Abstract
We herein report the results of the New TARGET study 2nd-line, which collected data on patients with chronic-phase (CP) chronic myeloid leukemia (CML) who received a 2nd-line tyrosine kinase inhibitor (TKI) because of resistance and/or to a 1st-line TKI. A total of 98 patients were enrolled intolerance between April 2010 and March 2013, and 82 patients were analyzed. The median age was 54 years (range 22–88 years). Seventy-six patients (93%) received imatinib as the 1st-line TKI. Forty-five (55%) and 37 (45%) patients began nilotinib and dasatinib treatments at entry, respectively. First-line TKI treatment achieved complete hematological response in 79 patients (96%) and complete cytogenetic response (CCyR) in 49 patients (60%), respectively. Nine patients (11%) had BCR-ABL1 kinase domain point mutations at enrollment. The estimated 3-year progression-free-survival rate after enrollment was 98.7% (95% CI 91.1–99.8%). Overall, the probabilities of achieving CCyR and a major molecular response were 89.3% (95% CI 81.4–94.8%) and 87.2% (95% CI 78.1–93.8%), respectively. There were no new safety issues. This study demonstrated that CML-CP patients in Japan who are resistant and/or intolerant to a 1st-line TKI can achieve an extremely good outcome by 2nd-line TKI treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Imatinib has dramatically improved the treatment outcomes of patients with chronic myeloid leukemia (CML) in chronic phase (CP). In the 10-year follow-up data of the International Randomized Study of Interferon and STI571 (IRIS) study, the rates of complete cytogenetic response (CCyR) and estimated overall survival (OS) with imatinib were 82.8% and 83.3%, respectively [1]. However, some patients with CML-CP develop resistance and/or intolerance to imatinib. Mechanisms of resistance to imatinib involve multiple factors including treatment adherence, drug bioavailability, and pharmacodynamics, with the most common cause being BCR-ABL1 kinase domain (KD) mutations [2, 3]. To overcome these issues, the second-generation (2G) tyrosine kinase inhibitors (TKIs)—i.e., nilotinib, dasatinib, and bosutinib—were developed, and patients with resistance and/or intolerance to imatinib achieved a good response by 2G-TKIs [4,5,6]. In Japan, nilotinib and dasatinib were approved by the Ministry of Health, Labor and Welfare for CML patients who showed resistance or intolerance to imatinib in January 2009. Subsequently, bosutinib and ponatinib were approved for such patients in September 2014 and in September 2016, respectively.
Randomized-controlled trials (RCTs) are the backbone of clinical evaluations of the efficacy and safety of new therapies. However, clinical questions in daily practice will be best answered by evaluating the real-world clinical outcomes. Prospective observational cohort study can extend the knowledge beyond the clinical trial setting. Moreover, in the case of CML, individual hematologists will have only limited treatment experience, since CML is a rare hematological malignancy with an age-adjusted incidence of only 0.5 per 100,000 persons in Japan [7].
With this background, the Japanese Society of Hematology (JSH) established the TARGET (Timely and Appropriate Registration System for GLIVEC Therapy) system to share clinical practice data with the aim of improving the quality of care for CML in Japan [8]. The TARGET system is an online database that can show the collected data on the web site in real time, and the results of a large-scale observational study of newly diagnosed CML patients treated with imatinib using this system were previously published [9].
The JSH further conducted two prospective observational studies for CML-CP patients receiving the 1st-line TKI therapy (designated as the New TARGET study 1st-line) [10] and 2nd-line TKI therapy (designated as the New TARGET study 2nd-line), respectively. We herein report the results of the New TARGET study 2nd-line, which enrolled CML-CP patients who exhibited resistance and/or intolerance to the 1st-line TKI.
Patients and methods
Study design and objectives
The New TARGET study 2nd-line is a multicenter prospective observational study for CML-CP patients administered a 2nd-line TKI due to resistance and/or intolerance to a 1st-line TKI (UMIN000003582). Physicians prospectively enter clinical data with regard to the prescription status of TKI, treatment outcomes, response, and occurrence of adverse events (AEs) using the New TARGET system. The choice of 2nd-line and subsequent TKIs—i.e., imatinib, nilotinib, dasatinib, or bosutinib—was at the discretion of each physician. The study was conducted in accordance with the Declaration of Helsinki and was approved by local ethical committees of all participating institutions. All patients gave written informed consent according to institutional guidelines.
The primary endpoint was the 3-year OS rate after switching to the 2nd-line or subsequent TKI treatment, and secondary endpoints were the 3-year progression-free survival (PFS) rate, event-free survival (EFS) rate, and cumulative incidence of cytogenetic and molecular response after switching to the 2nd-line or subsequent TKI treatment. Events were defined as loss of complete hematological response (CHR), loss of partial cytogenetic response (PCyR), loss of CCyR, progression to accelerated phase/blast crisis, and death by any cause.
We also assessed the treatment outcomes according to the Sokal risk classification at diagnosis, type of TKI selected as the 2nd-line or subsequent treatment, and BCR-ABL1 mutations.
