Abstract
Few studies have focused on the expression of heat shock proteins (HSPs) after chronic heat stress. The objective of this study was to investigate the effect of chronic high temperature–humidity index treatment on the expressions of HSP60, HSP70, HSP90, HSPA2 and HSC70, in the Rex rabbit testis and the expressions of these proteins after recovery from the chronic heat shock. Thirty mature male rabbits of the same age were randomly divided into three groups: control, heat stress, and recovery. The western blot results showed that the expressional levels of HSP60, HSP90, and HSC70 increased significantly and HSPA2 was elevated slightly after a 9-week heat treatment. HSP70 was absent in the control testis and had a high level of expression after heat stress. All of these proteins partially reverted back to normal levels after a 9-week recovery. The immunohistochemical results indicated that the expression patterns of HSP60, HSP90, HSPA2, and HSC70 did not change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All living organisms respond to environmental stresses, including hyperthermia, by synthesizing a set of proteins originally termed heat shock proteins (HSPs, Becker and Craig 1994). According to the molecular weights, mammalian HSPs are classified into different families including HSP105/110, HSP90, HSP70, HSP60, HSP40, and other small HSPs. HSPs act as molecular chaperones, assisting in the folding, assembly, and disassembly of other proteins (Wu 1995; Georgopoulos and Welch 1993). Through complex mechanisms, HSPs play a dual role in the regulation of apoptosis. They either inhibit the apoptotic response or directly promote apoptosis (Neuer et al. 2000; Sreedhar and Csermely 2004). Different HSPs have different expression profiles, are found in different cell types, and are localized to distinct cellular compartments.
HSP60 is a mitochondrial chaperon (Viitanen et al. 1992). Its major role is to facilitate the proper folding and assembly of newly imported proteins (Werner et al. 1997). HSP60 has been detected in the cytoplasm of spermatogonia, spermatocytes, and Sertoli cells in rat (Meinhardt et al. 1995) and human testis (Werner et al. 1997). It is also expressed in spermatids and Leydig cells in rabbit testis (Wu et al. 2011). A previous study indicates that it is constitutively expressed and is moderately induced in response to environmental insults such as heat (Welch 1993).
The expression of the 70-kDa heat shock cognate (HSC70) gene is both constitutively expressed and stress inducible at both the mRNA and protein levels (Brown et al. 1993; Chen et al. 1996). It is widely distributed in the spermatogonia, Sertoli cells, and round spermatids in the rabbit testis (Wu et al. 2011). In contrast, the expression of stress-inducible HSP70 is hardly detectable under normal growth conditions, but greatly elevated after exposure to environmental or physiological stresses (Brown et al. 1993; Chen et al. 1996). HSP70 is involved in cellular repair and protective mechanisms (Mosser et al. 2000). HSPA2 is developmentally regulated and is specifically expressed in spermatogenic cells. HSPA2 was specifically detected in meiotic and postmeiotic male germ cells, mainly in pachytene spermatocytes and round spermatids (Vydra et al. 2009; Wu et al. 2011).
HSP90 executes its chaperone function as part of a complex with a large number of cofactors (Wegele et al. 2003). Biggiogera et al. (1996) showed that HSP90 is present in the cytoplasm of all male germ cell types during mouse spermatogenesis. HSP90 has been detected mainly in spermatogonia and elongated spermatids in rabbit testis (Wu et al. 2011). A porcine study showed that HSP90 transcripts are constitutively expressed in porcine tissues including kidney, liver, brain, and heart, and their levels are markedly enhanced after a 30-min hyperthermia treatment at 43°C (Huang et al. 1999). Currently, there are many reports about the changes of heat shock proteins under acute heat stress conditions (Bukau and Horwich 1998; Cao et al. 2009), but there are very few reports about chronic heat treatment, especially in rabbits. Rabbits are extremely sensitive to heat stress. A rise in testicular temperature in rabbits leads to reduced spermatogenesis; temporary sterility; decreased sexual desire, ejaculate volume, motility, sperm concentration, and total number of spermatozoa in an ejaculate; and increased sperm abnormalities and dead sperm (Marai et al. 2002). And our previous study demonstrated the specific expression of HSP60, HSP90, HSPA2, and HSC70 in rabbit testis in normal condition (Wu et al. 2011). So, in the present study, we explore the expressional changes of HSP60, HSP70, HSP90, HSPA2, and HSC70 after chronic heat stress and after a 9-week recovery.
