Abstract
The cadherin–catenin proteins have in common with heat shock proteins (HSP) the capacity to bind/interact proteins of other classes. Moreover, there are common molecular pathways that connect the HSP response and the cadherin–catenin protein system. In the present study, we have explored whether in breast cancer the HSP might interact functionally with the cadherin–catenin cell adhesion system. β-Catenin was immunoprecipitated from breast cancer biopsy samples, and the protein complexes isolated in this way were probed with antibodies against HSP family members. We are thus the first to demonstrate a specific interaction between β-catenin and Hsp27. However, β-catenin did not bind Hsp60, Hsp70, Hsp90, gp96, or the endoplasmic reticulum stress response protein CHOP. To confirm the finding of Hsp27-β-catenin interaction, the 27-kDa immunoprecipitated band was excised from one-dimensional polyacrylamide gel electrophoresis gels and submitted to liquid chromatography–tandem mass spectrometry with electrospray ionization, confirming a role for Hsp27. In addition, β-catenin interacted with other proteins including heat shock transcription factor 1, P-cadherin, and caveolin-1. In human breast cancer biopsy samples, β-catenin was coexpressed in the same tumor areas and in the same tumor cells that expressed Hsp27. However, this coexpression was strong when β-catenin was present in the cytoplasm of the tumor cells and not when β-catenin was expressed at the cell surface only. Furthermore, murine breast cancer cells transfected with hsp25 showed a redistribution of β-catenin from the cell membrane to the cytoplasm. When the prognostic significance of cadherin–catenin expression was examined by immunohistochemistry in breast cancer patients (n = 215, follow-up = >10 years), we found that the disease-free survival and overall survival were significantly shorter for patients expressing P-cadherin and for patients showing expression of β-catenin in the cytoplasm only (not at the cell surface). The interactions of β-catenin with Hsp27 and with HSF1 may explain some of the molecular pathways that influence tumor cell survival and the clinical significance in the prognosis of the breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Numerous prognostic factors have been evaluated in breast cancer patients to predict the clinical outcome of the disease. If the disease is detected early, most of the patients with invasive carcinomas will have a high survival rate (about 70–90% in axillary lymph node-negative), although a certain number of patients will develop distant metastases making the disease practically incurable. In contrast, when the disease is detected at later stages, the probability of distant metastases increases considerably. However, even patients with axillary lymph node-positive cancer have variable prognosis, and not all of them will develop distant metastases. Therefore, it is desirable to improve the prognostic factors evaluated in the primary tumor; such information provided to the physician is very valuable because, for instance, patients with an aggressive disease should receive chemotherapy and/or be placed in special clinical protocols (more aggressive treatments and frequent follow-ups). Prognostic gene expression profiles detected by microarrays are under intensive investigation because hundreds of genes can be evaluated simultaneously (Van’t Veer et al. 2002). However, as with other molecular assays, the tissue submitted for gene expression analysis is different to the tissue examined under the microscope. For example, the tissue may contain invasive and noninvasive tumor, normal tissues, and necrotic areas. Immunohistochemistry allows this discrimination. Moreover, gene amplification or expression (at ribonucleic acid level) does not necessarily correspond to protein expression. Protein localization is important for explaining functional roles; for example, we have found that nuclear localization (but not cytoplasmic content) of heat shock protein (HSP) Hsp70 is related to poor prognosis (Vargas-Roig et al. 1998). In a previous study, we have integrated estrogen and progesterone receptors (ER and PR, respectively) with pathological and molecular prognostic factors in breast cancer patients (Gago et al. 1998). When the pathological prognostic factors were combined with the molecular factors, 82% of the patients who developed distant metastases were identified (follow-up of 5 years). In the present study, we have used this tumor bank, which now has a median follow-up of 10 years to analyze another molecular factor, cell adhesion molecules.
Cell adhesion molecules are essential during development and for the maintenance of tissue integrity in adult organisms. They limit cell movement and proliferation and are also implicated in several signal transduction pathways. The cadherins constitute a superfamily of adhesion molecules and are transmembrane glycoproteins responsible for calcium-dependent cell–cell adhesion. The extracellular domain forms homo–heterophilic bonds with cadherins on adjacent cells, which specify cell–cell recognition and sorting of mixtures of cells (Gumbiner 2000; Foty and Steinberg 2005). The classical cadherins include E- (epithelial) and P- (placental) cadherins, which are the best characterized members of this superfamily. In normal human breast tissues, these molecules show distinct patterns of expression: E-Cadherin is expressed by luminal epithelial cells, whereas P-cadherin is restricted to myoepithelial cells (Rasbridge et al. 1993). α-, β-, γ-, and p120 catenins are the cytoplasmic partners of cadherins, and the prevailing dogma is that cadherins are linked to the actin cytoskeleton through beta catenin and α-catenin strengthening its adhesive force. Recent studies show that α-catenin does not interact with both actin filaments and the E-cadherin–catenin complex simultaneously. These results suggest that the linkage between the cadherin–β-catenin complex and actin filaments is more dynamic rather than being static (Yamada et al. 2005). β-Catenin was first found to be part of the cell–cell adhesion complex but has subsequently been shown to be a downstream signaling molecule in the Wnt/wingless signaling pathway (Dann et al. 2001). Normally, the cytoplasmic level of β-catenin is downregulated through interaction with a protein complex comprised of normal adenomatous polyposis coli (APC), Axin, and glycogen synthase kinase 3β (GSK3β). Under these circumstances, β-catenin is phosphorylated by GSK3β and activates the ubiquitination pathway leading to degradation of cytoplasmic β-catenin levels. Activation of Wnt signaling leads to the inactivation of GSK3β resulting in accumulation of cytoplasmic β-catenin. At high cytoplasmic levels, β-catenin translocates into the nucleus controlling the transcription of genes in concert with TCF/LEF proteins (Rubinfeld et al. 1993; Morin et al. 1997). Several mechanisms have been reported to mediate this transcripitional event, including mutations of β-catenin, deletion of the APC gene, and activation of the Wnt signal pathway (Morin et al. 1997). Absence or altered expression of the cadherin–catenin complexes results in decreased adhesion, and this might be and important factor in tumorigenesis, particularly in tumor cell invasion.
