Abstract
BCR-ABL1 plays a key role in the pathogenesis of chronic myeloid leukemia (CML), and it has been investigated as a druggable target of tyrosine kinase inhibitors (TKIs) over two decades. Since imatinib, the first TKI for anti-cancer therapy, was successfully applied in CML therapy, further generation TKIs and a novel allosteric inhibitor targeting the myristate binding site have been developed as alternative options for CML management. However, significant concerns regarding toxicity profiles, especially in long-term treatment, have emerged from TKI clinical data. Efforts to reduce adverse events and serious complications are warranted not only for survival, but also quality of life in CML patients. A better understanding of the mechanism of action will help to identify on- and off-target effects of TKIs, and guide personalized TKI drug selection in each individual CML patient. Herein, this review summarizes the biologic mechanism of BCR-ABL1 inhibition and differential target spectra, and related off-target effects of each TKI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic myeloid leukemia (CML) is a clonal disorder associated with the Philadelphia chromosome (Ph), the result of a balanced translocation between chromosomes 9 and 22. The Ph chromosome was first described in bone marrow cells obtained from CML patients using light microscopy in 1960 [1]. Thereafter, advances in molecular biology deciphered that Ph is the result of a reciprocal gene rearrangement of the Abelson 1 (ABL1) gene on chromosome 9q34 to the Breakpoint Cluster Region (BCR) gene on chromosome 22q11 [2, 3]. The resultant product protein of the fusion transcript, BCR-ABL1, induces the constitutional activation of a tyrosine kinase (TK) and consequently results in hematopoietic cell proliferation and leukemic transformation.
Based on the early recognition of pathogenesis in CML development, therapeutic strategies targeting this disease-specific and druggable kinase have been extensively investigated. As a result imatinib (STI-571), the first selective tyrosine kinase inhibitor (TKI), was successfully developed by a chemical screening approach [4]. Imatinib led to a new era in CML management in the early 2000s, and thus the history of CML set paradigm-shifting milestones for targeted anti-cancer strategies.
Survival outcomes in CML patients treated with imatinib have drastically improved compared to conventional therapy [5]. However, immediately following the widespread application of imatinib in clinical practice, several issues became apparent, such as drug resistance and intolerance to imatinib, which prompted the development of other new drugs with better efficacy and tolerability. Currently, at least four different second-generation TKIs have been successfully developed. These have shown superior efficacy compared to imatinib, with a higher rate of major and deeper molecular responses [6,7,8,9]. On the other hand, all TKIs are reported to have a variety of toxicities and serious potential long-term side effects. Accordingly, more attention is warranted when selecting TKI drugs, in particular regarding the off-target effects and related toxicities.
The life expectancy of CML patients in chronic phase (CP) has reached 98% of that in the general population [10]. For CML patients, not only survival but also quality of life has become a significant factor in TKI treatment decision-making. In line with this consideration, TKI discontinuation had been attempted successfully in selected patients who attained deep and durable molecular responses to TKI therapy [11]. In contrast, CML patients in advanced phase still suffer from significantly poorer outcomes [12]. Intolerance of TKIs can affect quality of life adversely, and can result in inadequate response and increasing risk of treatment failure. The European LeukemiaNet (ELN) guideline recommends that possible TKI-related toxicities be considered during the TKI drug selection in a comorbid patient [13].
This review summarizes the biologic mechanism of BCR-ABL1 inhibition and the target spectrum with related off-target effects of currently available TKIs, to update the differential efficacy and safety profile of each TKI.
Structure and regulation of tyrosine kinase
The exact genomic breakpoint determines the resultant BCR-ABL1 protein conformation and ultimate domain composition. DNA breakage occurs in a relatively limited region, primarily on the major BCR (M-BCR), resulting in an 8.5 kb mRNA product and constitutional 210 kDa BCR-ABL1 protein (p210BCR−ABL1) in CML cells [14, 15]. The Ph chromosome can be frequently detected in B-cell acute lymphoblastic leukemia (B-ALL), but the constitutional breakpoint of Ph is different from that of CML [16]. DNA breakage within the minor BCR (m-BCR) produces a smaller mRNA and 185/190 kDa protein (p190BCR−ABL1). However, these protein–disease correlations are not always matched. DNA breakage and subsequent splicing can affect the formation of various BCR-ABL1 fusion transcripts.
