; (c) canine OS tumor negative for expression of HSP60; (d) granular, cytoplasmic expression in multinucleated cells and tumor osteoblasts of canine OS; (e) moderate to high expression in canine OS; (f) heterogeneous expression within the same tissue section of a canine OS; (g) and (h) scattered, weak intensity in osteoblasts; (i) canine liver tissue (positive control)
Downregulation of HSP60 expression by siRNA
To investigate the biological roles of HSP60 in canine OS, canine-specific siRNA from predicted HSP60 sequences were custom designed to selectively inhibit gene expression. Two canine OS cell lines, HMPOS and KOS-003, with metastatic characteristics were used. HSP60 siRNA-transfected cells clearly demonstrated downregulation of HSP60 mRNA from as early as 12 to 60 h post-transfection (Fig. 4a). KOS-003 was observed to have a maximum of approximately 70 % HSP60 mRNA downregulation compared to 90 % in HMPOS (Fig. 4b), and obvious protein downregulation was observed from 48 to 96 h for both cell lines on western blot assay (Fig. 4c).
HSP60 mRNA and protein expression upon targeting with canine-specific HSP60 siRNA in two OS cell lines. a HSP60 mRNA expression was normalized to reference genes (RPS5 and GAPDH), and the relative expression is depicted from pooled expression data from four experimental RNA samples assayed in duplicate by qPCR. b HSP60 siRNA efficiently downregulated HSP60 mRNA expression by up to 70 % for KOS-003 and by 90 % in HMPOS relative to mock-transfected cells. c Western blot analyses reveal a consistent decrease in HSP60 total protein expression at 72 h post-transfection compared to mock-transfected cells and cells grown under normal culture conditions
Cell proliferation of both canine OS cell lines was significantly inhibited by HSP60 siRNA, as demonstrated by a decrease of cell counts and total DNA content in time (Fig. 5). Cells transfected with siRNA were subjected to cell cycle analysis after 72 h of transfection. Tritium-thymidine incorporation after normalization for DNA content revealed significantly lower numbers of siRNA-treated HMPOS cells in the S phase of the cell cycle after 4.5 h of incubation, suggesting that the growth velocity of HMPOS was inhibited. Intriguingly, KOS-003 cells did not show a significant difference in tritium-thymidine incorporation. Both cell lines demonstrated enhanced caspase-3/7 activity after siRNA transfections, suggesting that downregulation of HSP60 in these two OS cells promotes apoptosis. The most significant activation of caspase-3/7 was observed at 24 h post-transfection (p < 0.05) for both cell lines; however, the activity decreased over time in both HMPOS and KOS-003 (Fig. 6).
HSP60 siRNA inhibition of cell proliferation observed from different assays. a, b Pico green DNA content assay. A significant decrease in DNA content was measured from 72–96 h post-transfection (bars represent triplicate measurements (±) SD). c MTT assay demonstrates significant cell growth inhibition in both cell lines over time. d Tritium-thymidine incorporation was significantly decreased in HMPOS (p < 0.001), but no differences were observed in KOS-003 cells treated with siRNA compared to mock controls (bars represent average of 6 experimental wells repeated twice, and the ratios of tritium incorporation (in counts per minute) to the total DNA content (in micrograms per milliliter) are averaged and presented with ±SD)
Apoptosis was increased significantly as observed by induction of caspase-3/7 in both a HMPOS (57 % relative to mock) and b KOS-003 (48 % increase relative to mock) cells at 24 h post-transfection. A subsequent decline in caspase-3/7 activity was observed at 48 and 72 h. (*p < 0.05, two-sided unpaired Student’s t test). The caspase activity was calculated after subtracting background luminescence
Discussion
Clinical and pathological relevance for HSP expression in canine OS
In the present study, we evaluated the expression of several heat shock proteins in a cohort of 58 canine primary appendicular tumors and 8 OS cell lines. In accordance with previous clinicopathological studies (Garzotto et al. 2000; Kirpensteijn et al. 2002; Misdorp and Hart 1979; Selvarajah et al. 2009), the osteoblastic subtype and increased serum alkaline phosphatase were associated with poor survival in dogs with OS in this dataset. The relative mRNA expression of HSP60 and -10 had significant overexpression in canine OS with predominant osteoblastic histology compared to mixed histology (osteoblastic with chondroblastic, fibroblastic, or telangiectatic combinations). In reports examining human OS, a strong correlation between HSP27 and histological subtype has been found (Moon et al. 2010). Studies on canine malignant mammary tumors have revealed enhanced HSP27 and -90 expressions compared to normal mammary gland tissues, while HSP27 is associated with invasive stages and correlates with shorter post-surgical survival in dogs. HSP27 expression in canine OS was not associated with clinicopathological parameters (Romanucci et al. 2006).