In breast carcinomas, a number of immunohistochemical studies have analyzed the expression of E-cadherin and have linked aberrant E-cadherin expression with development of metastases and poor prognosis (Siitonen et al. 1996; Heimann et al. 2000). This protein is lost or reduced in infiltrating lobular carcinomas (Berx et al. 1996). However, today, there are conflicting reports with regard to the significance of this altered expression (Gonzalez et al. 1999; Peralta Soler et al. 1999; Cobanoglu et al. 2004). The relative penetrance of P-cadherin expression in ductal carcinomas appears to be controversial. For instance, in one study, this cadherin was not detected (Rasbridge et al. 1993), while in others, the expression ranged from 20 (Palacios et al. 1995), to 40 Kovács et al. (2003), to 52% (Peralta Soler et al. 1999) suggesting a role in disease progression and poor prognosis (Peralta Soler et al. 1999; Kovács et al. 2003). The expression of P-cadherin in invasive lobular carcinomas (ILCs) was not detected in some studies (Peralta Soler et al. 1999; Kovács et al. 2002), while another showed a low cytoplasmic staining (Kovács et al. 2003). Finally, reports of β-catenin expression patterns in breast carcinomas are limited and inconsistent. Some authors show that loss of E-cadherin expression is associated with loss of α-, β-, and γ-catenin immunostaining (Gonzalez et al. 1999). Aberrant cytoplasmic/nuclear expression of α- and β-catenin was correlated with poor prognosis in the studied patients (Nakopoulou et al. 2002; Lin et al. 1999), while membranous normal β-catenin expression was associated with the highest survival rate and survival length (Lim and Lee 2002). In this study, we have analyzed the expression patterns of E-cadherin, P-cadherin, and β-catenin in breast cancer by immunohistochemistry to evaluate the prognostic significance of these cell adhesion molecules. We found a surprisingly high expression level of β-catenin in the cytoplasm of several of the tumor samples. Subsequent immunoprecipitation and coexpression studies examined the potential interaction of this protein with another class of cytoplasmic proteins with elevated levels and prognostic significance in breast cancer, the HSP (Ciocca and Calderwood 2005). Our study also indicate the interaction of β-catenin with Hsp27 and with heat shock transcription factor (HSF) 1, the transcriptional inducer of Hsp gene expression in response to stress (Christians et al. 2002; Khaleque et al. 2005).
2 Materials and methods
2.1 Patients
This study involved 215 consecutive breast cancer patients from the province of Mendoza, Argentina (ethnic group principally formed by descendants of Spanish and Italian immigrants). All patients were free of previous breast cancer treatments and presented stage I, II, or III operable primary cancer. The patients were first seen (initial diagnosis) during the period of 1989 to 1995, and all of them approved and signed an informed consent. After surgery (conservative tumor resection or mastectomy, with axillary lymph node dissection), patients were treated with radiotherapy (those treated with lumpectomy) and received systemic adjuvant therapy when indicated (according to tumor size, lymph node involvement, patient age, clinical situation). Adjuvant therapy consisted of CMF (cyclophosphamide, metotrexate, and 5-fluorouracil) or FAC (5-fluorouracil, adriamycin, cyclophosphamide); tamoxifen was indicated according to ER and PR status. The condition of the patients was periodically evaluated clinically and by especial examinations (including X-ray of the chest, bone scintigraphy, and liver ultrasound). The data were computerized and regularly updated. The study endpoints were the distant disease-free survival (DFS) and the overall survival (OS), which were calculated as the period from diagnosis until the date of the first distant recurrence or death, respectively.
Unique features of this tumor bank are: These are consecutive cases with a long follow-up, all patients were surgically treated by the same surgeon (Gago), the pathological study was performed by the same pathologist (Tello), and the molecular markers were all evaluated in the same laboratory. The main clinical data of the patients entered into this study are presented in Table 1.
2.2 Tissue processing and cell line evaluations
Tumor size was determined by the pathologist on fresh tissue. Tissues were fixed in 10% buffered formalin at room temperature for 24–48 h, dehydrated, and included in paraffin. Hematoxylin-and-eosin-stained tissue sections (5 μm thickness) were used for histopathological studies. Serial 5-μm-thick sections were mounted onto 3-aminopropyltrietoxysilane (Sigma-Aldrch, St. Louis, MO)-coated slides for subsequent immunohistochemical analysis. The following primary antibodies were used: (1) mouse MAb anti-P-cadherin, cat. no. C24120 (Transduction Laboratories, Lexington, KY), (2) mouse MAb anti-E-cadherin; cat. no. 13-1700 (Zymed, San Francisco, CA), and (3) mouse MAb anti-β-catenin, cat. no. 18-0226 (Zymed). The antigen retrieval protocol with a microwave oven was used to unmask the antigens (30 min in citrate buffer, pH 6.0). Tissue sections were incubated with the primary antibodies overnight at 4°C in humidity chambers at the following dilutions: P-cadherin, 1:200; E-cadherin, 1:20; and β-catenin, 1:400. A commercial kit to detect mouse and rabbit primary antibodies was used (Dako EnVision system, horse radish peroxidase, diaminobenzidine [DAB], from Dako, Carpinteria, CA). Slides were lightly counterstained with hematoxylin to reveal nuclei and observed with an IM35 microscope (Zeiss, Oberkochen, Germany). Negative control slides were processed excluding the primary antibody but including all other steps of the procedure. The immunostaining procedures to detect ER, PR, proliferating cell nuclear antigen (PCNA), p53, HER-2/neu, and P170 (mdr1) were described previously (Gago et al. 1998). Serial sections were used to evaluate the coexpression of proteins; in these cases, the antibodies used were: (a) rabbit, polyclonal against a hybrid Hsp27/Hsp25 protein (Ciocca et al. 2003) at 1:2,000, and (b) rabbit, polyclonal against HSF1 at 1:7,500 (kindly provided by Dr. Calderwood SK, Beth Israel Deaconess Medical Center, Boston, MA). Double immunostaining was performed on selected cases using as first chromogen diaminobenzidine (DAB) and then as second chromogen alkaline phosphatase (AP).
The immunostaining was semiquantitatively scored as follows: (1) P-cadherin: 0 = no staining or membrane staining in less than 1% of the invasive tumor cells, 1 = membrane staining in greater than 1% of tumor cells; 2) E-cadherin: 0 = membrane staining in greater than or equal to 30% of tumor cells, 1 = membrane staining in less than 30%, 2 = no staining or membrane staining in less than 1% of the invasive tumor cells (the scoring chosen for E-cadherin as a lower value [o, for membrane staining in greater than or equal to 30% of tumor cells] indicates good prognosis as was observed for P-cadherin and β-catenin); β-catenin: 0 = no staining or membrane staining in less than 1% of the invasive tumor cells, 1 = membrane staining (completed or fragmented) in greater than 1% of the invasive tumor cells (in this score, those samples that also presented cytoplasmic or nuclear staining but with very weak staining intensity were also included), 2 = cytoplasmic or nuclear staining (even in presence of weak membrane staining). These scores were obtained by two independent observers (Fanelli and Ciocca) blinded regarding clinical evaluation, and a few conflicting scores where resolved by consensus.