Resultant domains transcribed from the mutated BCR may include an N-terminal (oligomerization) domain, a serine/threonine kinase domain (including a docking site, Y177), a RAS homolog gene family/guanine nucleotide exchange factors (Rho/GEF) kinase domain, and/or calcium-binding domain (CalB) [17]. Domains from ABL1 consist of 3 SRC homology domains (SH3, SH2, and SH1) and a C-terminal domain. SH1 has the ABL1 kinase activity, which plays a major role in CML leukemogenesis, and SH2 and SH3 are associated with the regulation of SH1 kinase activity. Whereas normal ABL1 kinase is tightly regulated, BCR-ABL1 is constitutively activated by several mechanisms. Of those, the loss of the N-terminal sequence for myristoylation (N-cap) is a major critical event. Myristoylated N-cap interacts with the C-terminal lobe and maintains self-inhibition; however, this may be lost during fusion with the BCR gene may lead to kinase deregulation.
The 3-dimensional structure of BCR-ABL1 protein consists of two lobes: an N-terminal lobe composed of 5 β-sheets and a conserved α-helix, and a C-terminal lobe comprising α-helices [17]. In between the N- and C-terminal lobes, there are three components that link the two lobes: termed the catalytic segment, the P-loop (phosphate-binding loop), the A-loop (activation loop), and the hinge region, consisting of a ‘cleft’ between the two lobes. The ATP-binding and catalytic sites in this cleft are highly conserved. When ATP-binding occurs, the A-loop located on the C-terminal lobe alters its conformation and moves away from the catalytic center of the ABL1 kinase, forming an open conformation opposite to the inactivated-closed conformation. Tyrosine 393 (Y393) on A-loop is a key residue of activation and substrate binding. Unphosphorylated Y393 typically makes a hydrogen bond with Asparagine 363 (D363) on catalytic segment, which makes the A-loop bend toward the catalytic center and blocks substrate binding. Additionally, the A-loop has the highly conserved amino acid residue sequence at positions 381–383, called the DFG motif, which is essential for kinase activation. It coordinates with free magnesium ions, which are cofactors for catalysis.The DFG motif stays away from the catalytic center in inactivated condition, whereas it moves inside the catalytic site when the A loop is activated.
Biologic function of ABL1 kinases
The ABL1 kinase is a ubiquitous and non-receptor type kinase. Physiologically, it interacts with various cellular processes associated with cell proliferation, differentiation, survival, retraction, migration, cell adhesion, and stress response [18, 19]. It is also involved in the regulation of specialized functions in lymphocytes, neurons, and the intestinal epithelium. The function of the ABL1 kinase is not confined to a limited area, but serves as a shuttle or hub in a wide range of cellular environments. For this, ABL1 interacts with many other proteins associated with signalling pathways, other kinases, transcription factors or cell cycle regulators.
When the mutant BCR-ABL1 protein arises, loss of auto-inhibition and constitutional activation of ABL1 may occur, with consequential effects on signalling pathways related to cell cycle and apoptosis, such as the RAS/RAF/MEK/ERK pathways, the JAK2/STAT pathway, and the PI3K/AKT/mTOR pathway; and finally, it promotes the malignant transformation of hematopoietic cells [20].
Targeting tyrosine kinases in CML management
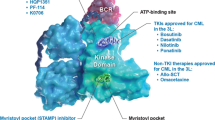
As BCR-ABL1 has been considered a druggable target exclusively expressed in CML cells and a key driver of leukemogenesis [21,22,23], it has been the main therapeutic target over the last two decades. Although remains a matter of debate whether the BCR-ABL1 fusion protein alone is sufficient to initiate and maintain the leukemogenic process of CML, the inhibition of the tyrosine kinase activity caused by BCR-ABL1 is one of the most successful therapeutic strategies in the history of cancer drug development. Based on their mechanism of action, BCR-ABL1 inhibitors can be classified into two types: the ATP-competitive inhibitors and the allosteric inhibitor.
ATP-competitive inhibitors
ATP-competitive inhibitors compete with ATP for binding to the ABL1 kinase domain through the cleft between the N- and C-terminal lobes. Because inactivated conformations of various kinases are highly similar, these have been the major target of TKIs, such as imatinib, nilotinib, and ponatinib (type 2 inhibition) [24]. On the other hand, dasatinib and bosutinib can bind to the active conformation and inhibit kinase activity (type 1 inhibition). Threonine 315 (T315) located in this region is known as a ‘gatekeeper’ residue, and its substitution with Isoleucine (T315I) blocks the binding of most 1st- and 2nd-generation TKIs [25].