One of the major challenges of managing dogs with OS is to inhibit metastasis, as metastasis is almost inevitable and is commonly associated with resistance to conventional chemotherapies. In the canine OS tumors in this study, HSP22 was found to be significantly over-expressed in tumors from dogs that responded poorly to postoperative chemotherapy regimes. Because limited investigations have focused on the roles of HSP22 in cancer and resistance to chemotherapy, this finding deserves further exploration. However, HSP27, which functions closely with HSP22 (a small heat shock protein) (Benndorf et al. 2001), was observed to be associated with resistance to chemotherapy and poor prognosis in human OS (Uozaki et al. 1997), while inhibition of this protein was found to enhance the response to zoledronic acid therapy (Morii et al. 2010).
Of the eight HSPs investigated for prognosis, only HSP60 mRNA expression was significantly and inversely associated with survival time. In a previous microarray study on canine OS tissues, HSP60, -70, and -90α mRNA expression was found to be associated with poor survival (Selvarajah et al. 2009). Although we found an overexpression of HSP70 or HSP90α in poor survivors in the present study, these associations were not statistically significant in the larger series used for qPCR. Further immunohistochemical staining for HSP70 and HSP90 on canine tumor tissue sections should clarify the prognostic value of these HSPs in canine OS.
Apart from the HSP expression in canine OS tumors, we have shown that the various HSP mRNAs are also expressed in canine OS cell lines. Additionally, among the clinically relevant HSPs from the mRNA screening in canine tumors, HSP60 was selected for further protein expression and functional analyses. HSP60 expression in all canine OS cell lines was comparable to the three human OS cell lines by western blot. This examination was essential to prove the specificity of the antibody for canine tissues. HSP60 protein expression was observed in osteoblast-type tumor cells, and it was highly expressed in giant cell types (osteoclasts), as demonstrated by immunohistochemistry performed on both dog and human OS tumor sections. However, not all tumors were found to express this protein, and due to the heterogeneity of expression within individual tumor sections, the expression alone did not reveal statistical significance upon prognostic evaluations. There was a clear trend of higher expression correlating with poorer outcome in dogs with OS. Unfortunately, the small number of cases made statistical analyses of the complicated data cumbersome. Although HSP60 was associated with survival time, this result should be confirmed using a larger study. Analysis of large-scale prognosis-associated gene expression is limited because of heterogeneous histology characteristics, the variable HSP60 protein expression within each tumor, the limited clinical data available, and the non-standardized chemotherapy protocols and follow-up times. Prognostic studies for HSP60 protein expression have been performed on several human tumors, including OS (Cappello 2003; Cappello et al. 2003; 2005; Faried et al. 2004; Moon et al. 2010), and have been associated with discrepancies in the prognostic evaluation of HSP60 on human OS tissue sections by means of immunohistochemistry (Moon et al. 2010; Uozaki et al. 2000). However, the great similarity in tumor histology, characteristics, and gene expression found between dog and human OS (Paoloni et al. 2009) suggests that the molecular function of HSP60 in OS pathogenesis contributes to the aggressive phenotype and deserves further exploration, including its subcellular localization and complex formation with HSP10.
HSP60 as a relevant therapeutic target for subset of canine OS
Among the different approaches for cancer therapy, RNA interference offers a mechanism that facilitates the sequence-specific gene expression silencing of a protein that is highly expressed in tumors. This approach is normally evaluated on cancer cells in vitro, and the specificity for targeting a tumor cell type from the surrounding normal cells needs to be determined before they can be further translated into the clinical setting. In this study, we used small interfering RNA (siRNA) to investigate the cellular effects of HSP60 downregulation in two canine OS cell lines. The hypothesis was that HSP60 is essential for cell cycle and proliferation and that downregulation would elicit apoptotic effects in OS. Inhibiting HSP60 in canine cells is novel, but this approach has already shown promising effects on human cancer cells, including OS. A study on human OS cells has demonstrated that a stable transfected short hairpin RNA that targets HSP60 resulted in cell growth arrest (Kaul et al. 2006). By down regulating this protein in vitro, both canine OS cell lines demonstrated increased apoptosis for the first 24 h, which declined over time. Cell proliferation of both KOS-003 and HMPOS was significantly inhibited by downregulation of HSP60. However, only in HMPOS, also a lower tritium-thymidine incorporation was observed at 72 h suggesting that lower cell numbers were a combined effect of transiently increased apoptosis and a lower amount of cells in S-phase whereas in KOS-003 cells the transient increased apoptosis at 24 h seemed to be solely responsible for cell growth inhibition.