2.3 Western blotting and immunoprecipitations
Western analysis was performed on selected breast cancer samples to check the specificity of the antibodies used in the immunohistochemical study and for immunoprecipitation. Breast cancer samples stored at −70°C were homogenized with lysis buffer (25 mM Tris–HCl, pH 7.5, 5 mM ethylenediamine tetraacetic acid, pH 7.5, 250 mM NaCl, 1% Triton X-100) and for centrifuged 30 min to 13,000 rpm at 4°C. Protein (40–60 μg) from the supernatant and a cell lysate prepared from the A431 human epidermoid carcinoma cell line (positive control for E-cadherin, P-cadherin, and β-catenin) were subjected to 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by transfer onto nitrocellulose filters, as previously described (Fanelli et al. 1998). The antibodies were diluted as follows: E-cadherin, 2 μg/ml; P-cadherin, 1:250; and β-catenin, 1:500. For detection of the immunocomplexes, chemiluminescence reagents were used following the manufacturer’s instructions (Dupont NEN, Boston, MA).
Monoclonal anti-β-catenin was covalently coupled to M-280 tosylactivated Dynabeads (DYNAL, Biotech ASA, Oslo, Norway) according to the manufacturer’s protocol. A human breast cancer sample was subjected to immunoprecipitation with the β-catenin-coated beads for 1 h with tilting and rotation. After washing with phosphate-buffered saline, the beads were resuspended in sample buffer, and the target protein bound was eluted and concentrated by boiling for 5 min at 97°C. For Western blotting, proteins were separated in 7.5 or 12.5% SDS-PAGE and transferred to nitrocellulose. Blots were then probed with anti-β-catenin (to check the success of the immunoprecipitation, 1:500), anti-P-cadherin (1:250), anti-HSF1 (1:15,000), anti-Hsp27 (1:4,000), anti-Hsp60 (1:1,000), anti-Hsp70 (1:2,000), anti-Hsp90 (1:1,000), anti-gp96 (1 μg/ml), anti-CHOP (1:250), and anti-caveolin 1(1:400).
2.4 Protein identification by liquid chromatography–tandem mass spectrometry with electrospray ionization
Proteins separated by SDS-PAGE were stained with Coomassie blue. The excised protein bands were digested by trypsin (Promega, Madison, WI) in gel digestion at 37°C overnight. The following day, the tryptic peptide solution was transferred to a microcentrifuge tube. The protein spots were first extracted with 1% formic acid (Sigma-Aldrich), 2% acetonitrile (high-performance liquid chromatography grade; Fisher Scientific, Pittsburg, PA) in water, followed by an extraction with 50% acetonitrile–water. Both extracts were combined with the tryptic peptide solution and concentrated (in a SpeedVac Termo Electron, Waltham, MA) and suspended in 1% formic acid and 2% acetonitrile. Peptide analysis was performed using liquid chromatography–tandem mass spectrometry with electrospray ionization (LC-ESI-MS/MS). The peptides were inline desalted and concentrated with RP-Trap column Symmetry 300C18, 5 μm NanoEase (Waters, Milford, MA), and separated using C18 RP PepMap, 75-μm (internal diameter) capillary column (LC Packing, Dionex, San Francisco, CA). The standard gradient used was: 0–2 min, 3% B isocratic and 2–40 min, 3–80% B linear. Mobile phase A was water/acetonitrile/formic acid (98.9:1:0.1, v/v/v), and phase B was acetonitrile/water/formic acid (99:0.9:0.1, v/v/v). The solvent flow rate was 8 μl/min, and the separation was performed using CapLC (Waters). Spectra were obtained in the positive ion mode with Q-tof micro-mass spectrometer (Micromass, UK), deconvoluted, and analyzed using the MassLynx software 4.0 (Micromass). The identification of peptide matching (peptide masses and sequence tags) and protein searches were performed using ProteinLynx Global Server v 2.1 (Micromass) against NCBinr v20060512. The data were also confirmed using Mascot open source (www.matrix-science.com) in tandem mass spectrometry ion search mode at NCBInnr v20060512 and Swiss Prot v49.6. The parameters for the search were as follows: The modification on cysteine residue by carbamidomethylation was set as fixed, and arginine and glutamine deamidation and methionine oxidation were considered as variable modifications. The maximum number of missed cleavages was one; the monoisotopic masses were considered, and the peptide and fragment tolerances were 100 ppm and 0.25 Da, respectively.
2.5 Cells and cell culture
The adherent 4T1 murine mammary adenocarcinoma cell line was used. The cells were cultivated, in accordance with American Type Culture Collection recommendation in complete Roswell Park Memorial Institute medium supplemented with 10% heat-inactivated fetal calf serum (GIBCO) and 100 IU/ml penicillin and 100 μg/ml streptomycin (GIBCO) at 37°C in an incubator with 5% CO2 and 100% humidity. Subconfluent cells were split twice a week at a ratio of 1:20.
2.6 Creation of a vector carrying hsp25
The plasmid (Bausero et al. 2006) containing a 4.4-kb XbaI fragment consisting of the genomic sequence of murine hsp25 is ligated into the multiple cloning site of the retroviral vector plasmid pLXSN, which contains a sequence conferring neomycin resistance under the control of the SV40 promoter.
2.7 Transfection and selection of target cells
Subconfluent cell cultures of 4T1 were transfected with 20 μg of the plasmid of interest using the calcium phosphate precipitation procedure (100-mm culture dish, Mammalian Transfection Kit; Stratagene, La Jolla, CA). Cells were selected for neoresistance by Geneticin, a neomycin analog (1.5 mg/ml G418; Sigma, Munich, Germany). Stably transfected and untransfected control cells were used for our immunohistochemical study to evaluate hsp27 and β-catenin expression following the described methodology.
2.8 Statistical analysis
Analyses of DFS and OS were performed by the Kaplan–Meier method. The difference between curves was evaluated with the log-rank test for censored survival or event observations. Contingency tables were analyzed by the Fisher’s exact test and Chi-square with Yates’ correction. Statistical analyses were performed using the Prism computer program (Graph Pad Software, San Diego, CA); a p < 0.05 was considered significant. The weightiness of the parameters was studied by Cox proportional hazard regression analysis. The interactions between molecular and pathological factors were analyzed by the Pearson Chi-square test.