Materials and methods
Experimental animals
The experiment was conducted using artificial climate chambers at the College of Animal Science and Technology at the China Agricultural University. A 12-h light, 12-h dark (12L:12D) daily schedule was used. Thirty male Rex rabbits, 18-week (adolescent) old and with an initial body weight of ~3,000 g, were subjected to the treatments. All the rabbits were housed in 54 × 60 × 40-cm wire cages arranged in a flat-deck system, with similar management and hygienic conditions. Commercial feed pellets and water were provided ad libitum to each rabbit. The thirty rabbits were placed at 24°C with 60% relative humidity for 4 weeks prior to the start of the experiment. After that, the rabbits were adult.
To ensure that an exact temperature and humidity were maintained in each climatic chamber, hydrothermographs (TES-1361C, Taiwan) were used to record temperature and relative humidity every 5 min. From these data, mean daily air temperature and relative humidity were calculated. temperature–humidity index (THI) was estimated according to the equation developed by Marai et al. (2001) for rabbits: THI = db (in °C) − {(0.31 − 0.31 RH) × [db (in °C) − 14.4]}, where db is dry bulb temperature in degrees Celsius and RH is the relative humidity percentage/100. Calculated THI values were subsequently classified as follows: <27.8 = absence of heat stress, 27.8–28.9 = moderate heat stress, 28.9–30.0 = severe heat stress, and >30.0 = extremely severe heat stress.
Experimental design
Thirty male Rex rabbits were randomly divided into three groups: control (CON), heat stress (HS), and heat stress recovery (RE). CON rabbits were housed at a constant 24°C with 60% relative humidity. HS and RE rabbits were subjected to temperatures that fluctuated between 26°C and 36°C on a daily basis. For a detailed description of the daily temperature regimen, see Fig. 1. Relative humidity for these animals was held at 80%. These conditions imitate the daily summertime conditions of Beijing.
The temperature cycle during a single day of heat stress. HS rabbits were subjected to a heat regimen that imitates the summer climate of Beijing. From 7:00 to 11:00 hours, the temperature was gradually increased from 26°C to 36°C. The temperature was maintained at 36°C for 4 h and then gradually decreased to 26°C between 15:00 and 19:00 hours. Between 19:00 and 7:00 hours, the temperature was maintained at 26°C
Rabbits were subjected to these conditions for 9 weeks. At the end of week 9, CON and HS rabbits were sacrificed using intravenous pentobarbital sodium salt (50 mg/kg; Merck KGaA, Darmstadt, Germany). One testis from each rabbit was collected, fixed in Bouin's solution, and embedded in paraffin. The other testis was collected and frozen in liquid nitrogen for western blot analysis. Immunohistochemical staining and western blot analysis for the expression of HSP60, HSP70, 90, HSPA2, and HSC70 were respectively performed using the protocols as previously described (Wu et al. 2011).The expressions of HSPs were quantified using Gel-Pro analyzer software (Version 4.0, Media Cybernetics, America) and normalized against expressions of β-actin.
After the 9-week test period, RE rabbits were allowed to recover for an additional 9 weeks at 24°C and 60% relative humidity. Following the recovery period, their testes were collected as described above.
The THI for the CON and RE (the last 9 weeks) groups was 22.7, which indicates that rabbits living in these conditions were free of heat stress. The THI for the HS and RE (the first 9 weeks) groups was 29.3, which indicates that these rabbits suffered severe heat stress.
Statistical analysis
The data were presented as the mean ± SD of three independent experiments. Significant differences (P values <0.05) with control group were determined using general linear model with Statistical Analysis System (Version 8 of the SAS System).