The potential role for cytosolic HSP60 in carcinogenesis has been suggested; however, its role in tumor cell survival is controversial because both pro-apoptotic and anti-apoptotic (pro-survival) functions have been reported. HSP60 is involved in forming a complex with HSP10 and the apoptotic protein pro-caspase-3 (Samali et al. 1999). In vitro experiments have demonstrated a stimulatory effect of HSP60 on pro-caspase-3 activation by cytochrome c and dATP. Therefore, HSP60 may be involved in activating pro-caspase-3 by folding/maintaining this protein in a protease-insensitive conformation. Other investigations have revealed that cytosolic HSP60 accumulation is commonly seen during apoptosis induction. However, this accumulation occurs with or without apparent mitochondrial HSP60 release. It seems that HSP60 possesses a pro-apoptotic role when a significant mitochondrial HSP60 release is present. In contrast, a pro-survival role is present when HSP60 accumulates without mitochondrial release. These variations are the consequence of differential interactions with caspase-3 (Chandra et al. 2007). The canine OS cells demonstrated early caspase-3/7 induction, which was apparent within 24 h of siRNA targeting. However, this induction decreased over time. The ratio and composition of HSP60 within the mitochondrial or cytosolic compartments in these canine cells was not investigated in this study, and further exploration is needed to determine whether the differences in caspase activity and cell cycle are due to differences in the role of HSP60 resulting from the different cellular localization.
In conclusion, canine OS primary tumors and cell lines express several HSP members. HSP60 mRNA was associated with poor clinical outcome. Heterogeneous protein expression was observed in OS of both humans and dogs. Gene silencing using siRNA approaches against HSP60 promotes apoptosis and inhibits cell proliferation of canine OS cells in vitro. These studies therefore affirm the suggested development of HSP60 targeting drugs for anticancer therapies (Pace et al. 2012) which could be tested in dogs and also used for human OS studies.
References
Akyol S, Gercel-Taylor C, Reynolds LC, Taylor DD (2006) HSP-10 in ovarian cancer: expression and suppression of T-cell signaling. Gynecol Oncol. doi:10.1016/j.ygyno.2005.11.014
Benndorf R, Sun X, Gilmont RR, Biederman KJ, Molloy MP, Goodmurphy CW, Cheng H, Andrews PC, Welsh MJ (2001) HSP22, a new member of the small heat shock protein superfamily, interacts with mimic of phosphorylated HSP27 ((3D)HSP27). J Biol Chem. doi:10.1074/jbc.M103001200
Burel C, Mezger V, Pinto M, Rallu M, Trigon S, Morange M (1992) Mammalian heat shock protein families. Expression and functions. Experientia
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. doi:10.1016/j.tibs.2006.01.006
Cappello F, Conway de Macario E, Marasa L, Zummo G, Macario AJ (2008) Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol Ther
Cappello F, David S, Rappa F, Bucchieri F, Marasa L, Bartolotta TE, Farina F, Zummo G (2005) The expression of HSP60 and HSP10 in large bowel carcinomas with lymph node metastase. BMC Cancer. doi:10.1186/1471-2407-5-139
Cappello F (2003) HSP60 and HSP10 as diagnostic and prognostic tools in the management of exocervical carcinoma. Gynecol Oncol
Cappello F, Rappa F, David S, Anzalone R, Zummo G (2003) Immunohistochemical evaluation of PCNA, p53, HSP60, HSP10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Res
Chandra D, Choy G, Tang DG (2007) Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: evidence that its pro-apoptotic or pro-survival functions involve differential interactions with caspase-3. J Biol Chem. doi:10.1074/jbc.M702777200
Chu RM, Sun TJ, Yang HY, Wang DG, Liao KW, Chuang TF, Lin CH, Lee WC (2001) Heat shock proteins in canine transmissible venereal tumor. Vet Immunol Immunopathol