3 Results
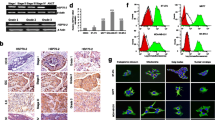
E-Cadherin and P-cadherin immunostaining was observed mainly at the cell membrane of the tumor cells as a homogeneous, fragmented, or dotted halo around the cells (Fig. 1a,b). Membrane immunostaining was consistently strong, and for this reason, we did not evaluate the intensity but the proportion of positive cells for scoring. Weak immunostaining for E-cadherin or P-cadherin in the cytoplasm or in the nuclei of the tumor cells was sporadically observed but was not included in the score. The percentages of positive cases for these two markers in patients with/without metastases are shown in Table 2. The prognostic significance of E-cadherin expression did not reach statistical differences. In contrast, P-cadherin expression was increased in the membrane more frequently in patients with distant metastases (p < 0.0001). Immunoreactivity for β-catenin was also detected at the cell membrane (Fig. 1c), but in most cases, β-catenin was present as granules in the cytoplasm of tumor cells (Fig. 1d). These granules were of different sizes and reached large magnitudes in several tumor samples. Table 2 shows the percentage of β-catenin immunostaining in the cell membrane and in cytoplasmic granules. A greater percentage of patients without metastases showed membrane β-catenin expression (p = 0.0016).
Human breast cancer biopsy samples immunostained to reveal E-cadherin (a), P-cadherin (b, e) and β-catenin (c, d). Note that E-cadherin and P-cadherin immunoreactivities appeared mainly at the cell membrane (black stain), while β-catenin appeared at the cell membrane (c) but also in the cytoplasm of the tumor cells (d). There is only one cell stained for P-cadherin in the middle of the tissue section; other areas of the tumor are negative (e). The tissue samples were lightly counterstained with hematoxylin to reveal nuclei. Original magnification, ×128. Bar = 20 μm
The DFS and OS were significantly shorter both for patients with P-cadherin-positive immunostaining and cytoplasmic β-catenin-positive tumors (Fig. 2). E-Cadherin expression did not discriminate the prognosis of the patients.
Disease-free survival (DFS) and overall survival (OS) curves according to: a, b membrane staining of β-catenin in greater than 1% of the invasive tumor cells (squares, β-catenin 1) and cytoplasmic or nuclear staining of β-catenin (triangles, β-catenin 2). c, d No staining or membrane staining of P-cadherin in less than 1% of the invasive tumor cells (squares, P-cadherin 0) and membrane staining of P-cadherin greater than 1% (triangles, P-cadherin 1)
P-Cadherin, β-catenin, and erbB-2 status reached statistical significance in the univariate analyses, while in the multivariate analyses, only c-erbB-2 status was identified as a significant prognostic factor (Table 3).
The relationship between E-cadherin, P-cadherin, and β-catenin scores and pathological and molecular factors are shown in Table 4. E-Cadherin expression showed an inverse correlation with PCNA levels. The majority of tumors with high E-cadherin expression (81.5%) were not proliferative (p = 0.0111). P-Cadherin expression showed a statistically significant correlation with cerbB-2 and with mutant p53 status (in immunohistochemistry p53 staining represents a subrogate marker of mutant or inactivated p53). An important percentage of P-cadherin-negative cases were negative for erbB-2 (80%, p = 0.0005) and for p53 (74%, p = 0.0001), respectively. Statistical analysis revealed a significant inverse correlation between P-cadherin expression and receptor status; 85% (86 of 101) of P-cadherin-negative cases were ER and/or PR positive (p = 0.0045). Additionally, a significant association between P-cadherin-negative cases and low tumor grade (p = 0.0056) was also observed. A marginally significant difference was shown between P-cadherin negative expression and absence of lymph node metastases (p = 0.0555). β-Catenin expression showed a statistically significant correlation with the lymph node. The majority of membrane β-catenin-positive cases (68.6%) were free of lymph node metastases (p = 0.0483). Additionally, an important association between membrane β-catenin expression and receptor status was observed: 73.5% membrane β-catenin-positive cases were receptor positive (p = 0.0751). The relationship between P-cadherin and β-catenin was also evaluated but did not reach significant differences. We also have found that P-cadherin and E-cadherin immunostaining were absent in ILC.
In the Western blot studies, P-cadherin detected two closed bands with a molecular weight (MW) of ≅120 kDa, E-cadherin showed a MW band of 120 kDa (Fig. 3a), while β-catenin detected mainly a single band with a MW of ≅92 kDa (Fig. 3b,c). In the immunoprecipitation/Western blot studies, β-catenin was immunoprecipitated with magnetic particles, and the resulting product was probed with different antibodies against the Hsp family of proteins (Fig. 3d–f). The results showed that β-catenin was interacting with Hsp27. To confirm this finding, the 27-kDa immunoprecipitated band was excised and submitted to LC-ESI-MS/MS, and in this study, the band was identified as Hsp27 protein 1 (Homo sapiens). In addition, Fig. 3 shows that β-catenin interacted with HSF1, P-cadherin, and caveolin-1 in the immunoprecipitation/Western blot studies. However, there was no interaction of β-catenin with Hsp60, Hsp70, Hsp90, gp96 (also known as GRP94), and CHOP (also known as GADD 153). The Hsp27/β-catenin interaction was quantified; approximately 40% of the total cellular content of Hsp27interacts with β-catenin, while approximately 70% of β-catenin interacts with Hsp27.