Result
Expression of testicular HSP60, HSP70, HSP90, HSPA2, and HSC70
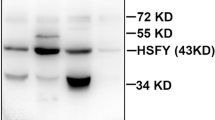
Using specific antibodies and western blot analyses, we demonstrated that HSP60, HSP90, HSPA2, and HSC70 were expressed in all rabbit testis of the three groups (Fig. 2a, lane 1). The expression levels of HSP60 (Fig. 2b), HSP90 (Fig. 2f), and HSC70 (Fig. 2c) increased significantly (P < 0.001), and the expression of HSPA2 elevated slightly (Fig. 2e, P < 0.05) after a 9-week heat treatment. HSP70 was not expressed in the testis of the control group, but was expressed at a high level after chronic heat exposure (Fig. 2a, lane 1; Fig. 2d). The expression of HSP60, HSP70, HSP90, and HSC70 decreased partially after the 9-week recovery but were still higher than the levels of the control group (Fig. 2b–d, f, P < 0.05); however, the expression level of HSPA2 recovered to the normal rate (Fig. 2e, P > 0.05).
Expression of HSPs after heat stress by western blot. a HSP60, HSC70, HSP70, HSPA2, and HSP90 were detected in the testes of control (lane 1), heat stress (lane 2), and recovery (lane 3) rabbits. β-Actin was used as a reference. Statistical analysis the expression levels of HSP60 (b), HSC70 (c), HSP70 (d), HSPA2 (e), and HSP90 (f) in CON, HS, and RE rabbits. Band intensity was measured using Gel-Pro analyzer software and normalized against that of β-actin. Data were presented as the mean ± SD and analyzed using general linear model. **P < 0.001, *P < 0.05
Immunolocalization of HSP60, HSPA2, HSP90, and HSC70 in the testis
The immunohistochemistry (IHC) result revealed that the distributions of HSP60, HSP90, HSPA2, and HSC70 did not change after the high temperature treatment and the 9-week recovery. In the testis of the three groups, HSP60 was localized in the cytoplasm of the spermatogonia, Sertoli cells, Leydig cells, and in the apical pole of the spermatids (Fig. 3a–c). HSC70 staining was seen in the cytoplasm of the spermatogonia, spermatocytes, and spermatid heads (Fig. 3d–f). The HSPA2 protein immunoreactivity was detected in the cytoplasm of pachytene spermatocytes and round spermatids (Fig. 3g–i). IHC results showed specific staining of HSP90 in elongated spermatids and spermatogonia (Fig. 3j–l).
Localization of HSP60, HSC70, HSPA2, and HSP90 proteins in the rabbit testes of control rabbits (CON), 9-week heat stress treated rabbits (HS), and recovery rabbits (RE). a HSP60 of control, b HSP60 of heat stress, c HSP60 of recovery, d HSC70 of control, e HSC70 of heat stress, f HSC70 of recovery, g HSPA2 of control, h HSPA2 of heat stress, i HSPA2 of recovery, j HSP90 of control, k HSP90 of heat stress, and l HSP90 of recovery. HSP60, HSC70, HSPA2. Positive staining is shown as brown. Spg spermatogonium, Spc spermatocyte, R round spermatids, S Sertoli cell, E elongated spermatid. Bar 20 μm
Immunolocalization of HSP70 in the testis
Immunohistochemical results showed that HSP70 was not expressed in the rabbit testis of the control and recovery groups (Fig. 4a, c). However, HSP70 was expressed in the nuclei of the Leydig cells, spermatids, and spermatocytes after heat stress (Fig. 4b).
Discussion
We investigated the expression levels and cellular localizations of heat shock proteins 60, 70, 90, A2, and HSC70 in the normal, heat-treated, and recovery rabbit testis. The results show that chronic heat stress affects the expressional levels but not the localizations of HSP60, HSP70, HSP90, HSPA2, and HSC70 in the rabbit testis.
The expression pattern of HSP60 did not change, and the HSP60 signals were elevated significantly after chronic heat stress. That is in agreement with previous observations in the testis of cryptorchid monkeys (Tao et al. 2006) and in rat skeletal muscles after heat stress (Oishi et al. 2002). In the present study, the expression level of HSP60 after the 9-week recovery period was still higher than that of the control. A possible reason for that is because the recovery period was not long enough. Zhang et al. (2005) have shown that HSP60 levels did not reach pretreatment levels until 84 days after cessation of the heat treatment in adult cynomolgus monkey testis. Considering the protective function of HSP60 as to cellular apoptosis (Lin et al. 2001) and the wide expression of HSP60 in the rabbit semiferous probably suggests that the increase in HSP60 exerts a multifunctional protective role in the testis during heat shock.