The term ‘TKI’ has been widely used for ATP-competitive inhibitors until the recent development of an allosteric inhibitor, asciminib. The list of targets of currently approved ATP-competitive inhibitors is summarized in Table 1. The inhibition of BCR-ABL1 kinase activity is the main action mechanism of TKIs; however, the potency of each drug against the kinase varies considerably (Table 2). Besides, there are various kinases other than ABL1 kinase negatively regulated by TKIs, and extensive investigation to identify additional targets has been performed for decades [26,27,28,29]. TKIs with a broad spectrum of inhibition interact not only with tyrosine kinases, but also other kinomes. A differential target profile of commonly used TKIs is shown in Fig. 1. Each target inhibited by TKI therapy for CML has a unique biologic function in normal conditions, and its altered function possibly correlates with distinct clinical manifestations. With the development of various TKIs, knowledge of the differential mechanism of action and target profile of each drug should be considered in the management of patients with CML. It may guide the selection of the optimal TKI to exploit the better clinical outcome and tolerability related to on- and off-target effects of the drug.
Selectivity of ATP-competitive tyrosine kinase inhibitors. This figure shows the differential inhibition profile between imatinib (a), nilotinib (b), dasatinib (c), bosutinib (d), and ponatinib (e). The size and color density of each node represent the degree of inhibition by each kinase. Targets which were inhibited 80% or more by each kinase are labelled. Data were adapted from Uitdehaag et al. [26] and figure was generated using CORAL website (http://phanstiel-lab.med.unc.edu/CORAL/. Accessed on 16 Feb 2021)
Imatinib
Imatinib mesylate (STI-571), a first-generation TKI targeting BCR-ABL1, was developed using a screening strategy of candidate chemical compounds which selectively bound to the inactive conformational structure of BCR-ABL1 (type 2 inhibition). Based on the improved clinical activity in CML patients compared to the standard of care at the time, initial approval was given in May 2001, by the Food and Drug Administration (FDA) [4]. The IRIS trial showed that imatinib improved progression-free survival (PFS) and overall survival (OS), up to 80 – 90% and 90 – 95%, respectively, and was associated with higher rates of response over the control arm, which was cytarabine with interferon-alpha [5, 30]. However, its use can result in diverse toxicities, such as fluid retention, gastrointestinal complications, musculoskeletal pain, drug eruption, chronic fatigue, and myelosuppression. However, various mutations in ABL1 kinase domain have been found conferring resistance to imatinib [31]. The success and limitations of imatinib have led to the further development of the second-generation agents with improved efficacy [6,7,8, 32].
Importantly, imatinib can inhibit KIT and PDGFR, which gives anti-tumor effects against some malignancies carrying these targets [33, 34]. KIT is also expressed in hematopoietic stem cells, resulting in imatinib-related cytopenia. The inhibition of PDGFR, expressed in subcutaneous tissue, may frequently cause subcutaneous edema during imatinib therapy. It also inhibits DDR1/2, which is related to immunity in some solid tumors, and oxidoreductase NQO2, which is a non-kinase protein related to potential drug–drug interactions [28].
Nilotinib
Nilotinib and dasatinib are second-generation TKIs with improved efficacy against wild-type and imatinib-resistant mutant BCR-ABL1 cells. Nilotinib shares a similar target spectrum with imatinib, but has a 30-fold higher potency in vitro against BCR-ABL1 (type 2 inhibition) [35]. It can overcome some imatinib-resistant ABL1 domain-mutated CML cells [13]. Its major molecular response (MMR) and deep molecular response (DMR, defined as 4.0 log reduction of BCR-ABL1 transcript (MR4.0) or deeper) rate in TKI- naïve patients with CML CP are 77 and 66% in 5 years, and 82.6% and 73% in 10 years, respectively [6, 36].