Western blot (WB) and immunoprecipitation (IP) studies in human breast cancers and a human tumor cell line. a WB for P-cadherin (lanes 1–4) and E-cadherin (lanes 5–7). Two close bands were detected with the P-cadherin antibody, lanes 1–3: cytosol from three different human breast cancer samples with different P-cadherin expression levels, lane 4: cytosol from the A431 cell lysate (positive control, human epidermoid carcinoma). E-Cadherin expression (lanes 5–7) in the same human breast tumors shown in lanes 1–3. b WB for β-catenin. Lane 1 and 3: cytosol from human breast cancers with different β-catenin expression levels, lane 2: A431 cell lysate; c β-catenin expression in a human breast cancer sample (lane 3); lane 2: IP of β-catenin using the same tumor shown in lane 3, followed by WB for β-catenin (positive control); lane 1: IP of β-catenin (same tumor shown in lane 3) followed by WB for P-cadherin; note the positive P-cadherin bands (arrow). d IP for β-catenin and WB for different probes. Note that in the IP studies, the proteins that coimmunoprecipitated with β-catenin were: P-cadherin (arrow in lane 1, c), Hsp27 (arrow in lane 1, d), caveolin-1 (arrow in lane 3, d), and HSF1 (arrow in lane 1, f). Equal amounts of proteins were loaded and subjected to SDS-PAGE (7.5 and 12.5%) and Western blotting. For comparative purposes, a nonspecific (NS) band (MW of ≅83 kDa) indicating similar loading of protein lysates in the different lanes is shown in d–f. g Negative control for IP
To further explore the β-catenin/HSF1 and β-catenin/Hsp27 associations, we performed double immunostaining to evaluate the coexpression of proteins in the human breast cancer biopsy samples (n = 54). Cytoplasmic β-catenin and nuclear HSF1 coexpressed in the same tumor cells as was revealed by one chromogen only (nuclear and cytoplasmic staining with DAB, Fig. 4a) and by the use of two chromogens (cytoplasmic staining with DAB and nuclear staining with AP, Fig. 4b). Additionally, β-catenin was observed in the same tumor areas and in the same tumor cells that expressed Hsp27. This coexpression could be visualized by DAB (cytoplasmic β-catenin staining) and AP (cytoplasmic Hsp27 staining; Fig. 4c). This association was strong when β-catenin was expressed in the cytoplasm of the tumor cells but not when β-catenin was expressed at the cell membrane only (Fig. 4d). β-Catenin and Hsp27 were both present in 61% of the tumors and were absent in 11% of the cases. Evaluation of the contingency tables showed a statistically significant association between both proteins (p < 0.005). It may be significant that in 28% of the cases, β-catenin was expressed mainly at the membrane and in these cases there was absence of Hsp27.
Coexpression of HSF1 and Hsp27 with β-catenin in human breast cancer biopsies. a HSF1 (nuclear staining) and β-catenin (granules/cytoplasmic staining) using DAB (bar = 20 μm). b using Two different chromogens: DAB (cytoplasmic β-catenin) and AP (nuclear HSF1). c Coexpression of cytoplasmic Hsp27 (AP chromogen substrate) and cytoplasmic β-catenin (DAB substrate). Arrows indicate cytoplasmic β-catenin expression like brown granules. d Only membrane β-catenin immunostaining was detected; Hsp27 was absent. e Costaining of β-catenin (DAB) with cytokeratin (AP). Original magnification: b, ×100, and inserts in b, ×157.5; c, ×157.5
We also examined these interactions in murine mammary carcinoma cells in vitro
Immunocytochemistry of 4T1 cells showed that in untransfected control cells, β-catenin was mainly localized at the cell surface (Fig. 5a,c), while after forced expression of hsp25, β-catenin relocated to the cytoplasm (Fig. 5b,d). The differential localization of β-catenin observed in vitro suggests a potential functional role for the interaction between Hsp27 with β-catenin observed in clinical samples.
Immunocytochemistry of 4T1 cells. a Untransfected control 4T1 cells immunostained to reveal Hsp25. b hsp25 4T1-transfected cells showing an increase in Hsp25 content. c Membrane β-catenin expression in untransfected control cells. d Cytoplasmic β-catenin expression in hsp25 4T1-transfected cells. Original magnification: a, b, ×100; c, d, ×157.5
4 Discussion
Understanding the metastatic cascade of breast cancer based on identification of the proteins involved in this process offers a greater repertoire of useful molecular markers and the opportunity of tailoring therapy to individual tumor characteristics. We have evaluated P-cadherin, E-cadherin, and β-catenin as potential prognostic markers in breast cancer biopsies samples. We show that P-cadherin expression is a valuable indicator of poor prognosis in breast cancer patients. In addition, when P-cadherin expression was considered in relation to other molecular and pathological markers, we found a direct link of P-cadherin expression with erbB-2, p53 and tumor grade and a significant inverse correlation with receptor status. These findings are in agreement with those of previous authors (Gonzalez et al. 1999; Gamallo et al. 2001; Paredes et al. 2002, 2005). The frequency of P-cadherin expression in invasive breast tumors in our study was similar to the 35% found by others (Palacios et al. 1995; Paredes et al. 2005). In normal breast tissue, P-cadherin is expressed in myoepithelial rather than in epithelial cells (Wheelock et al. 2001). However, a subset of mammary tumors showed aberrant expression of this protein. It is not clear what mechanism might lead to the activation of P-cadherin expression in tumor cells whose normal progenitors do not express this protein. Changes in transcriptional regulation or deoxyribonucleic acid (DNA) methylation might lead to the silencing of the P-cadherin gene in normal cells. Changes in the tumor environment might also be involved in P-cadherin expression in cancer cells. A recent study has suggested that P-cadherin expression is repressed in normal epithelial cells by this specific mechanism and that the aberrant expression in breast cancer might be regulated by gene promoter hypomethylation (Paredes et al. 2005). The biological aggressiveness of P-cadherin expression in breast cancer is still poorly understood. It seems that a change in cadherin family members in neoplastic cells modifies the behavior of such cells when compared with normal epithelial cells. Perhaps the new interactions between adjacent cells lead to novel intercellular communication and mitogenic signaling (Wheelock et al. 2001). We also show an indirect link between P-cadherin and hormone receptor expression. Such an inverse correlation was also described by others (Palacios et al. 1995; Paredes et al. 2005). Paredes et al. (2004) showed that P-cadherin expression depends on an estrogen-independent cell environment and suggest a role for P-cadherin in invasion involving interaction with proteins bound to the juxtamembrane domains. Taniuchi et al. (2005) have observed that the motility of pancreatic cancer cells is promoted by overexpressed P-cadherin interacting with p120ctn and activating the rho-family GTPases. It will be of interest to explore the relationship between the overexpression of P-cadherin and the rho family of small GTPases in breast cancer (proteins). Another signaling pathway associated with poor prognosis that may be activated by P-cadherin overexpression is the erbB-2 pathway. The erbB-2 receptor is expressed at a high level in 30% of human breast cancer patients and plays an important role in the transformation and the prognosis of breast cancer. In a complementary DNA microarray analysis, the P-cadherin (CDH3) gene (among others) was differentially regulated under erbB-2 overexpression in breast cancer (Mackay et al. 2003). Perhaps crosstalk between P-cadherin and erbB-2 is initiated under these conditions. This could explain, at least in part, the poor survival of patients with P-cadherin-positive tumors. Although the clinical correlations between P-cadherin expression and cancer progression appear to be robust, the biological pathways have not yet been determined and need further investigations.