Our results in the rabbit testis showed that inducible HSP70 protein was induced by chronic heat stress. This finding has been confirmed by studies in murine testis (Zaprjanova et al. 2011) and rat skeletal muscles (Oishi et al. 2002). Cao et al. (2009) demonstrated that the dramatic increase of HSP70 expression after heat stress might be responsible for the protection of cells from high temperature. Our unpublished data suggest that chronic heat shock triggers apoptosis in germ cells, especially in the spermatogonia. The immunohistochemical findings in the present study showed an intensive expression of HSP70 in the spermatogonia. This induction of HSP70 is to potentially reduce apoptosis.
HSC70 is a constitutively expressed protein, and it can also be induced by heat stress. The expression of HSC70 was shown to increase significantly in porcine embryos after hyperthermia treatment (Bernardini et al. 2004). Our current results in the rabbit testis agree with these results. With regard to its function, Zaprjanova et al. (2011) reported that in heat stress conditions, HSC70 is mobilized to prevent apoptosis in testis and to assist HSP70 in its protective function by repairing proteins whose conformation is altered by stress.
In this study, HSPA2 levels increased slightly after chronic heat shock without changes in localization. However, Ścieglińska et al. (2008) found that at physiological temperature, HSPA2 was localized primarily in the cytoplasm whereas, during heat shock, localization shifted to the nucleus and nucleoli. Zhou et al. (2001) found that there was no difference in the levels of HSPA2 expression between the contralateral scrotal testis and normal testis at any times. HSPA2 has a modest role in the maintenance of germ cell viability during the middle part of the pachytene spermatocyte development and is essential for the viability of late pachytene spermatocytes (Eddy 1999). The continuous presence of HSPA2 is necessary for preventing apoptosis, enabling the cells to complete the cell cycle in normal and heat-treated spermatogenesis (Zhou et al. 2001).
The localization of HSP90 did not change in our study, and the western blot results show that the expression of HSP90 is elevated after chronic heat stress. These results are in agreement with previous reports in mouse testis (Biggiogera et al. 1996), rat liver (Cvoro and Matić 2000), and porcine tissues (Huang et al. 1999). Grad et al. (2010) speculated that HSP90 may be required to maintain and activate the meiotic regulators and HSP90 interactors (HSPA2, NASP, and Cdc2) and/or to disassemble the synaptonemal complex that holds homologous chromosomes together. Therefore, elevated expression of HSP90 may have a protective role in spermatogenesis under thermal stress.
In the present study, we used the heating condition that imitated the summer climate in Beijing because data from practical breeding showed reproductive ability of male Rex rabbit was decreased apparently under this climate. And our result demonstrated this heat stress condition significantly influenced the expressions of HSPs in rabbit testes. This conformed the finding in human testes that variation of HSPs is associated to abnormal spermatogenesis and male infertility (Feng et al. 2001; Adly et al. 2008).
Actually, this summer climate also probably has an adverse effect on human fertility because data from Hebei province, which abuts Beijing, indicated that sperm concentration, sperm count per ejection, sperm progressive motility (%), and sperm viability (%) of healthy men were lowest in summer (Gao et al. 2007). Although the mechanism that seasonal change influences man semen quality has not been explored yet, it probably has a close relation with the expression of HSPs in the testes as mentioned above. However, presently, there is no report about the expressional changes of HSPs in the human testes under heat stress. The present study has potential as an important reference for human study because the distribution patterns of HSP60 (Werner et al. 1997), HSP70 (Widłak 2006), HSP90 (Liu et al. 2004), and HSPA2 (Son et al. 1999) in male testes at normal condition are similar with those of rabbit. Thus, this study will be valuable for detecting the changes and functions of HSPs in human testes under heat stress and be helpful to further understand the mechanism of hyperthermia that affects the human fertility.
In conclusion, the present study shows that all the expression levels of HSP60, HSP70, HSP90, HSPA2, and HSC70 increased after a 9-week heat treatment and are partially restored to normal levels after an additional 9-week recovery, although to different degrees. Their elevated expressions after heat treatment is most likely due to their protective roles in repairing the damage caused by heat stress. However, further studies are necessary to further elucidate their exact roles.