Faried A, Sohda M, Nakajima M, Miyazaki T, Kato H, Kuwano H (2004) Expression of heat-shock protein Hsp60 correlated with the apoptotic index and patient prognosis in human oesophageal squamous cell carcinoma. Eur J Cancer. doi:10.1016/j.ejca.2004.08.013
Garzotto CK, Berg J, Hoffmann WE, Rand WM (2000) Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. J Vet Intern Med
Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. doi:10.1126/science.1068408
Helmbrecht K, Zeise E, Rensing L (2000) Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif
Hendrick JP, Hartl FU (1993) Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. doi:10.1146/annurev.bi.62.070193.002025
Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. doi:10.1007/s12192-008-0068-7
Kaul Z, Yaguchi T, Kaul SC, Wadhwa R (2006) Quantum dot-based protein imaging and functional significance of two mitochondrial chaperones in cellular senescence and carcinogenesis. Ann N Y Acad Sci
Kirpensteijn J, Kik M, Teske E, Rutteman GR (2008) TP53 gene mutations in canine osteosarcoma. Vet Surg
Kirpensteijn J, Kik M, Rutteman GR, Teske E (2002) Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet Pathol
Kumaraguruparan R, Karunagaran D, Balachandran C, Manohar BM, Nagini S (2006) Of humans and canines: a comparative evaluation of heat shock and apoptosis-associated proteins in mammary tumors. Clin Chim Acta. doi:10.1016/j.cca.2005.08.018
Levine RA, Forest T, Smith C (2002) Tumor suppressor PTEN is mutated in canine osteosarcoma cell lines and tumors. Vet Pathol
McCleese JK, Bear MD, Fossey SL, Mihalek RM, Foley KP, Ying W, Barsoum J, London CA (2009) The novel HSP90 inhibitor STA-1474 exhibits biologic activity against osteosarcoma cell lines. Int J Cancer. doi:10.1002/ijc.24660
Misdorp W, Hart AA (1979) Some prognostic and epidemiologic factors in canine osteosarcoma. J Natl Cancer Inst
Mitra A, Shevde LA, Samant RS (2009) Multi-faceted role of HSP40 in cancer. Clin Exp Metastasis. doi:10.1007/s10585-009-9255-x
Moon A, Bacchini P, Bertoni F, Olvi LG, Santini-Araujo E, Kim YW, Park YK (2010) Expression of heat shock proteins in osteosarcomas. Pathology. doi:10.3109/00313025.2010.493866
Morello E, Martano M, Buracco P (2010) Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differences with human osteosarcoma. Vet J. doi:10.1016/j.tvjl.2010.08.014
Morii T, Ohtsuka K, Ohnishi H, Mochizuki K, Satomi K (2010) Inhibition of heat-shock protein 27 expression eliminates drug resistance of osteosarcoma to zoledronic acid. Anticancer Res
Norrdin RW, Powers BE, Torgersen JL, Smith RE, Withrow SJ (1989) Characterization of osteosarcoma cells from two sibling large-breed dogs. Am J Vet Res
Pace A, Barone G, Lauria A, Martorana A, Piccionello AP, Pierro P, Terenzi A, Almerico AM, Buscemi S, Campanella C, Angileri F, Carini F, Zummo G, de Macario EC, Cappello F, Macario AJ (2012) Hsp60, a novel target for antitumor therapy: Structure-function features and prospective drugs design. Curr Pharm Des
Paoloni M, Davis S, Lana S, Withrow S, Sangiorgi L, Picci P, Hewitt S, Triche T, Meltzer P, Khanna C (2009) Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics. doi:10.1186/1471-2164-10-625
Romanucci M, D'Amato G, Malatesta D, Bongiovanni L, Palmieri C, Ciccarelli A, Buracco P, Morello E, Maniscalco L, De Maria R, Martano M, Della Salda L (2012) Heat shock protein expression in canine osteosarcoma. Cell Stress Chaperones. doi:10.1007/s12192-011-0294-2
Romanucci M, Bastow T, Della Salda L (2008) Heat shock proteins in animal neoplasms and human tumours—a comparison. Cell Stress Chaperones. doi:10.1007/s12192-008-0030-8
Romanucci M, Marinelli A, Sarli G, Della Salda L (2006) Heat shock protein expression in canine malignant mammary tumours. BMC Cancer. doi:10.1186/1471-2407-6-171