We have also confirmed that P-cadherin expression is absent in ILC; possibly, the P-cadherin/CDH3 gene may undergo mutation or allelic loss as has been observed for the E-cadherin gene in this histological type of tumors (Droufakou et al. 2001).
Cytoplasmic β-catenin expression was also demonstrated to play a role in the discernment of prognosis; DFS and OS were significantly shorter for patients with cytoplasmic β-catenin in their tumors. Elevated levels of β-catenin are associated with poor prognosis in breast cancer (Lin et al. 1999). A recent study has also shown that a low expression level of membranous β-catenin is associated with a significantly worse outcome in breast cancer (Dolled-Filhart et al. 2006). However, the mechanisms by which β-catenin confers the more aggressive biological behavior are poorly understood in breast cancer. In this study, 84% of tumors showed abnormal β-catenin expression, mainly in cytoplasmic granules, although 16% of tumors had membrane expression. Previous reports have described an aberrant pattern of β-catenin expression in different kinds of tumors such as colorectal, breast, and pancreatic cancer and melanoma, indicating alterations in the wnt signaling pathway (Karayiannakis et al. 2001; Rhan et al. 2001; Li et al. 2005; Kariola et al. 2005; Larne and Delmas 2006). While some studies have demonstrated a nuclear/cytoplasmic localization of β-catenin in breast cancer biopsies, we and others (Rhan et al. 2001) were unable to detect β-catenin in the nucleus of breast cancer cells by immunohistochemistry. The mechanistic connections associated with aberrant expression of β-catenin are not really understood, specifically in breast cancer. It is known that in colon carcinoma, APC is one of the components most affected in the Wnt/β-catenin signaling pathway (80–95%) allowing β-catenin to translocate to the nucleus and turn on the transcription of c-myc and cyclin D1 oncogenes. Mutations in the amino-terminal region of β-catenin gene represent the second major mechanism of β-catenin accumulation (Morin et al. 1997). However, the β-catenin signaling pathway does not follow the same pattern in breast cancer where APC or β-catenin mutations are not common. β-Catenin is a promiscuous protein, and the search of possible partners could help to elucidate the aggressive behavior of cells with aberrant expression of this protein. Several β-catenin-associated proteins have been described and may be involved in signaling pathways in cancer. Previous studies in breast cancer showed that overexpression of Pin-1 contributes to the upregulation of β-catenin by inhibiting its interaction with APC, decreasing β-catenin turnover, and increasing its nuclear translocation (Ryo et al. 2001). A specific protein–protein interaction has been demonstrated between β-catenin and the androgen receptor in both prostatic and neuronal cells (Yang et al. 2002; Pawlowski et al. 2002). Likewise, interaction between β-catenin and estrogen receptor α (Kouzmenko et al. 2004) and Galectin-3 (Shimura et al. 2004) have been found to be new binding partners for β-catenin. In this study, we have found novel interactions of β-catenin with Hsp27 and with HSF1. The β-catenin/Hsp27 association was strong when β-catenin was expressed in the cytoplasm of the tumor cells, not when β-catenin was expressed at the cell membrane only. This stresses the importance to study the protein localization in tumor cells by immunohistochemistry. Adittionally, cytoplasmic β-catenin and nuclear HSF1 were coexpressed in the same tumor cells. Moreover, in hsp25-transfected 4T1 murine mammary adenocarcinoma cells (highly metastatic cell line), the β-catenin expression was localized in the cytoplasm of tumor cells, while in control cells, the β-catenin expression was mainly found in the membrane of tumor cells. Previously, Bausero et al. (2006) showed that silencing the hsp25 gene has a profound effect on 4T1 cells through decreasing cell proliferation and inhibiting cell migration. We have shown the first direct evidence of interaction of β-catenin with the HSP. This newly identified interaction may help to clarify the mechanism underlying poor prognosis associated with aberrant β-catenin expression. Interactions of β-catenin with caveolin 1 and P-cadherin were also identified by immunoprecipitation in this study as shown previously (Galbiati et al. 2000; Patel et al. 2003).
HSP are overexpressed in a wide range of human cancers and are implicated in tumor cell proliferation, differentiation, invasion, metastasis, death, and recognition by the immune system (Ciocca and Calderwood 2005). Increased HSP expression may also predict the response to some anticancer treatments, and Hsp27 is implicated in resistance to chemotherapy in breast cancer (Ciocca and Calderwood 2005). In addition, there are common molecular pathways for regulation of the HSP response and for the cadherin–catenin system involving phosphatidylinositol 3′-kinase (PI3K). In breast cancer cells, this pathway is activated by the membrane estrogen receptors and/or by heterodimerization of the Her-2/neu oncoproteins; PI3K can then positively regulate HSP transcription through activation of HSF1 (Ciocca et al. 2006). On the other hand, the PI3K/Akt pathway also regulates intracellular β-catenin levels. Akt is a downstream target of PI3K, phosphorylating and inactivating GSK3β, thereby accumulating unphosphorylated β-catenin (Cross et al. 1995; Monick et al. 2001). Recently, Khaleque et al. (2005), have shown that HSF1 activation by heregulin β1 leads to protection from apoptosis and to anchorage-independent growth. There is evidence that HSF1 activation leads to hsp70 upregulation (Calderwood et al. 2007). Heregulin β1 binding to c-erbB receptors on the cell surface leads to the inhibition of intracellular HSF1 antagonist glycogen synthase kinase 3. In addition, this protein is part of a complex that mediates β-catenin degradation, and if it is inhibited in heregulin-treated cells, we observe both HSF1 activation and cytoplasmic β-catenin accumulation (Adam et al. 2001). GSK3β may thus be the point of crosstalk between HSF1 and HSP with β-catenin in this tumorigenic pathway.
Unlike the P-cadherin and β-catenin, E-cadherin levels were not useful to discriminate the prognosis of patients. The role played by this protein is less clear. Reports in the literature regarding the relationship between E-cadherin and prognosis are contradictory. Some authors report reduced expression of E-cadherin to be associated with tumor recurrence, metastases, and poor prognosis in breast cancer (Siitonen et al. 1996; Asgeirsson et al. 2000). However, Gillet et al. (2001) found that conserved E-cadherin expression persists into the later stages of breast carcinoma, although it may be inactivated functionally. A recently study had found that E-cadherin is preserved in aggressive tumors in a high-risk population, but statistical analysis revealed no correlation between high E-cadherin expression and OS (Howard et al. 2005). In this study, 53.2% of tumors were E-cadherin positive evaluated with 0 (membrane expression in greater than 30% of tumor cells) and 1 (membrane staining in less than 30% of tumor cells) scores but did not reach statistical differences with prognosis. Marginally statistical differences were found in patients with/without metastases. We found an inverse relationship between PCNA and E-cadherin. The role played by this protein in breast cancer is unclear at this point and in this study did not add prognostic information.
