Abstract
It has been previously reported that the plasma levels of autoantibodies against heat shock protein 70 (HSP70) are elevated in atherosclerosis. The aim of the present study was to elucidate whether anti-HSP70 antibodies are involved in the pathogenesis of atherosclerosis. To determine this, we chose rats as an atherosclerosis model. Titers of plasma anti-HSP70 autoantibody were determined by ELISA. After the intravenous administration of antibody into the tail, the damaged areas of aorta were stained with Evans Blue, atheromatous plaque were stained by Oil Red O, and then they were measured and quantified with AxioVision computer software. The number of macrophages (\( {{\hbox{M}}_\Phi } \)), smooth muscle cells (SMCs), and T cells were determined by immunocytochemistry. The level of anti-HSP70 IgG1 antibody was apparently increased in the AS group at the tenth week, and one hybridoma of HSP70 antibody (BD091, IgG1, recognizing C-terminal) had the same binding epitope as plasma anti-HSP70 autoantibodies. After intravenous administration, the lesion area of aorta with BD091 was significantly larger than those of IgGmouse and SPA-810. Moreover, injection of BD091 resulted in significant endothelium damage, followed by a greater accumulation of \( {{\hbox{M}}_\Phi } \), T cells, and SMCs in lesions than in the control. In conclusion, BD091 reaction with HSP70 expressed on arterial endothelial cells inducing endothelium damage triggers the inflammatory response in the vessel wall that accelerates atherosclerosis in rats. BD091 shares the same binding epitope with HSP70 autoantibodies. These data indicated that a specific epitope of anti-HSP70 autoantibody participated in the pathogenesis of atherosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Atherosclerosis, in which inflammation and autoimmunity have been associated with pathogenesis of the disease (Libby 2002; Wick et al. 2004), is a leading a cause of morbidity and mortality. Different autoantigens, such as oxidized low-density lipoprotein (LDL; Binder et al. 2002), glycoproteins, and heat shock proteins (HSPs; Xu 2002), have implicated HSPA1A in the development of atherosclerosis by induction of an autoimmune process. HSP70, a member of the HSP family, physiologically protects the cells from damage induced by different intracellular stimuli. Higher serum Hsp70 levels were associated with a reduced risk of atherosclerosis. Hsp70 is released in myocardial infarction; serum levels after acute myocardial infarction were higher in patients with angina. Some researchers have found a protective role for HSP70 in atherosclerosis and myocardial ischemia (Xu 2002; Williams and Benjamin 2000). Hsp70 can be recognized by the immune system, which generates autoantibodies in various rheumatic and autoimmune disorders (Wu and Tanguay 2006). The induction of self-HSP immune reactivity is thought to be involved in the pathogenesis of atherosclerosis. Thus, once Hsps are released from the cell, they may become the target of humoral and T-cell-mediated immune responses (Williams and Benjamin 2000). Accumulating evidence from human seroepidemiological studies show that HSP70 autoantibodies are associated with the progression and severity of atherosclerosis (Bobryshev and Lord 2002; Pockley et al. 2003; Chan et al. 1999). Immunization with HSP70 has been known to prevent disease in experimental models of autoimmunity (Tanaka et al. 1999; Wendling et al. 2000). Until now, however, there is no direct evidence to demonstrate that HSP70 autoantibodies were directly involved in atherosclerosis. Moreover, the exact effect of HSP70 autoantibodies in atherosclerosis was unclear. Previous studies have found that different epitopes of autoantibodies play different roles in the progression of atherogenesis, such as autoantibodies to oxidized low-density lipoprotein (OxLDL) and HSP60. These results may give us a new way to think about HSP70 autoantibodies. The epitope of HSP70 autoantibodies may be changed in the progression of atherogenesis.
We therefore hypothesized that autoantibodies to HSP70 may be involved in the pathogenesis of atherosclerosis. In the present study, we aimed to identify whether anti-HSP70 autoantibodies play a role in the induction of atherosclerosis in animal models and to describe the effect of anti-HSP70 autoantibody in the formation of atherosclerosis.
2 Materials and methods
2.1 Animals
All animal experiments were performed according to protocols approved by the Institutional Committee for the Use and Care of Laboratory Animals. The mice and rats were maintained on a light/dark (12/12 h) cycle at 22°C and received food and water ad libitum. The hybridoma cell lines producing the mouse monoclonal antibody (mAb) used in all mice in the present study was C57BL/6 bred. Rats used in the present study were 10-week-old male Sprague-Dawley rats of 200-g weight and bred in our laboratory. All the animals used in this work received humane care in compliance with institutional animal care guidelines. Animal care was approved by the Local Institutional Committee.
2.2 Induction of atherosclerosis and pathological study
Each experimental group consisted of eight rats that were fed either a normal diet or, with the addition of a single dose of vitamin D3 (600,000 unit/kg, i.p.; Sigma, China), were fed with a high-cholesterol diet for 10 weeks to induce atherosclerosis (Cai et al. 2005). The high-cholesterol diet contained 3% cholesterol, 0.5% cholic acid, 0.2% 6-propyl 2-thiouracil, 5% sucrose, 3% (w/w) lard (Sigma, China), and 81.3% regular rat chow. At the end of treatment, rats were anesthetized by intraperitoneal injection of pentobarbital natrium (50 mg/kg body weight; Sigma, China). Plasma was separated from blood samples, and total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL) were measured by using corresponding kits supplied by Boehringer Mannheim (Boehringer Mannheim, France S.A.). The aorta was fixed and removed for freezing section preparation and stained with Oil Red O (Paigen et al. 1987) and hematoxylin and eosin (HE staining). Aortas were opened longitudinally and fixed on a silicon bed with stainless steel minutien pins (Fine Science Tool) with the intima exposed. The Oil-Red-O-stained areas were measured and quantified with AxioVision computer software.