Romanucci M, Bongiovanni L, Marruchella G, Mara M, di Guardo G, Preziosi R, della Salda L (2005) Heat shock proteins expression in canine intracutaneous cornifying epithelioma and squamous cell carcinoma. Vet Dermatol. doi:10.1111/j.1365-3164.2005.00436.x
Ru G, Terracini B, Glickman LT (1998) Host related risk factors for canine osteosarcoma. Vet J
Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S (1999) Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. doi:10.1093/emboj/18.8.2040
Selvarajah GT, Kirpensteijn J (2010) Prognostic and predictive biomarkers of canine osteosarcoma. Vet J. doi:10.1016/j.tvjl.2010.04.010
Selvarajah GT, Kirpensteijn J, van Wolferen ME, Rao NA, Fieten H, Mol JA (2009) Gene expression profiling of canine osteosarcoma reveals genes associated with short and long survival times. Mol Cancer. doi:10.1186/1476-4598-8-72
Sun X, Fontaine JM, Bartl I, Behnam B, Welsh MJ, Benndorf R (2007) Induction of Hsp22 (HspB8) by estrogen and the metalloestrogen cadmium in estrogen receptor-positive breast cancer cells. Cell Stress Chaperones
Trieb K, Gerth R, Holzer G, Grohs JG, Berger P, Kotz R (2000a) Antibodies to heat shock protein 90 in osteosarcoma patients correlate with response to neoadjuvant chemotherapy. Br J Cancer. doi:10.1054/bjoc.1999.0881
Trieb K, Gerth R, Windhager R, Grohs JG, Holzer G, Berger P, Kotz R (2000b) Serum antibodies against the heat shock protein 60 are elevated in patients with osteosarcoma. Immunobiology
Trieb K, Lechleitner T, Lang S, Windhager R, Kotz R, Dirnhofer S (1998) Heat shock protein 72 expression in osteosarcomas correlates with good response to neoadjuvant chemotherapy. Hum Pathol
Uozaki H, Ishida T, Kakiuchi C, Horiuchi H, Gotoh T, Iijima T, Imamura T, Machinami R (2000) Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathol Res Pract
Uozaki H, Horiuchi H, Ishida T, Iijima T, Imamura T, Machinami R (1997) Overexpression of resistance-related proteins (metallothioneins, glutathione-S-transferase pi, heat shock protein 27, and lung resistance-related protein) in osteosarcoma. Relationship with poor prognosis. Cancer
Wolf M, El-Rifai W, Tarkkanen M, Kononen J, Serra M, Eriksen EF, Elomaa I, Kallioniemi A, Kallioniemi OP, Knuutila S (2000) Novel findings in gene expression detected in human osteosarcoma by cDNA microarray. Cancer Genet Cytogenet
Acknowledgments
The authors would like to acknowledge the following individuals for their various contributions to the project: Malaysian Ministry of Higher Education (MOHE) for funding the PhD candidate working on this project; Peter van Kooten (Department of Infectious Diseases and Immunology, Utrecht University) for providing the LK-1 HSP60 antibody; Monique E. Van Wolferen for assisting in RNA isolation and cDNA synthesis; Annette van Drie (Department of Pathobiology, Utrecht University) for assistance with preparation of histology slides; Frank Riemers for valuable input on cell transfection protocols, siGlo transfection optimizations, western blotting and QPCR analyses; Ellen Martens for help maintaining cells in culture; Dr. Chand Khanna, Christina Mazcko (National Cancer Intsitute, NIH, USA) and Prof. Farese (University of Florida, USA) for kindly providing the canine OS cell lines utilized in this study. We also thank Jantine Posthuma de Boer from the University of Amsterdam and Dr. A.M.Cleton-Jansen from the Department of Pathobiology at the University of Leiden for providing the human OS cell lines and paraffin-embedded human OS tissue sections, respectively, for the comparative analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selvarajah, G.T., Bonestroo, F.A.S., Kirpensteijn, J. et al. Heat shock protein expression analysis in canine osteosarcoma reveals HSP60 as a potentially relevant therapeutic target. Cell Stress and Chaperones 18, 607–622 (2013). https://doi.org/10.1007/s12192-013-0414-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-013-0414-2