However, nilotinib is known to increase the risk of cardiovascular events (CVE) including cardiac failure, arrhythmia, QT prolongation, and ischemic heart disease. Thus, nilotinib is not recommended in patients with a history of cerebrovascular accidents, coronary artery disease, or peripheral arterio-occlusive disease [37], and it should be used cautiously in patients with cardiovascular or metabolic comorbidities, such as diabetes mellitus. The mechanism of how nilotinib accelerates atherosclerotic processes and increases the risk of cardiovascular toxicity is not fully elucidated. Frequent hyperglycemia and dyslipidemia during nilotinib therapy might contribute to the development of atherosclerosis and coronary arterial disease, rather than cardiomyopathy. It is also known to be associated with an increased risk of pancreatitis. Grade 3–4 ischemic heart and cerebrovascular events were observed in 6.1% and 2.2%, respectively, in patients treated with nilotinib 400 mg twice daily for 5 years [6]. From the same study, nilotinib dose modification to 300 mg twice daily was shown to reduce CVE risk. In long-term follow-up data, nilotinib-associated CVE risk has been shown to gradually increase over 5 – 10 years [36].
Dasatinib
Dasatinib binds the activated and open conformation of BCR-ABL1 (type 1 inhibition) [38], and it has more than 300-fold increased potency of kinase inhibition compared to imatinib in vitro [39]. It can overcome P-loop mutations, such as Y255H, E255V/K, as well as the F359V/I/V mutation [13]. As a front-line therapy for CML CP, MMR and MR4.5 (4.5 log reduction of BCR-ABL1 transcript or deeper) rates of dasatinib therapy in 5 years are 76 and 42%, respectively [7]. The target spectrum of dasatinib is much broader than for imatinib or nilotinib, including the KIT, PDGFR, SRC family kinases, EPHA, EGFR and MAP kinases (Fig. 1c) [28]. Unlike the former two drugs, pleural or pericardial effusions are common during dasatinib treatment in newly diagnosed CML in CP. Effusions can be reversed with temporary interruption of dasatinib, diuretics or a short-term course of steroid therapy. Pulmonary arterial hypertension (PAH) is a rare but serious event associated with dasatinib, which should be permanently discontinued.
Bosutinib
Bosutinib is another 2nd-generation TKI which mainly inhibits BCR-ABL1 (type 1 inhibition) and SRC family kinases. Its ability to inhibit ABL1 kinase is 200-fold greater than imatinib [40, 41]. The recent BFORE study which compared the efficacy of bosutinib and imatinib in the front-line setting of CML CP showed higher MMR rates with bosutinib at 1 year [8]. Toxicity profiles were not significantly different between the two TKIs except for more frequent diarrhea and hepatic toxicity with bosutinib. Diarrhea is associated with serotonin re-uptake transporter (SERT) inhibition by bosutinib, increasing the level of circulating serotonin [42]. Elevation of hepatic enzymes is also commonly observed, especially in the early period of treatment with bosutinib, but may persist beyond 12 months [43] and lead to discontinuation of the drug in some patients. Notably, bosutinib does not have clinically relevant activity against KIT or PDGFRA, but it does inhibit additional molecules associated with cell cycle regulation and calcium/calmodulin-dependent protein kinases (CAMK) (Fig. 1d) [44, 45]. Long-term efficacy and adverse event profiles remain to be further investigated.
Ponatinib
Ponatinib is a third-generation TKI which has the broadest spectrum of its targets, covering ABL1, KIT, PDGFR, SRC family, VEGFR, EGFR, HER2, FLT3, FGFR, and JAK2. The purpose of its initial development was to overcome the resistance to prior generation TKIs, especially of the T315I mutation, by the introduction of a triple bond ethynyl linker which allows ponatinib to span the bulky T315I isoleucine residue side chain in the ATP-binding site. As a multi-kinase inhibitor, the potential efficacy of ponatinib against various cancers as well as CML has been investigated. Ponatinib was approved by FDA in 2012 based on excellent efficacy against CML in the PACE trial [46]. However, clinical use should be undertaken with caution due to the concerning increased arterial occlusive events (AOEs), which is related to multiple targets involving endothelial function and atherosclerosis [9, 46]. The phase 3 EPIC trial comparing the front-line use of imatinib and ponatinib for CML was terminated early due to concerns of up to 6% serious AOEs in the ponatinib arm. Therefore, the European LeukemiaNet (ELN) 2020 guideline recommends that ponatinib should be confined to a third-line treatment option after failure of two or more TKIs or for the treatment of highly resistant disease carrying the T315I mutation [13]. Close monitoring of cardiac function and adequate management of risk factors, such as hypertension, dyslipidemia, and DM, are required, including smoking cessation. Dose adjustment should be considered in less-resistant disease or in intolerant patients. Recently, the OPTIC trial evaluated a response-based dose adjustment, suggesting that ponatinib dose can be reduced to 15 mg in patients who achieved BCR-ABL1 transcript level ≤ 1% [47]. The safety profile, particularly hematologic toxicities and AOEs, was much improved compared to the 45 mg ponatinib dose group. Based on these data, the dose of ponatinib should be reduced when an appropriate molecular response is attained.