2.3 SDS–PAGE and Western blotting
Binding epitopes of nonreduced and reduced rat endothelial cell (EC) HSP70 were assessed by Western blot analysis. Equal amounts of protein preparation (20 μg in 25 μl buffer) were run on sodium dodecyl sulfate (SDS)–polyacrylamide gels (PAGE), electrotransferred to polyvinylidene difluoride membranes, and blotted with an additional primary antibody against β-tubulin (Boster, China), anti-HSP70 antibody (SPA-810), and HSP70 monoclonal antibody (CG024, DB107, BC082, BD091, CD032, DE104). Cross-reactivity with HSP60 was assessed identically as previously described, and 50 μg protein preparation was loaded onto SDS–polyacrylamide gels. After electrotransferred, the membranes were incubated with BD091 or anti-HSP60 antibody (Hsp60 monoclonal antibody (Mab11-13) Stressgen, SPA-829). Molecular band intensity was determined by densitometry using image software.
To express N-terminal (amino acid (aa) 1–383, N70) or C-terminal (aa 384–642, C70) substrate binding domain of HSP70 (Multhoff et al. 2001), the DNA encoding N- and C-terminal fragment Hsp70 was amplified by polymerase chain reaction with the following primers,: 5′-CGGAATTCATGGCCAAGAAAACAGCGATC-3′ (the EcoRI site underlined) and 5′-CGGGATCCTCCCCCATCAGGATGGCCGCC-3′ (the BamHI site underlined) for N70; 5′-CGGAATTCCAAGTCGGAGAACGTGCAGG-3′ (the EcoRI site underlined) and 5′-CGGGATCCCTAATCCACCTCCTCGATGG-3′ (the BamHI site underlined) for C70. The amplified product was digested with EcoRI and BamHI and inserted into the complementary sites in pET28a vector (Novagen) to generate pet-N70 and pet-C70. DNA sequences were determined by Invitrogen. All these recombinant plasmids were transformed into DE3, and expression was induced by IPTG (1 mmol/l, 25°C, 4 h). The recombinant protein was transferred onto nitrocellulose membranes. The membranes were probed with different primary antibodies. Molecular band intensity was determined by densitometry using image software.
2.4 Purification of antibody and intravenous administration
To create monoclonal antibodies, splenocytes and draining LN cells from a C57BL/6 mouse immunized with HSP70 were fused with the SP2/0-AG14 myeloma cell line by the standard protocol to establish hybridomas. Successful fusions were screened by Western blot and selected based on specific reactivity with rat HSP70. Six positive IgG-producing hybridomas, CG024, DB107, BC082, BD091, CD032, DE104, were cloned further and studied. Purification of ascites anti-HSP70 antibodies was performed following an established method (Rahbarizadeh et al. 2000). Reactivity of the purified monoclonal antibody was confirmed by application of Western blots and competition enzyme-linked immunosorbent assay (ELISA). Purification of atherosclerotic plasma anti-HSP70 autoantibodies was performed following an established method (Mayr et al. 1999). Pooled rat plasma was precipitated by a standard (NH4)2SO4 procedure and incubated in a chromatography column with 2 ml agarose gel beads (Affi-Gel Kit, Bio-Rad) coupled with 3 mg recombinant HSP70 (Stressgen). Specific Igs were recovered by 20 mmol/l HCl acid elution, pooled, and equilibrated with phosphate-buffered saline (PBS), pH 7.2. Titers of purified Igs were similar to original plasma (1:2,560). Rats received an intravenous injection every week. All the groups followed the administration protocol described above and were fed with a high-cholesterol diet.
2.5 Autoantibody measured by ELISA
Titers of plasma HSP70 antibody isotypes were determined by ELISA. In brief, microtiter plates were coated with recombinant HSP70 (Hsp70 Recombinant Rat Protein, Stressgen, SPP-758D), dissolved in PBS at a concentration of 1 μg/ml overnight at 4°C, and then washed three times. After blocking with 5% BSA overnight at 4°C, plates were washed three times. One-hundred-microliter samples in duplicate were added to wells of plate and incubated at 37°C for 30 min. Wells were washed three times with 1× PBST (PBS/0.5% Tween-20). Then, 100 µl (1:1,000 in PBS; horseradish peroxidase (HRP)-conjugated) goat antirat IgM (Bethyl Laboratories, A110-100P), IgA (A110-102A), IgG1 (A110-106P), IgG2a (A110-109P), IgG2b (A110-111P), and IgG (Abcam, UK, ab6120) were added to the plates, which were then incubated at 37°C for 20 min. One-hundred-microliter TMB substrate was added to each well and incubated at 37°C for 15 min. ELISA was developed using 100 μl per well and 2 M H2SO4 and read at 405 nm with a 492-nm reference filter.