Abbreviations
- HSP:
-
Heat shock protein
- THI:
-
Temperature–humidity index
- HSC:
-
Heat shock cognate protein
- CON:
-
Control
- HS:
-
Heat stress
- RE:
-
Heat stress recovery
- IHC:
-
Immunohistochemistry
References
Adly MA, Assaf HA, Hussein MR (2008) Heat shock protein 27 expression in the human testis showing normal and abnormal spermatogenesis. Cell Biol Int 32:1247–1255
Becker J, Craig EA (1994) Heat-shock proteins as molecular chaperones. Eur J Biochem 219:11–23
Bernardini C, Fantinati P, Zannoni A, Forni M, Tamanini C, Bacci ML (2004) Expression of HSP70/HSC70 in swine blastocysts: effects of oxidative and thermal stress. Mol Reprod Dev 69:303–307
Biggiogera M, Tanguay RM, Marin R, Wu Y, Martin TE, Fakan S (1996) Localization of heat shock proteins in mouse male germ cells: an immunoelectron microscopical study. Exp Cell Res 229:77–85
Brown CR, Martin RL, Hansen WJ, Beckmann RP, Welch WJ (1993) The constitutive and stress inducible forms of hsp70 exhibit functional similarities and interact with one another in an ATP-dependent fashion. J Cell Biol 120:1101–1112
Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92:351–366
Cao W, Huang P, Zhang L, Wu HZ, Zhang J, Shi FX (2009) Acute heat stress increases HSP70 expression in the testis, epididymis and vas deferens of adult male mice. Nat J Androl 15:200–206
Chen MS, Featherstone T, Laszlo A (1996) Amplification and altered expression of the hsc70/U14 snoRNA gene in a heat resistant Chinese hamster cell line. Cell Stress Chaperones 1:47–61
Cvoro A, Matić G (2000) Glucocorticoid receptor interaction with Hsp90 remains unaltered after whole body hyperthermia. Stress 3:257–260
Eddy EM (1999) Role of heat shock protein HSP70–2 in spermatogenesis. Rev Reprod 4:23–30
Feng HL, Sandlow JI, Sparks AE (2001) Decreased expression of the heat shock protein hsp70-2 is associated with the pathogenesis of male infertility. Fertil Steril 76:1136–1139
Gao J, Gao ES, Yang Q, Walker M, Wu JQ, Zhou WJ, Wen SW (2007) Semen quality in a residential, geographic and age representative sample of healthy Chinese men. Hum Reprod 22:477–484
Georgopoulos C, Welch WJ (1993) Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Dev Biol 9:601–634
Grad I, Cederroth CR, Walicki J, Grey C, Barluenga S, Winssinger N, de Massy B, Nef S, Picard D (2010) The molecular chaperone hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS One 5:e15770
Huang HC, Lee WC, Lin JH, Huang HW, Jian SC, Mao SJT, Yang PC, Huang TY, Liu YC (1999) Molecular cloning and characterization of porcine cDNA encoding a 90-kDa heat shock protein and its expression following hyperthermia. Gene 226:307–315
Lin KM, Lin B, Lian IY, Mestril R, Scheffler I, Dillmann WH (2001) Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation 103:1787–1792
Liu Z, Wang G, Pan Y, Zhu C (2004) Expression of androgen receptor and heat shock protein 90alpha in the testicular biopsy specimens of infertile patients with spermatogenic arrest. Zhonghua Nan Ke Xue 10:662–666
Marai IFM, Ayyat MS, Abd El-Monem UM (2001) Growth performance and reproductive traits at first parity of New Zealand white female rabbits as affected by heat stress and its alleviation under Egyptian conditions. Trop Animal Health Prod 33:451–462
Marai IFM, Habeeb AAM, Gad AE (2002) Rabbits' productive, reproductive and physiological performance traits as affected by heat stress: a review. Livest Prod Sci 78:71–90
Meinhardt A, Parvinen M, Bacher M, Aumuller G, Hakovirta H, Yagi A, Seitz J (1995) Expression of mitochondrial heat shock protein 60 in distinct cell types and defined stages of rat seminiferous epithelium. Biol Reprod 52:798–807
Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massi B (2000) The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biochem 20:7146–7159
Neuer A, Spandorfer SD, Giraldo P, Dieterle S, Rosenwaks Z, Witkin SS (2000) The role of heat shock proteins in reproduction. Hum Reprod Update 6:149–159
Oishi Y, Taniguchi K, Matsumoto H, Ishihara A, Ohira Y, Roy RR (2002) Muscle type-specific response of HSP60, HSP72, and HSC73 during recovery after elevation of muscle temperature. J Appl Physiol 92:1097–1103
Ścieglińska D, Wojciech P, Mazurek A, Małusecka E, Zebracka J, Filipczak P, Krawczyk Z (2008) The HspA2 protein localizes in nucleoli and centrosomes of heat shocked cancer cells. J Cell Biochem 104:2193–2206
Son WY, Hwang SH, Han CT, Lee JH, Kim S, Kim YC (1999) Specific expression of heat shock protein HspA2 in human male germ cells. Mol Hum Reprod 5:1122–1126
Sreedhar AS, Csermely P (2004) Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy—a comprehensive review. Pharmacol Ther 101:227–257
Tao SX, Guo J, Zhang XS, Li YC, Hu ZY, Han CS, Liu YX (2006) Germ cell apoptosis induced by experimental cryptorchidism is mediated by multiple molecular pathways in Cynomolgus Macaque. Front Biosci 11:1077–1089
Viitanen PV, Lorimer GH, Seetharam R, Gupta RS, Oppenheim J, Thomas JO, Cowan NJ (1992) Mammalian mitochondrial chaperonin 60 functions as a single toroidal ring. J Biol Chem 267:695–698
Vydra N, Winiarski B, Rak-Raszewska A, Piglowski W, Mazurek A, Scieglinska D, Widlak W (2009) The expression pattern of the 70-kDa heat shock protein Hspa2 in mouse tissues. Histochem Cell Biol 132:319–330
Wegele H, Haslbeck M, Reinstein J, Buchner J (2003) Sti1 is a novel activator of the Ssa proteins. J Biol Chem 278:25970–25976
Welch WJ (1993) Heat shock proteins functioning as molecular chaperones: their roles in normal and stressed cells. Philos Trans R Soc Lond B Biol Sci 339:327–333
Werner A, Meinhardt A, Seitz J, Bergmann M (1997) Distribution of heat-shock protein 60 immunoreactivity in testes of infertile men. Cell Tissue Res 288:539–544
Widłak W (2006) The heat shock response and HSP70 gene expression in male germ cells. Postepy Biochem 52:289–295
Wu C (1995) Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol 11:441–469
Wu YJ, Pei YL, Qin YH (2011) Developmental expression of heat shock proteins 60, 70, 90, and A2 in rabbit testis. Cell Tissue Res 344:355–363
Zaprjanova S, Rashev P, Zasheva D, Martinova Y, Mollova M (2011) Electrophoretic analysis of Hsp72 and Hsp73 expression in male reproductive tissues. CR Acad Bulg Sci 64:529–534
Zhang XS, Lue YH, Guo SH, Yuan JX, Hu ZY, Han CS, Sinha Hikim AP, Swerdloff RS, Wang C, Liu YX (2005) Expression of Hsp105 and Hsp60 during germ cell apoptosis in the heat-treated testes of adult cynomolgus monkeys (Macaca fascicularis). Front Biosci 10:3110–3121
Zhou XC, Han XB, Hu ZY, Zhou RJ, Liu YX (2001) Expression of Hsp70-2 in unilateral cryptorchid testis of rhesus monkey during germ cell apoptosis. Endocrine 16:89–95
Acknowledgments
This work was supported by the Earmarked Fund for Modern Agro-Industry Technology Research System (CARS-44) and the Natural Science Foundation of China (31001009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yangli Pei and Yingjie Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Pei, Y., Wu, Y. & Qin, Y. Effects of chronic heat stress on the expressions of heat shock proteins 60, 70, 90, A2, and HSC70 in the rabbit testis. Cell Stress and Chaperones 17, 81–87 (2012). https://doi.org/10.1007/s12192-011-0287-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-011-0287-1