The allosteric inhibitor - Asciminib
Allosteric inhibition is a more recently identified strategy investigating druggable targets other than the ATP-binding site. One of the targets is the myristoylated N-cap of ABL1, which regulates ABL1 kinase activity by binding to a hydrophobic myristate pocket of the C-terminal lobe. This binding leads to a conformational change of the kinase domain, which is essential for interaction with the SH3–SH2 domains, keeping the kinase in an inactive state. As mentioned above, the loss of the N-cap myristoyl group occurs during gene translocation and transcription of BCR-ABL1, inducing the constitutional activation of the kinase. Compounds that bind to the myristate pocket may restore the natural regulation of ABL1 kinase activity. This mechanism is independent of the conformational change of the A loop, the target of ATP-competitive inhibitors. This suggests potential synergistic activities of the two inhibitors overcoming the resistance to pre-existing TKIs which bind to the ATP-binding pocket.
Asciminib (ABL001) is the only described allosteric inhibitor showing clinical efficacy in CML. It showed feasible activity against CML that failed three or more ATP-competitive TKIs and harboured resistant mutations [48]. As it specifically binds to the myristate pocket, rather than other orthosteric sites, asciminib has a highly specific on-target effect against the ABL1 kinase without any significant off-target effects [49]. Notably, it does not inhibit ABL1-dependent cellular proliferation [50]. The MMR rate in CML CP at 1 year was 48% in patients who failed at least two previous lines of TKI therapy, and in those with the T315I mutation, the MMR rate was 24% [48]. Common adverse events related to asciminib were rash, fatigue, nausea, headache, arthralgia, and pancreatitis. Preclinical studies suggested that asciminib can be combined with other TKIs, which can synergistically increase efficacy against CML with resistant mutations [51, 52]. Recently, the result of a randomized trial comparing asciminib to bosutinib in CML patients who failed two lines of TKI therapy or beyond showed better outcomes with asciminib [53]. Further clinical and experimental data on the target spectrum of asciminib are required.
Specific targets of tyrosine kinase inhibitors and related adverse events
Wild-type ABL inhibition reported with all TKIs is reported to be associated with cardiac toxicity. Left ventricular dysfunction related to imatinib was first described in 2006, although serious events reported in clinical trials were rare [54]. Despite little clinical evidence of imatinib-induced cardiotoxicity, cardiovascular adverse events associated with other TKIs have been reported consistently, likely due to additional inhibition of kinases other than ABL1.
PDGFR is a major target of most TKIs except bosutinib and asciminib [29], and fluid retention, serositis, and pleural/pericardial effusion are significant off-target effects of PDGFR inhibition. PDGFR is primarily expressed in pericytes and pulmonary tissue, and PDGFR inhibition leads to altered fluid homeostasis between tumor, vascular, and interstitial compartments, resulting in peripheral edema and third space loss of fluid, associated with the use of most TKIs [55, 56].
The incidence of pleural or pericardial effusions with dasatinib is much higher than imatinib and nilotinib [5,6,7], and this may be explained by more potent PDGFR inhibition as well as additional inhibition of kinases, such as SRC family kinases [57]. SRC family kinases which are differentially suppressed by dasatinib, bosutinib, and ponatinib, are predominantly expressed in hematopoietic cells, and play key roles in various signal transduction pathways associated with cell survival and differentiation, angiogenesis, and vascular permeability [58]. Among the SRC family kinases, YES and SRC are widely expressed in the lung, and are directly associated with vascular endothelial growth factor (VEGF)-mediated vascular permeability [59]. Interestingly, bosutinib, a dual-ABL1/SRC inhibitor, has been associated with a low incidence of pleural effusion (1.5%) owing to the relatively low activity of bosutinib against PDGFR [8].