Competition ELISA was developed to compare the monoclonal antibody binding to the solid-phase antigen in the competing plasma HSP70 autoantibodies. Microtiter plates coated with HSP70 were the same as ELISA. Then, the wells were incubated overnight at 4°C with a variety of concentrations of anti-HSP antibodies, including Ig with CG024, DB107, BC082, CD032, DE104, IgGmouse (SC-2026 Santa Cruz Biotechnology, Inc.), SPA-810 (Stressgen, C92F3A-5), IgAs (atherosclerotic rat Igs), and IgNor (normal diet rat Igs). Plates were washed three times with PBST and incubated with BD091 conjugated with biotin for 1 h at 37°C. Plates were washed three times again with PBST. Subsequently, streptavidin–HRP was visualized with substrate. A sample was considered positive for BD091 if the optical density at 450 nm exceeded 0.200.
2.6 Endothelial damage assays in vivo
Evans blue dye stains blue in the areas where the endothelium is damaged or dysfunctional (Kwei et al. 2004). Eight rats were treated intravenously with BD091 five times or once intraperitoneally with lipopolysaccharide (LPS) (5 mg/kg), which is a reagent known to induce endothelial damage. At the end of 5 weeks, rats received intravenous 1% Evans blue dye injection into the tail vein 48 h after treatment. Their aortas were harvested and opened. Blue areas were measured with AxioVision.
2.7 Endothelial cytotoxicity assays
For LDL isolation and oxidation, EDTA-treated plasma was pooled from normolipemic, fasting (12 to 14 h) male donors, aged 20 to 30 years. Lipoproteins were prepared by differential centrifugation using solid KBr to adjust the density, as described previously (Jürgens et al. 1989; Xu et al. 1992). LDL were obtained in fraction between 1.019 and 1.055 g/ml. Concentrations of LDL were determined gravimetrically by aliquot weight after drying, and quantities of lipoproteins were expressed as total weights (Jürgens et al. 1989; Xu et al. 1992). LDL oxidation was performed by incubation of LDL (1 mg/ml PBS) with 10 mmol/l CuCl 2 at 37°C for 18 h (Amberger et al. 1997). The extent of oxidation was assessed by measurement of thiobarbituric acid reactive substances (9.6 ± 1.1 nmol/mg; Buege and Aust 1978). The OxLDL preparation was filtered through 0.22-μm filters and stored at 4°C. The protein content of OxLDL was determined by BCA reagents (Pierce).
Rat aortic endothelial cells were prepared as described previously (Kreisel et al. 2001). For antibody-mediated cytotoxicity, endothelial cells were preincubated with 20 μg/ml OxLDL (ox pretreatment) at 37°C for 12 h and then kept in M199 medium at 37°C for another 90 min, whereas control cells were preincubated with PBS (endothelial cells were not obviously damaged by OxLDL at 20 μg/ml concentration, data not shown). After three washes with medium 199, different antibodies (BD091, SPA-810, IgAs, IgNor, IgGmouse; 200) were added and incubated for 7 h at 37°C in the presence of guinea pig serum (50 μl, 1140; Behringwerke) as a complementary source. Guinea pig serum was selected to be free of anti-hsp70 Ab (ELISA). At the end of the incubation period, 100 μl of cold (4°C) 10% FCS was added, and the plates were centrifuged at 800×g for 10 min at 4°C. One hundred microliters of supernatant was removed, and lactate dehydrogenase (LDH) was determined spectrophotometrically at 340 nm using a commercial kit as recommended by the manufacturer (Sigma).
2.8 Immunostaining
Cryosections of aortic roots from the BD091- and SPA-810-treated groups were used. The sections were stained for smooth muscle cells with a mouse monoclonal antibody against α-actin (SMαA) antibodies (Boster, China, 1:10) by immunohistochemistry. SMαA is considered the most sensitive, although not a specific, marker for plaque SMCs (Hoofnagle et al. 2006) because of its high occurrence in atheromatous plaque. For macrophage and T cell staining, a similar protocol was used. The section was probed at 4°C overnight with MAC-1 antibody (Boster, China, 1:10) or T cell marker antibody (SC52711, Santa Cruz Biotechnology, Inc., 1:100). The secondary antibody was goat antimouse conjugated with horseradish peroxidase (DAKO Corp; 1:50). Total and positive-stained cells were counted under the microscope.
2.9 Statistical analysis
Values are expressed as medians and quartiles or mean ± SEM when appropriate. Statistical analyses were performed with the Mann–Whitney U test and ANOVA, respectively. Results are given as mean ± SEM. A value of p < 0.05 was considered significant.
3 Results
3.1 The titers of anti-HSP70 autoantibody isotypes in atherosclerosis
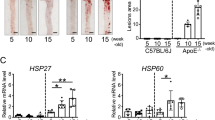
Atherosclerosis was observed in all the SD rats in the atherosclerotic group at 10 weeks. At 10 weeks, the pathological changes that occurred in rats were very similar to those in humans (Fig. 1a), and plaques were observed in the aorta in the As group (Fig. 1b). Because the rat is an atherosclerosis-resistant species (Moghadasian 2002), we found that a high-cholesterol diet alone did not induce rat atherosclerosis (data not shown). So in this experiment we combined vitamin D3 and a high-cholesterol diet to induce rat atherosclerosis (Cai et al. 2005). We found that there was no significant difference between the control in Fig. 1d and 0w in Fig. 1e (data not shown). We concluded that the level of plasma HSP70 autoantibodies was not significantly changed after 10 weeks of normal diet.