5 Conclusion
Identification of prognostic factors for breast cancer are taking place at an increasing rate, mainly because of the technical advances that permit the study of several molecules simultaneously using gene expression or protein expression profiling (Van’t Veer et al. 2002; Jacquemier et al. 2005). In this study, we have shown that P-cadherin and β-catenin levels as well as protein localization are useful molecular/morphological markers that should be added to the list of prognostic factors in breast cancer. Moreover, we have found an association of β-catenin with molecules associated with the heat shock cascade (HSF1 and Hsp27). Understanding the interaction networks of these proteins in aggressive tumors could permit identification of novel, molecularly based therapeutic targets in breast cancer.
References
Adam L, Vadlamudi RK, McCrea P, Kumar R (2001) Tiam1 overexpression potentiates heregulin-induced lymphoid enhancer factor-1/β-catenin nuclear signaling in breast cancer cells by modulating the intercellular stability. J Biol Chem 276:28443–28450
Asgeirsson KS, Jonasson JG, Tryggvadottir L, Ólafsdóttir K, Sigurgeirsdóttir JR, Ingvarsson S, Ögmundsdóttir HM (2000) Altered expression of e-cadherin in breast cancer: patterns, mechanisms and clinical significance. Eur J Cancer 361:098–1106
Bausero MA, Bharti A, Page DT et al (2006) Silencing the hsp25 gene eliminates migration capability of the highly metastatic murine 4T1 breast adenocarcinoma cell. Tumor Biol 7:17–26
Berx G, Cleton-Jansen AM, Strumane K, de Leeuw WJ, Nollet F, Van Roy F, Cornelisse C (1996) E-cadherin is inactivated in a majority of invasive lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene 13:1919–1925
Calderwood SK, Khaleque MA, Bharti A, Ciocca DR (2007) HSF1: an emerging factor in cancer. In: Calderwood SK, Sherman MY, Ciocca DR (eds) Heat shock proteins in cancer. Springer, The Netherlands 53–71
Christians ES, Yan LJ, Benjamin IJ (2002) Heat shock factor 1 and heat shock proteins: critical partners in protection against acute cell injury. Crit Care Med 30:S43–S50
Ciocca DR, Calderwood S (2005) Heat shock proteins in cancer: diagnostic, prognostic, predictive and treatment implications. Cell Stress Chaperones 10:86–103
Ciocca DR, Rozados VR, Cuello-Carrión FD, Gervasoni SI, Matar P, Scharovsky OG (2003) Heat shock proteins 25 and 70 in rodent tumors treated with doxorubicin and lovastatin. Cell Stress Chaperones 8:26–36
Ciocca D, Gago F, Fanelli M, Calderwood S (2006) Co-expression of steroid receptors (estrogen receptor alpha and/or progesterone receptors) and Her-2/new: clinical implications. J Steroid Biochem Mol Biol 102:32–40
Cobanoglu U, Ersoz S, Turgutalp H, Reis A, Ozoran Y (2004) Correlation of E-cadherin expression with clinicopathological parameters in breast carcinoma. Saudi Med J 25:1024–1027
Cross DA, Alessi DE, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789
Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ (2001) Insights into WNT binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature (Lond) 412:86–90
Dolled-Filhart M, McCabe A, Giltnane J, Cregger M, Camp RL, Rimm DL (2006) Quantitative in situ analysis of beta-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res 66:5487–5494
Droufakou S, Deshmane V, Roylance R, Hanby A, Tomlinson I, Hart I (2001) Multiple ways of silencing E-cadherin gene expression in lobular carcinoma of the breast. Int J Cancer 92:404–408
Fanelli MA, Cuello-Carrión FD, Dekker J, Schoemaker J, Ciocca DR (1998) Serological detection of heat shock protein hsp27 in normal and breast cancer patients. Cancer Epidemiol Biomarkers Prev 7:791–795
Foty RA, Steinberg MS (2005) The differential adhesion hypothesis: a direct evaluation. Dev Biol 278:255–263
Gago FE, Tello OM, Diblasi AM, Ciocca DR (1998) Integration of estrogen and progesterone receptors with pathological and molecular prognostic factors in breast cancer patients. J Steroid Biochem Mol Biol 67:431–437
Galbiati F, Volonte D, Brown AM, Weinstein DE, Ben-Zeèv A, Pestell RG, Lisanti MP (2000) Caveolin-1 expression inhibits Wnt/ β-catenin/Lef-1 signaling by recruiting β-catenin to caveolae membrane domains. J Biol Chem 275:23368–23377
Gamallo C, Moreno-Bueno G, Sarrio D, Calero F, Hardisson D, Palacios J (2001) The prognostic significance of P-cadherin in infiltrating ductal breast carcinoma. Mod Pathol 14:650–654
Gillet CE, Miles DW, Ryder K, Skilton D, Liebman RD, Springall RJ, Barnes DM, Hanby AM (2001) Retention of the expression of e-cadherin and catenins is associated with shorter survival in grade III ductal carcinoma of the breast. J Pathol 93:433–441
Gonzalez MA, Pinder SE, Wencyk PM et al (1999) An immunohistochemical examination of the expression of E-cadherin, and α and β/γ- catenin and α2- and β1 integrins in invasive breast cancer. J Pathol 187:523–529
Gumbiner BM (2000) Regulation of cadherin adhesive activity. J Cell Biol 148:399–404
Heimann R, Lan F, McBride R, Hellman S (2000) Separating favorable from unfavorable prognostic markers in breast cancer: the role of E-cadherin. Cancer Res 60:298–304
Howard EM, Lau SK, Lyles RH, Birdsong GG, Umbreit JN, Kochhar R (2005) Expression of e-cadherin in high-risk breast cancer. J Cancer Res Clin Oncol 131:14–18
Jacquemier J, Ginester C, Rougemont J et al (2005) Protein expression profiling identifies subclasses of breast cancer and predicts prognosis. Cancer Res 65:767–769
Kovács A, Walker R, Nagy A, Gomba S, Jones L, Dearing S (2002) Immunohistochemical study of P-cadherin in breast cancer. Orv Hetil 143:405–409
Kovács A, Dhillon J, Walker R (2003) Expression of P-cadherin, but not E-cadherin or N-cadherin, relates to pathological and functional differentiation of breast carcinomas. Mol Pathol 56:318–322
Karayiannakis A, Nakopoulou L, Gakiopoulou H, Keramopoulous A, Davaris P, Pignatelli M (2001) Expression patterns of β-catenin in in situ and invasive breast cancer. EJSO 27:31–36