Ponatinib can inhibit not only PDGFR and SRC family kinases but also KDR (VEGFR) and FGFR activities, and ponatinib has been associated with a higher frequency of hypertension, AOEs, and venous thromboembolism (VTE), rather than fluid retention or effusions [60]. VEGFR is expressed in vascular endothelial cells and hematopoietic cells, mediating angiogenesis and vascular permeability as well as bone metabolism, hematopoiesis, wound healing, and development [61]. FGFR regulates various cellular signalling pathways involving cell growth, survival, and angiogenesis [62, 63]; LYN and FYN, of the SRC family, are involved in the regulation of platelet function [64] and TEK (Tie2) is important to endothelial cell survival [65]. Broad-spectrum inhibition of regulators of vascular wall and platelet function leads to ponatinib-induced thrombosis. The risk of cardiovascular and thrombotic events in patients treated with ponatinib, which has the broadest target spectrum, is higher than those of other TKIs [66, 67]. Unlike nilotinib cardiotoxicity, which is mainly contributed by metabolic and atherosclerotic changes, ponatinib therapy induces endothelial damage and arterial thrombosis leading to subsequent AOEs.
Another consequence of anti-PDGFR therapy is altered bone metabolism [29]. The development and activation of osteoclasts and osteoblasts can be decreased by TKIs, resulting in decreased bone resorption, altered bone remodelling, and compensatory parathyroid hormone secretion accompanied by serum hypophosphatemia. Imatinib can induce a generalized decrease in osteoclast activity, which cannot be compensated by decreased osteoblast activity, leading to a reduction in bone mineral density (BMD) [68, 69]. Imatinib may require regular monitoring of the BMD in patients treated over the long term. CSF1R, which regulates the maturation of macrophages into osteoclasts, may be inhibited by TKIs, especially by dasatinib and ponatinib. CSF1R inhibition also contributes to the dysregulation of bone metabolism and is associated with myelosuppression. Relatively, high rates of grade 3–4 myelosuppression by dasatinib and ponatinib correlate with their differential mechanism.
The inhibition of KIT by most TKIs except bosutinib leads to myelosuppression, as KIT has a critical role in the development of hematopoietic cells, melanocytes, and mast cells [70]. The varied potency of KIT inhibition among TKIs may correlate with the severity of drug-induced myelosuppression and cytopenias. Concurrent SRC kinase inhibition by dasatinib and ponatinib appears to be responsible for their high rate of myelosuppression [7, 46].
Finally, alterations in KIT functioning by TKIs are also associated with dermatologic toxicity, commonly including skin rash, superficial edema, and changes in pigmentation [5, 30].
Summary
Although the entire list of adverse events cannot be necessarily integrated with specific off-target effects and the potency of each TKI, the pattern of differential TKI activity is linked to the safety profiles in the treatment of CML patients. Understanding the mechanism of action and specific off-target profiles of clinically available TKIs will help guide personalized management for patients with CML. Future investigation of selective kinase inhibition by new drugs, including asciminib, should be undertaken.
References
Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85–109.
Groffen J, Stephenson JR, Heisterkamp N, Bartram C, de Klein A, Grosveld G. The human c-abl oncogene in the Philadelphia translocation. J Cell Physiol Suppl. 1984;3:179–91.
Rowley JD. Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–3.
Cohen MH, Williams G, Johnson JR, Duan J, Gobburu J, Rahman A, et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res. 2002;8:935–42.
O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004.
Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of front-line nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54.
Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boque C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333–40.
Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim DW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231–7.
Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Assouline S, et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:612–21.
Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851–7.
Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17–23.
Shah NP. Advanced CML: therapeutic options for patients in accelerated and blast phases. J Natl Compr Canc Netw. 2008;6(Suppl 2):S31–6.
Hehlmann R. The new ELN recommendations for treating CML. J Clin Med. 2020;9:3671.
Clark SS, McLaughlin J, Crist WM, Champlin R, Witte ON. Unique forms of the abl tyrosine kinase distinguish Ph1-positive CML from Ph1-positive ALL. Science. 1987;235:85–8.
Groffen J, Stephenson JR, Heisterkamp N, de Klein A, Bartram CR, Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984;36:93–9.
Hermans A, Heisterkamp N, von Linden M, van Baal S, Meijer D, van der Plas D, et al. Unique fusion of bcr and c-abl genes in Philadelphia chromosome positive acute lymphoblastic leukemia. Cell. 1987;51:33–40.
Panjarian S, Iacob RE, Chen S, Engen JR, Smithgall TE. Structure and dynamic regulation of Abl kinases. J Biol Chem. 2013;288:5443–50.
Wang JY. The capable ABL: what is its biological function? Mol Cell Biol. 2014;34:1188–97.
Soverini S, Mancini M, Bavaro L, Cavo M, Martinelli G. Chronic myeloid leukemia: the paradigm of targeting oncogenic tyrosine kinase signaling and counteracting resistance for successful cancer therapy. Mol Cancer. 2018;17:49.