The level of plasma anti-HSP70 antibody in rat atherosclerosis. Atherosclerosis in rats on hyperlipidemic diet for 10 weeks. a The pathological changes of rat aortae (HE, ×200). b Representative photomicrographs of Oil Red O staining of the aortae. c Total plasma IgG, d the titers of HSP70 autoantibody isotypes at 10 weeks, and e the titers of HSP70 autoantibody isotypes in atherosclerosis group were measured by ELISA as described in “Materials and methods.” Values are mean ± SD from three independent experiments, *p < 0.05 compared with control
To further characterize the development of HSP70 reactivity in atherosclerosis, we analyzed the titers of HSP70-reactive antibody isotypes. Total levels of anti-HSP70 autoantibodies increased markedly in atherosclerosis (Fig. 1c), consistent with previous reports (Bobryshev and Lord 2002). Markedly elevated levels of IgG1 anti-HSP70 autoantibodies were found along with less pronounced levels of IgG2a, IgG2b, and IgA, while the level of IgM anti-HSP70 autoantibodies slightly increased (Fig. 1d). In the AS group, a similar trend was found between the 10th and 0th week (Fig. 1e), while in the control group, there was no significant difference between 10th and 0th week (data not shown). These results suggest that the isotype titers of anti-HSP70 autoantibodies changed in rats with atherosclerosis and increased significantly as the atherosclerosis developed (p < 0.05 compared with control).
3.2 Anti-HSP70 antibody induces endothelial damage
Observed titers of anti-HSP70 autoantibody changed in atherosclerosis. To investigate the role of different epitopes recognized by antibodies in atherosclerosis, we established six hybridomas according to standard protocol, including CG024, DB107, BC082, BD091, CD032, and DE104. We chose SPA-810 as control antibody (IgG1). Purified monoclonal antibodies were applied to demonstrate specific reactivity by Western blot analysis of nonreduced native HSP70 protein (Fig. 2a) and reduced native HSP70 protein (Fig. 2b). As a result, six clones recognized different epitopes of unreduced rat endothelial cell HSP70 (EC HSP70), while two hybridomas reacted with reduced rat EC HSP70, indicating that six monoclonal antibodies had different binding epitopes of HSP70 and were also different from SPA-810. In addition, we found that BD091 (IgG1) binding to HSP70 coated on ELISA plates was completely inhibited by the presence of rat “IgAs” purified from plasma from atherosclerotic rat by competition ELISA, whereas other mAbs or SPA-810 had no effect, indicating that BD091 shares the same binding epitope with autoantibodies in rat atherosclerosis (Fig. 2e). By using HSP70 deletion mutants that lack either the C- or N-terminal domain, the binding site of BD091 could be localized within the C-terminal substrate binding domain of HSP70 between aa 384 and 642 (Fig. 2d) and different from SPA-810 (with binding site aa 437–504; Botzler et al. 1998). These results also indicated that six HSP70 mAb had different binding sites. Schett et al. (1995) had found autoantibodies against HSP60-induced endothelial cytotoxicity by reactivity with HSP60. Can BD091 react with HSP60 and induce cell cytotoxicity? In Fig. 2c, we found that BD091 did not react with HSP60. These data suggest that the binding epitope of BD091 is different from anti-HSP60 antibody.
Representative Western blotting for (a nonreduced, b reduced) different binding epitopes of monoclonal antibodies against HSP70 (SPA-810 as control mAb antibody) and β-tubulin (as loading control) in REC and c cross-reactivity with HSP60 (1, 2 anti-HSP60 antibody as control; 3, 4 BD091; 1, 3 reduced loading buffer; 2, 4 nonreduced loading buffer). d Western blotting analysis of epitope specificities of HSP70 mAb. N-terminal fragment (N70, aa 1–383) and C-terminal fragment (C70, aa 384–642) of HSP70 were expressed in DE3. Thirty micrograms of whole bacterial lysed proteins was separated by SDS–PAGE and then transferred onto nitrocellulose membranes. The membranes were probed with different HSP70 mAb. SPA-810 (aa 437–504) was used as control mAb antibody. The data are representative of three independent experiments. e Competition ELISA using protocol described in “Materials and methods.” Purified HSP70 autoantibodies from normal diet group (IgNor) and of rats with atherosclerosis with high titers of antibodies (titer > 1:2,000; IgAs) including all Ig subtypes using HSP70-coupled column. f Comparison of complement-mediated cytotoxicity on ox-pretreated and untreated endothelial cells. Rat aortic endothelial cells in 96-well plates were treated as described in endothelial cytotoxicity assays. LDH activity was determined. g, h Evans blue dye leaking study. Rats were injected with BD091High (500 μg/100 g body weight) or LPS (100μg per animal) via the tail vein every week. At the fifth injection, rats were killed (n = 8), and aortas were harvested and washed. Blue areas, representing the damaged area of endothelium on the surface of aortas, were observed and quantified. Data are the mean of three experiments. *Significant difference from controls (p < 0.01)
To investigate the mechanisms of antibody-induced atherosclerotic lesions, vascular endothelial cells pretreated with OxLDL as described in “Materials and methods” were treated with a variety of antibodies in the presence of complement. IgGmouse was used as a control antibody described previously. In normal conditions, the activity of LDH in culture media was very low. The cellular injury caused by antibody treatment elevated the release of LDH to culture media. So the LDH activity in culture reflects the extent of cellular injury. Significant LDH activity by OxLDL-pretreated endothelial cells was induced by IgAs from high-titer plasma and BD091 but not by IgNor and SPA-810 (Fig. 2f). This effect depended on OxLDL-pretreated endothelial cells (LDH activity was not significantly different in non-OxLDL-pretreated endothelial cell groups). These data provide evidence that only Ig from high-titer plasma and BD091 has a cytotoxic effect on endothelial cells. In vivo administration of BD091 to rat resulted in extensive damage of the aortic endothelium as identified by Evans blue dye stains. After five weekly injections, blue areas (percent) on the surface of aortas were scanned and quantified after Evan’s blue injection, revealing extensive damage of the endothelium in animals treated with BD091High, twice that in untreated control (30.72% vs 12.33%; p < 0.05; Fig. 2g, h).