Kariola R, Abdel-Rahman W, Ollikainen M, Butzow R, Peltomaki P, Nystrom M (2005) APC and beta-catenin protein expression patterns in HNPCC-related endometrial and colorectal cancers. Fam Cancer 4:187–190
Khaleque A, Bharti A, Sawyer D, Gong J, BenjaminI B, Stevenson MA, Calderwood SK (2005) Induction of heat shock proteins by heregulin β1 leads to protection from apoptosis and anchorage-independent growth. Oncogene 00:1–10
Kouzmenko A, Takeyama K, Ito S et al (2004) Wnt/β-catenin and estrogen signaling converge in vivo. J Biol Chem 279:40255–40258
Larne L, Delmas Y (2006) The Wnt/Beta-catenin pathway in melanoma. Front Biosci 11:733–742
Li Y, Wei ZM, Meng YX, Ji XR (2005) β-Catenin up-regulates the expression of cyclin D1, c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis. World J Gastroenterol 11:2117–2123
Lim SCH, Lee MS (2002) Significance of E-cadherin/β-catenin complex and cyclin D1 in breast cancer. Oncol Rep 9:915–928
Lin S, Xia W, Wang J, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC (1999) β-Catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA 97:4262–4266
Mackay A, Jones CH, Dexter T, Silva RL, Bulmer K, Jones A, Simpson P, Harris RA, Jat PS, Neville AM, Reis LF, Lakhani SR, O’Hare MJ (2003) cDNA microarray analysis of genes associated with ERBB2 (HER2/neu) overexpression in human mammary luminal epithelial cells. Oncogene 22:2680–2688
Monick MM, Carter AB, Robeff PK, Flaherty DM, Peterson MW, Hunninghake GW (2001) Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of β-catenin. J Immunol 166:4713–4720
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Volgestein B, Kinzler KW (1997) Activation of β-catenin–Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275:1787–1790
Nakopoulou L, Gakiopoulou-Givalou H, Karayiannakis A, Giannopoulou I, Keramopoulos A, Davaris P, Pignatelli M (2002) Abnormal α-catenin expression in invasive breast cancer correlates with poor patient survival. Histopathology 40:536–46
Palacios J, Benito N, Pizarro A, Suarez A, Espada J, Cano A, Gamallo C (1995) Anomalous expression of P-cadherin in breast carcinoma. Correlation with E-cadherin expression and pathological features. Am J Pathol 147:605–612
Paredes J, Milanezi F, Reis-Filho JS, Leitao D, Athanazio D, Schmitt F (2002) Aberrant P-cadherin expression: is it associated with estrogen-independet growth in breat cancer? Pathol Res Pract 198:795–801
Paredes J, Stove CV, Milanezi F, Van Marck V, Derycke lL, Mareel M, Bracke M, Schmitt F (2004) P-cadherin is up-regulated by antiestrogen ICI 182,780 and promotes invasion of human breast cancer cells. Cancer Res 64:8309–8317
Paredes J, Albergaria A, Oliveira JT, Jerónimo C, Milanezi F, Schmitt FC (2005) P-Cadherin overexpression is an indicator of clinical outcome in invasive breast carcinomas and is associated with CDH3 promoter hypomethylation. Clin Cancer Res 11:5869–5877
Patel IS, Madan P, Getios S, Bertrand MA, MacCalman CD (2003) Cadhering switching in ovarian cancer progression. Int J Cancer 106:172–177
Pawlowski JE, Ertel JR, Allen MP, Xu M, Butler C, Wilson EM, Wierman ME (2002) Liganded androgen receptor interaction with beta-catenin: nuclear co-localization and modulation of transcriptional activity in neuronal cells. J Biol Chem 277:20702–20710
Peralta Soler A, Knudsen K, Salazar H, Han A, Keshgegian A (1999) P-cadherin expression in breast carcinoma indicates poor survival. Cancer 86:1263–1272
Rasbridge SA, Gillett CE, Sampson SA, Walsh FS, Mills RR (1993) Epithelial (E-) and placental (P-) cadherin cell adhesion molecules expression in breast carcinoma. J Pathol 169:245–250
Rhan JJ, Dabbagh L, Pasdar M, Hugh JC (2001) The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer 91:1973–1982
Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P (1993) Association of the APC gene product with β-catenin. Science 262:1731–1734
Ryo A, Nakamura M, Wulf G, Liou Y, Lu KP (2001) Pin1 regulates turnover and subcellular localization of β-catenin by inhibiting its interaction with APC. Nat Cell Biol 3:793–801
Shimura T, Takenaka Y, Tsutsumi S, Hogan V, Kikuchi A, Raz A (2004) Galectin-3, a novel binding partner of β-catenin. Cancer Res 64:6363–6367
Siitonen SM, Kononen JT, Helin HJ, Rantala IS, Holli KA, Isola JJ (1996) Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. Am J Clin Pathol 105:394–402
Taniuchi K, Nakagawa H, Hosokawa M, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y (2005) Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res 65:3092–3099
Van’t Veer LJ, Dai H, Van de Vijver MJ (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–546
Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR (1998) Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer (Pred Oncol) 79:468–475
Wheelock MJ, Peralta Soler A, Knudsen KA (2001) Cadherin junctions in mammary tumors. J Mammary Gland Biol Neoplasia 6:275–285
Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ (2005) Deconstructing the cadherin–catenin–actin complex. Cell 123:889–901
Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z (2002) Linking beta-catenin to androgen-signaling pathway. J Biol Chem 277:11336–11344
Acknowledgments
The authors appreciate the excellent technical support provided by Arturo O. Stati, Esteban Azar, and Remedios Marin. We are very grateful to Dr. Stuart Calderwood for his helpful assistance in the editing process.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the National Research Council of Argentina (PIP 5149-CONICET) and the Argentine Foundation for Cancer Research.
Rights and permissions
About this article
Cite this article
Fanelli, M.A., Montt-Guevara, M., Diblasi, A.M. et al. P-Cadherin and β-catenin are useful prognostic markers in breast cancer patients; β-catenin interacts with heat shock protein Hsp27. Cell Stress and Chaperones 13, 207–220 (2008). https://doi.org/10.1007/s12192-007-0007-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-007-0007-z