Melo JV, Deininger MW. Biology of chronic myelogenous leukemia—signaling pathways of initiation and transformation. Hematol Oncol Clin North Am. 2004;18:545–68 (vii–viii).
Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–30.
Kelliher MA, McLaughlin J, Witte ON, Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci USA. 1990;87:6649–53.
Ramaraj P, Singh H, Niu N, Chu S, Holtz M, Yee JK, et al. Effect of mutational inactivation of tyrosine kinase activity on BCR/ABL-induced abnormalities in cell growth and adhesion in human hematopoietic progenitors. Cancer Res. 2004;64:5322–31.
Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–42.
Azam M, Seeliger MA, Gray NS, Kuriyan J, Daley GQ. Activation of tyrosine kinases by mutation of the gatekeeper threonine. Nat Struct Mol Biol. 2008;15:1109–18.
Uitdehaag JC, de Roos JA, van Doornmalen AM, Prinsen MB, de Man J, Tanizawa Y, et al. Comparison of the cancer gene targeting and biochemical selectivities of all targeted kinase inhibitors approved for clinical use. PLoS ONE. 2014;9:e92146.
Kitagawa D, Yokota K, Gouda M, Narumi Y, Ohmoto H, Nishiwaki E, et al. Activity-based kinase profiling of approved tyrosine kinase inhibitors. Genes Cells. 2013;18:110–22.
Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk Lymphoma. 2008;49:615–9.
Giles FJ, O’Dwyer M, Swords R. Class effects of tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia. 2009;23:1698–707.
Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–27.
Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80.
Kwak JY, Kim SH, Oh SJ, Zang DY, Kim H, Kim JA, et al. Phase III clinical trial (RERISE study) results of efficacy and safety of radotinib compared with imatinib in newly diagnosed chronic phase chronic myeloid leukemia. Clin Cancer Res. 2017;23:7180–8.
McGary EC, Weber K, Mills L, Doucet M, Lewis V, Lev DC, et al. Inhibition of platelet-derived growth factor-mediated proliferation of osteosarcoma cells by the novel tyrosine kinase inhibitor STI571. Clin Cancer Res. 2002;8:3584–91.
Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–32.
Weisberg E, Manley P, Mestan J, Cowan-Jacob S, Ray A, Griffin JD. AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL. Br J Cancer. 2006;94:1765–9.
Kantarjian HM, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre P, et al. Long-term outcomes with front-line nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35:440–53.
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84.
Tokarski JS, Newitt JA, Chang CY, Cheng JD, Wittekind M, Kiefer SE, et al. The structure of Dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res. 2006;66:5790–7.
Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401.
Golas JM, Arndt K, Etienne C, Lucas J, Nardin D, Gibbons J, et al. SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res. 2003;63:375–81.
Puttini M, Coluccia AM, Boschelli F, Cleris L, Marchesi E, Donella-Deana A, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66:11314–22.
European Medicines Agency: EMEA/H/C/002373—https://www.ema.europa.eu/en/documents/assess-ment-report/bosulif-epar-public-assessment-report_en.pdf. Feb 2021
Brümmendorf TH, Cortes JE, Milojkovic D, Gambacorti-Passerini C, Clark RE, le Coutre PE, et al. Bosutinib (BOS) versus imatinib for newly diagnosed chronic phase (CP) chronic myeloid leukemia (CML): final 5-year results from the bfore tria. Blood. 2020;136:41–2.
Remsing Rix LL, Rix U, Colinge J, Hantschel O, Bennett KL, Stranzl T, et al. Global target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cells. Leukemia. 2009;23:477–85.
Mancini M, Brusa G, Zuffa E, Corrado P, Martinelli G, Grafone T, et al. Persistent Cdk2 inactivation drives growth arrest of BCR-ABL-expressing cells in response to dual inhibitor of SRC and ABL kinases SKI606. Leuk Res. 2007;31:979–87.
Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075–88.
Cortes JE, Lomaia E, Turkina A, Moiraghi B, Sutton MU, Pavlovsky C, et al. Interim analysis (IA) of OPTIC: a dose-ranging study of three ponatinib (PON) starting doses. J Clin Oncol. 2020;38:7502.
Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381:2315–26.
Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543:733–7.
Manley PW, Barys L, Cowan-Jacob SW. The specificity of asciminib, a potential treatment for chronic myeloid leukemia, as a myristate-pocket binding ABL inhibitor and analysis of its interactions with mutant forms of BCR-ABL1 kinase. Leuk Res. 2020;98:106458.