3.3 Anti-HSP70 antibody promoted atherosclerosis
As a further test of the pathogenic potential of antibodies against HSP70 and to explore the role of different epitopes recognized by antibodies, we introduced BD091 and SPA-810 in combination with a high-cholesterol diet into rats. SD rats were divided into four groups, normal mouse IgG (negative control, IgGmouse), SPA-810, BD091Low (100 μg/100 g body weight), BD091High (500 μg/100 g body weight). After ten injections of antibodies, a significant increase in atherosclerosis was observed in rats treated with high concentrations of BD091 antibody (Fig. 3a), and this increase was dramatic compared with the other groups. The area of atherosclerosis increased to 58.34% of the aorta. Several small lesions were also observed on the surface of the aorta treated with IgGmouse or SPA-810. However, the percentage of the lesion area was about 33.15% or 31.73%, while the lesion area was nearly 48.95% in rats treated with low concentrations of BD091 antibody (Fig. 3c). The lesion size at the thoracic aorta was evaluated by measuring the ORO-positive area present in the sections (Fig. 3b). The lesion area was lowest in rats treated with mouse IgG or SPA-810; but in the groups that received BD091 antibody injection, at either high or low concentrations, intimal thickening and accumulation of lipids were significant. The pattern was similar to that of the en face assay; thoracic aorta lesion area was nearly 42.36% and 32.16%, respectively (p < 0.05; Fig. 3d). Thus, antibody-induced endothelial damage triggers the inflammatory response in the vessel wall that accelerates atherosclerosis.
HSP70 monoclonal antibodies accelerated atherosclerosis. Representative photomicrographs of Oil Red O staining and quantitative analysis of atherosclerotic lesion size were analyzed. Two groups of rats were treated with total Ig from purified monoclonal antibody BD091 with either high (500 μg/100 g body weight) or low (250 μg/100 g body weight) concentrations of anti-HSP70 monoclonal antibody (n = 8 for both groups). Two control groups were also used. A normal mouse IgG-treated group (sc-2026 Santa Cruz Biotechnology, Inc. 500 μg/100 g body weight) was used as a negative control, and an HSP70 (SPA-810) mAb-treated group as an antibody control, each group receiving the five weekly injections described above. a Representative photograph of aortas (only one piece of aorta is shown); b representative photographs of thoracic aorta sections; c statistical data of lesions on aortic surface; d statistical data of lesions from frozen sections of thoracic aorta. e The level of blood lipids. *Significant difference from Rat IgG and SPA-810-treated controls (n = 8, p < 0.05)
Blood TC, HDL, LDL-C, and TG levels did not significantly differ between rats that received antibody injection and the untreated controls. Thus, Ig administration alone does not alter blood TC, HDL, LDL-C, and TG levels in rats (Fig. 3e).
3.4 Anti-HSP70 antibody increases artery cell accumulation
In the context of atherosclerosis (Ross 1999) or in response to arterial endothelial denudation (Lindner et al. 1993), the normally quiescent SMCs of the tunica media of the artery proliferate and migrate into the subendothelial intima and form neointimal lesions. Regulation of neointimal hyperplasia by BD091 could result from effects in two relatively distinct cellular compartments: (1) arterial SMCs, which proliferate and migrate into the neointima (Ross 1999; Lindner et al. 1993); and (2) monocyte/macrophages, which infiltrate intima and release chemokines/cytokines that induce arterial SMC proliferation and migration (Ross 1999). SMCs were considered to be the main promoter of atherosclerotic lesion formation. Proliferative response of vascular smooth muscle cells is the major cellular event in the development of the atherosclerotic plaque. Activated macrophages in lesions secrete abundant amounts of cytokines that in turn can activate EC, SMC, and macrophages/lymphocytes to foster cytokine production, leading to a self-perpetuating inflammatory process that participates in the development of atherosclerosis.
To investigate the mechanisms of anti-HSP70 antibodies that cause an acceleration in atherosclerotic lesions, we observed the numbers of SMCs and macrophages in atherosclerotic plaque. Immunostaining for the sections of aortas from BD091-treated animals displayed increased neointimal lesions as revealed by the large numbers of macrophages and smooth muscle cells (Fig. 4a). There was almost a sixfold difference in the numbers of SMCs (p < 0.05) between BD091-treated and untreated control. This result demonstrates that BD091 treatment resulted in more neointimal formation. A similar result was seen in the macrophage staining: the number of macrophages in atheromatous plaque from BD091 treatment was higher in the BD091High group than in control and SPA-810 groups (p < 0.05): Macrophages were 235 ± 35, 51 ± 6, and 52 ± 9, respectively (Fig. 4b). BD091 led to a reduction in macrophage accumulation in the lesions, a key step in atherosclerosis (Ross 1999). Meanwhile SPA-810 antibody treatment did not bring about any particular change in the number of smooth muscle cells and infiltrating macrophages compared with the untreated control animals. These data suggest that anti-HSP70-antibody-treated animals are characterized by increased neointimal formation, accompanied by significantly elevated levels of infiltrating macrophages, in the aortic roots of BD091-treated rats. More macrophages would secrete more cytokines, contributing to progression of atherosclerosis.
BD091 treatment induced increased number of cells infiltrating in atherosclerotic lesions. Rats were killed after ten injections of SPA-810, BD091High. Ten-week-old rats were injected with antibody BD091High or SPA-810 via the tail vein. Ten weeks after injection, the rats were killed, and aortic roots were prepared for sections. Frozen sections were labeled with antibodies against MAC-1, smooth muscle cell (SMC), or T cell marker antibody. The number of cells in the section was counted. Arrow indicates example of positive-stained cells. a Representative photographs of macrophage and SMC staining. c Representative photographs of T cells. b, d Quantitative data (mean ± SD) of eight animals per group. *Significant difference from controls. *p < 0.05
T lymphocytes, occurring concomitantly with macrophages, are found in human lesions in substantial numbers both in very early stages and in advanced plaques (Jonasson et al. 1986; Hansson et al. 1989). An additional possible mechanism for the acceleration of neointimal formation is the cellular immune response. As Hsp70 is a powerful immunogen, it is possible that anti-Hsp70 T cells were generated that localized to the areas of preferential Hsp70 expression (i.e., the arterial injury domains) where ligation of their receptor could have triggered a local production of cytokines that could promote smooth muscle migration and leukocyte chemoattraction. By immunohistochemistry, we found intima infiltration by large numbers of T cells in the sections of aortas from BD091-treated animals (Fig. 4c). The number of T cells was 314 ± 44, much higher than in SPA-810 and control groups (p < 0.05). They were 91 ± 17 and 78 ± 17, respectively (Fig. 4d). These data suggest that BD091 induced atherosclerosis by increasing intima infiltration by T cells and acceleration of artery wall inflammatory response.
4 Discussion
The present study examines for the first time the role of anti-HSP70 antibody in the development of atherosclerosis. We describe a damaging role for anti-HSP70 antibody during atherosclerotic lesion development in rats on a high-cholesterol diet. This damage was observed despite no obvious difference in blood lipid levels between the experimental and the control animals.
Previous studies have reported that elevated levels of HSP antibodies are detected in patients with atherosclerosis (Xu et al. 1999; Pockley 2002). The presence of antibodies to various Hsps is thought to be of significance in the generation, formation, and prognosis of diseases (Wu et al. 1998). HSP70 is one of the more widely studied HSPs. It shares many HSP class functions, seems to protect against ischemic injury, and has a cardioprotective role in several examples of myocardial stress (Suzuki et al. 2002; Yamashita et al. 1997). Zhu et al. (2003) and Pockley et al. (2003) have shown an inverse correlation between HSP70 levels and atherosclerotic disease progression. Martin-Ventura et al. also found that low plasma levels of HSP70 are found in patients with atherosclerosis. These findings prompted the notion that HSP70 may play a protective role in atherosclerosis. Despite their known protective effects, HSPs may contribute over the long term, through the development of autoantibodies, to chronic disease (as has been demonstrated for HSP60; Xu 2002; Wu et al. 1998; Martin-Ventura et al. 2007). In addition to Hsp60, Hsp65, 70 and 90 can be targeted by Abs in various rheumatic and autoimmune disorders (Wu and Tanguay 2006). Increasing evidence from human seroepidemiological studies supports our hypothesis that HSP70 autoantibodies are associated with the progression and severity of atherosclerosis (Pockley et al. 2003). Thus, we hypothesize that anti-HSP70 autoantibodies play a damaging role in the pathogenesis of atherosclerosis. Limited experimental or clinical data on the effects of HSP70 autoantibodies on atherogenesis are available. Nevertheless, we do not consider that these autoantibodies play an initial causal role in the development of atherosclerosis. Jacob et al. (2001) had found that rats immunized with HSP70 developed accelerated intimal thickening in balloon-injured carotid arteries. They observed that HSP70 IgG deposition in rat carotid arteries after balloon injury may have functional effects on the endothelial cells as has been shown for anti-Hsp65 antibodies. But animals in which the carotid arteries were balloon-injured are not really a pathophysiological model for atherosclerosis. We had found that immunization of rats with HSP70 was never shown to induce atherosclerosis in wild-type or hypercholesterolemic experimental rats. In this study, we used vitamin D3 (which can induce deposits of calcium that is involved in the process of atherogenesis) as an atherogenic agent, combined with a high-cholesterol diet in order to investigate the initiation, development, and progression of atherosclerotic lesions and advanced plaque that reproduce the classic stages already shown in human pathology (Cai et al. 2005). However, the model does not have completely similar pathogenic mechanisms to those of human atherosclerosis nor completely similar pathological changes in myocardium due to coronary atherosclerosis.