Lindström HJG, Friedman R. The effects of combination treatments on drug resistance in chronic myeloid leukaemia: an evaluation of the tyrosine kinase inhibitors axitinib and asciminib. BMC Cancer. 2020;20:397.
Eide CA, Zabriskie MS, Savage Stevens SL, Antelope O, Vellore NA, Than H, et al. Combining the allosteric inhibitor asciminib with ponatinib suppresses emergence of and restores efficacy against highly resistant BCR-ABL1 mutants. Cancer Cell. 2019;36(431–43):e5.
Hochhaus A, Boquimpani C, Rea D, Minami Y, Lomaia E, Voloshin S, et al. Efficacy and safety results from ASCEMBL, a multicenter, open-label, phase 3 study of asciminib, a first-in-class STAMP inhibitor, vs bosutinib (BOS) in patients (Pts) with chronic myeloid leukemia in chronic phase (CML-CP) previously treated with ≥2 tyrosine kinase inhibitors (TKIs). Blood. 2020;136(Suppl 2):LBA-4.
Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–16.
Ostman A, Heldin CH. PDGF receptors as targets in tumor treatment. Adv Cancer Res. 2007;97:247–74.
Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–95.
Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carbox-amide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–61.
Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000;100:293–6.
Quintas-Cardama A, Kantarjian H, O’Brien S, Borthakur G, Bruzzi J, Munden R, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25:3908–14.
Zeng P, Schmaier A. Ponatinib and other CML tyrosine kinase inhibitors in thrombosis. Int J Mol Sci. 2020;21:6556.
Alvarez-Aznar A, Muhl L, Gaengel K. VEGF receptor tyrosine kinases: key regulators of vascular function. Curr Top Dev Biol. 2017;123:433–82.
Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41.
Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. Biochem J. 2011;437:199–213.
Quek LS, Pasquet JM, Hers I, Cornall R, Knight G, Barnes M, et al. Fyn and Lyn phosphorylate the Fc receptor gamma chain downstream of glycoprotein VI in murine platelets, and Lyn regulates a novel feedback pathway. Blood. 2000;96:4246–53.
Milam KE, Parikh SM. The angiopoietin-Tie2 signaling axis in the vascular leakage of systemic inflammation. Tissue Barriers. 2015;3:e957508.
Cirmi S, El Abd A, Letinier L, Navarra M, Salvo F. Cardiovascular toxicity of tyrosine kinase inhibitors used in chronic myeloid leukemia: an analysis of the FDA adverse event reporting system database (FAERS). Cancers (Basel). 2020;12:826.
Jain P, Kantarjian H, Boddu PC, Nogueras-Gonzalez GM, Verstovsek S, Garcia-Manero G, et al. Analysis of cardiovascular and arteriothrombotic adverse events in chronic-phase CML patients after front-line TKIs. Blood Adv. 2019;3:851–61.
Vandyke K, Fitter S, Drew J, Fukumoto S, Schultz CG, Sims NA, et al. Prospective histomorphometric and DXA evaluation of bone remodeling in imatinib-treated CML patients: evidence for site-specific skeletal effects. J Clin Endocrinol Metab. 2013;98:67–76.
Aleman JO, Farooki A, Girotra M. Effects of tyrosine kinase inhibition on bone metabolism: untargeted consequences of targeted therapies. Endocr Relat Cancer. 2014;21:R247–59.
Ratajczak MZ, Luger SM, DeRiel K, Abrahm J, Calabretta B, Gewirtz AM. Role of the KIT protooncogene in normal and malignant human hematopoiesis. Proc Natl Acad Sci USA. 1992;89:1710–4.
Redaelli S, Mologni L, Rostagno R, Piazza R, Magistroni V, Ceccon M, et al. Three novel patient-derived BCR/ABL mutants show different sensitivity to second and third generation tyrosine kinase inhibitors. Am J Hematol. 2012;87:E125–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DK has received honoraria and research grant from Novartis, Pfizer, and Paladin, has served on advisory boards for Novartis, Pfizer, and Paladin, and has received research grant from BMS. HL and INB have no conflicts of interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Lee, H., Basso, I.N. & Kim, D.D.H. Target spectrum of the BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia. Int J Hematol 113, 632–641 (2021). https://doi.org/10.1007/s12185-021-03126-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03126-6