Our previous study observed that the level of rat plasma anti-HSP70 autoantibodies was associated with the progression and severity of the rat atherosclerosis model (data not shown). In this study, we found significantly higher plasma anti-HSP70 IgG1 concentrations in atherosclerosis. At the same time, Figueredo et al. (1996) found that the mean titers of IgG and IgM class anti-HSP70 did not differ significantly between diabetic patients and nondiabetic control subjects. In contrast, the mean titer of anti-HSP70 IgA antibodies was significantly higher in diabetic sera than in the control in both types of diabetes. It may be that, under different conditions (different disease), the autoantibodies present different isotypes. We also asked whether a specific epitope of anti-HSP70 autoantibodies could influence atherogenesis since epitope-specific anti-HSP70 autoantibodies may play a key role in the progression of atherosclerosis. But the level of plasma-specific epitopes of HSP70 autoantibodies was low, and it was impossible for us to purify it. Moreover, we had no information on the epitope mapping of HSP70 autoantibodies. However, we found some methods to purify autoantibodies and acquire epitope-specific HSP70 antibody by means of the hybridoma technique. By this means, six hybridomas of different epitopes were developed. It was found that only one hybridoma-BD091 shared the same binding epitope with the anti-HSP70 autoantibody in atherosclerotic rats. By Oil Red O staining, we found significant induction of atherosclerosis after BD091 administration, whereas SPA-810 did not induce it.
As mentioned, accumulating evidence has demonstrated the impact of HSPs on the pathogenesis of atherosclerosis via different mechanisms, including the involvement of soluble HSPs present in circulating blood in the induction of immune reactions. When overexpressed in response to stress, HSPs are released and translocate onto the cell surface, where cryptic antigens are detected by the immune surveillance system, thereby triggering an immune response. Establishing the increased anti-HSP70 autoantibodies in atherosclerosis, we hypothesize that atherosclerosis results from endothelial cell damage induced by antibody-mediated cytotoxicity. As a result, we demonstrated that BD091 (which was a binding site within the HSP70 C-terminal) has endothelial cytotoxic effects, significant endothelial damage in the aorta after BD091 treatment. SPA-810 which has a binding site on HSP70 of aa 437–504 does not have cytotoxic effects on cells in vitro, consistent with the result of Georg Schett et al. (1995). These results highlighted the importance of the specific epitope sequence for the process. After BD091 binding to endothelium, there was increased neointimal formation, accompanied by significantly elevated levels of infiltrating macrophages and T cells in the aortic roots of BD091-treated rats. Thus, antibody-induced endothelial damage triggers the inflammatory response in the vessel wall that accelerates atherosclerosis. More T cells would secrete more cytokines that contribute to the progression of atherosclerosis.
In conclusion, this is the first study to demonstrate that anti-HSP70 antibody plays a key role in the progression of atherosclerosis. It promotes atherosclerosis in rats through induced endothelium damage. Although our results provide evidence for a damaging effect of the anti-HSP70 antibody, we only studied the effect of BD091 (IgG1), and an increased level of IgM may also be involved in initiating the progression of atherosclerosis. IgM antibody increased markedly in the early phase in atherogenesis. In addition, we studied the effect of anti-HSP70 antibody in a rat model. Whether this antibody plays the same role in human atherosclerosis remains to be investigated. Studies to elucidate the specific epitope sequence of this potentially pathogenic Ab are under way. Regardless of whether anti-HSP70 autoantibodies act in the same manner as human HSP70 autoantibodies, their increased level in the plasma of atherosclerotic animals suggests that they play a role in the progression of atherosclerosis. Anti-HSP70 antibodies may be a new therapeutic target for atherosclerosis.
Abbreviations
- SMCs:
-
Smooth muscle cells
- IgG:
-
Immunoglobulin G
- SPA-810:
-
Anti-HSP70 monoclonal antibody (binding site, 437-504)
- mAb:
-
Monoclonal antibody
- \( {{\hbox{M}}_\Phi } \) :
-
Macrophage
- ELISA:
-
Enzyme-linked immunosorbent assay
- BD091:
-
Anti-HSP70 mAb antibody (which recognized HSP70 C-terminal)
- LDH:
-
Lactate dehydrogenase
- PBS:
-
Phosphate-buffered saline
- AS:
-
Atherosclerosis
- HRP:
-
Horseradish peroxidase
- TMB:
-
Tetramethyl benzidine
- OxLDL:
-
Oxidized low-density lipoprotein
- aa:
-
amino acid
References
Amberger A, Maczek C, Jürgens G, Michaelis D, Schett G, Trieb K, Eberl T, Jindal S, Xu Q, Wick G (1997) Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial venous endothelial cells in response to cytokines and oxidized low density lipoproteins. Cell Stress Chaperones 2:94–103
Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL (2002) Innate and acquired immunity in atherogenesis. Nat Med 8:1218–1226
Bobryshev YV, Lord RS (2002) Expression of heat shock protein-70 by dendritic cells in the arterial intima and its potential significance in atherogenesis. J Vasc Surg 35:368–375
Botzler C, Li G, Issels RD, Multhoff G (1998) Definition of extracellular localized epitopes of Hsp70 involved in an NK immune response. Cell Stress Chaperones 3:6–11
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cai GJ, Miao CY, Xie HH, LH LU, DF SU (2005) Arterial baroreflex dysfunction promotes atherosclerosis in rats. Atherosclerosis 183:41–47
Chan YC, Shukla N, Samee-M A, Berwanger CS, Stanford J, Singh M, Mansfield AO, Stansby G (1999) Anti-heat-shock protein 70 kDa antibodies in vascular patients. Eur J Vasc Endovasc Surg 18:381–385
Figueredo A, Ibarra JL, Rodriguez A, Molino AM, Gomez-de la Concha E, Fernandez-Cruz A, Patiño R (1996) Increased serum levels of IgA antibodies to HSP70 protein in patients with diabetes mellitus: their relationship with vascular complications. Clin Immunol Immunopathol 79:252–255
Hansson GK, Holm J, Jonasson L (1989) Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol 135:169–175
Hoofnagle MH, Thomas JA, Wamhoff BR, Owens GK (2006) Origin of neointimal smooth muscle: we’ve come full circle. Arterioscler Thromb Vasc Biol 26:2579–2581
Jacob G, Shai G, Iris B, Madhavir S, Sara P-C, Shlomo L, Gad K (2001) Accelerated intimal thickening in carotid arteries of balloon-injured rats after immunization against heat shock protein 70. JACC 38(5):1564–1569
Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK (1986) Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 6:131–138
Jürgens G, Xu QB, Huber LA, Böck G, Howanietz H, Wick G, Traill KN (1989) Promotion of lymphocyte growth by high density lipoproteins (HDL): physiological significance of the HDL binding site. J Biol Chem 264:8549–8556
Kwei S, Stavrakis G, Takahas M, Taylor G, Folkman MJ, Gimbrone MA Jr, García-Cardeña G (2004) Early adaptive responses of the vascular wall during venous arterialization in mice. Am J Pathol 164:81–89
Kreisel D, Krupnick AS, Szeto WY, Popma SH, Sankaran D, Krasinskas AM, Amin KM, Rosengard BR (2001) A simple method for culturing mouse vascular endothelium. J Immunol Methods 254:31–45
Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874
Lindner V, Fingerle J, Reidy MA (1993) Mouse model of arterial injury. Circ Res 73:792–796
Martin-Ventura JL, Leclercq A, Blanco-Colio LM, Egido J, Rossignol P, Meilhac O, Michel JB (2007) Low plasma levels of HSP70 in patients with carotid atherosclerosis are associated with increased levels of proteolytic markers of neutrophil activation. Atherosclerosis 194:334–341
Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q, Wick G (1999) Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation 99:1560–1566
Moghadasian MH (2002) Experimental atherosclerosis. A historical overview. Life Sci 70:855–865
Multhoff G, Pfister K, Gehrmann M, Hantschel M, Gross C, Hafner M, Hiddemann W (2001) A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones 6:337–344
Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA (1987) Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis 68:231–240
Pockley AG (2002) Heat shock proteins, inflammation, and cardiovascular disease. Circulation 105:1012–1017
Pockley AG, Georgiades A, Thulin T, de Faire U, Frostegård J (2003) Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension 42:235–238
Rahbarizadeh F, Rasaee MJ, Madani R, Rahbarizadeh MH, Omidfar K (2000) Preparation and characterization of specific and high-affinity monoclonal antibodies against morphine. Hybridoma 19:413–417
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126
Schett G, Xu Q, Amberger A, Van der Zee R, Recheis H, Willeit J, Wick G (1995) Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest 96:2569–2577
Suzuki K, Murtuza B, Sammut IA, Latif N, Jayakumar J, Smolenski RT, Kaneda Y, Sawa Y, Matsuda H, Yacoub MH (2002) Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia–reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation 106(12 Suppl 1):I270–I276
Tanaka S, Kimura Y, Mitani A, Yamamoto G, Nishimura H, Spallek R, Singh M, Noguchi T, Yoshikai Y (1999) Activation of T cells recognizing an epitope of heat-shock protein 70 can protect against rat adjuvant arthritis. J Immunol 163:5560–5565
Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W (2000) A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-HSP70 homologue. J Immunol 164:2711–2717
Wick G, Knoflach M, Xu Q (2004) Autoimmune and inflammatory mechanisms in atherosclerosis. Ann Rev Immunol 22:361–403
Williams RS, Benjamin IJ (2000) Protective responses in the ischemic myocardium. J Clin Invest 106:813–818
Wu T, Tanguay RM (2006) Antibodies against heat shock proteins in environmental stresses and diseases: friend or foe? Cell Stress Chaperones 11:1–12
Wu T, Yuan Y, Wu Y, He H, Zhang G, Tanguay RM (1998) Presence of antibodies to heat stress proteins in workers exposed to benzene and in patients with benzene poisoning. Cell Stress Chaperones 3:161–167
Xu Q (2002) Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol 22:1547–1559
Xu Q, Jürgens G, Huber LA, Böck G, Wolf H, Wick G (1992) Lipid utilization by human lymphocytes is correlated with high-density-lipoprotein binding site activity. Biochem J 285:105–112
Xu Q, Kiechl S, Mayr M, Metzler B, Egger G, Oberhollenzer F, Willeit J, Wick G (1999) Association of serum antibodies to heat shock protein 65 with carotid atherosclerosis: clinical significance determined in a follow-up study. Circulation 100:1169–1174
Yamashita N, Hoshida S, Nishida M, Igarashi J, Aoki K, Hori M, Kuzuya T, Tada M (1997) Time course of tolerance to ischemia-reperfusion injury and induction of heat shock protein 72 by heat stress in the rat heart. J Mol Cell Cardiol 29:1815–1821
Zhu J, Quyyumi AA, Wu H, Csako G, Rott D, Zalles-Ganley A, Ogunmakinwa J, Halcox J, Epstein SE (2003) Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol 23:1055–1059
Acknowledgements
This research was supported by the Key program of the Chinese National Natural Science Foundation (grant no. 30430590) and Tianjin Basic Research Program of Applied (grant no. 07JCYBJC08900).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leng, X., Zhan, R., Wang, Y. et al. Anti-heat shock protein 70 autoantibody epitope changes and BD091 promotes atherosclerosis in rats. Cell Stress and Chaperones 15, 947–958 (2010). https://doi.org/10.1007/s12192-010-0203-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-010-0203-0