Eligibility criteria
Patients with CML-CP who showed resistance and/or intolerance to the 1st-line TKI (imatinib, nilotinib, or dasatinib) were enrolled. CP was defined as follows: less than 15% blasts in the peripheral blood (PB), less than 30% blasts in the bone marrow (BM), less than 20% basophils in the PB, and no extramedullary involvement except for liver and spleen [11]. Resistance to TKIs was defined as failure or suboptimal response in European LeukemiaNet (ELN) recommendations in 2009 [12]. Intolerance to TKI was defined as any toxicity at grade 2 or higher severity lasting more than 1 month despite the optimal supportive care or at grade 3/4 which led to reduction or discontinuation of TKI.
Patient evaluation
Patients were evaluated on the basis of hematological, cytogenetic, and molecular examinations. Complete blood counts and biochemistries were performed at enrollment and every 3 months. Cytogenetics analyses on BM aspiration were performed at enrollment and every 6 months. Fluorescent in situ hybridization (FISH) assessments of peripheral blood were done every 6 months. Achievement of a major molecular response (MMR) was assessed at the BCR-ABL1 transcript level using real-time quantitative reverse-transcriptase polymerase chain reactions (RQ-PCR), which were performed every 3 months for the first 12 months and then every 6 months afterward. Hematological, cytogenetic, and molecular responses were evaluated according to the definition of ELN recommendations [12].
Additionally, peripheral blood cell mRNA was subjected to BCR-ABL1 gene mutation analysis ranging from codon 225–505 by direct sequencing at enrollment, 12 months, and when the copy number of RQ-PCR showed a more than a fivefold increase over the lowest level. If mutation was detected, this analysis could be repeated at 24 and 36 months. All mutational assessments were performed at BML Inc. (Tokyo).
AEs were assessed using the National Cancer Institute’s Common Terminology Criteria (NCI-CTC) for Adverse Events version 4.03.
Statistical analyses
All the efficacy analyses were performed on the basis of the intention-to-treat principle. Comparison of response rates among subgroups was performed using post hoc analyses. The P values for post hoc analyses were not adjusted for multiple comparisons and are provided for descriptive purposes. The differences between two groups were compared using Fisher’s exact test or the Mann–Whitney U test, as appropriate. OS, PFS, EFS, and the times to responses were estimated using Kaplan–Meier methods. Differences between subgroups in the times to responses were evaluated with the use of a log-rank test. The 95% confidence intervals (CIs) for survival and response rates were calculated using the Clopper–Pearson method. Statistical analyses were performed using SAS statistical software (version 9.3).
Results
Patients and treatment
A total of 98 patients from 46 institutions were enrolled in this study between April, 2010 and March, 2013 in Japan. Of these, 82 patients with at least 3 months of follow-up were included in the analysis. The cut-off date was March, 2016 (3 years after the last patient enrolled).
Patients’ demographics at enrollment are shown in Table 1. Their median age was 54 years (range 22–88 years), and 23 (28%) patients were over 65 years old. There was a male predominance. The median period from CML diagnosis was 33 months (range 3–214 months). With regard to the 1st-line TKI, 76 patients (93%) received imatinib as the first and only TKI, 4 patients received one 2G TKI in addition to imatinib (these 3rd-line patients were included because of the very short-term treatment of the 1st-line TKI and analyzed together with the other patients), 1 patient received dasatinib as the first and only TKI, and there was no data for 1 patient. Among 80 patients who received imatinib as the 1st-line TKI, 49 patients (60%) were imatinib-resistant (failure or suboptimal response), and 31 patients (38%) were imatinib-intolerant. At the baseline, 79 patients (96%) had achieved CHR in response to the 1st-line TKI. PCyR had been achieved in 63 patients (77%) and CCyR in 49 patients (60%), and 2 patients had already achieved MMR. Nineteen patients (23%) had comorbidities and the most common comorbidity was hypertension (7%). Comorbidities reported in more than 2 cases are listed in Table 1.
The type and dose of the 2nd-line TKI were at the discretion of each physician. At the time of enrollment, 45 patients were started on nilotinib, and 37 patients were started on dasatinib. The median starting daily dose of nilotinib was 600 mg (range 300–800 mg), and that of dasatinib was 100 mg (range 50–100 mg). During the follow-up period, the doses of TKIs were reduced in 26 patients (32%) (nilotinib: 17 patients; dasatinib: 9 patients) and the 2nd-line TKI was switched to the 3rd-line TKI in 13 (16%) (nilotinib: 10 patients; dasatinib: 3 patients). Among those 65 years old or older, dose reduction was done in 13 of 23 patients and a switch to the 3rd-line TKI was done in 7 of 23 patients. In other words, the proportion of the patients who needed dose-reduction and/or a switch to the 3rd-line TKI was 65% in the elderly (≥ 65 years old) and 25% in the younger (< 65 years old) patients. However, the reasons for dose reduction and the switch to the 3rd-line TKI were not collected in this study.
The patients’ demographics data by their 2nd-line TKI (nilotinib or dasatinib) are also shown in Table 1. There was a little difference between the two groups, but with regard to the reason for discontinuation of imatinib, patients with imatinib resistance were more frequent than those with imatinib intolerance in the dasatinib group (81% vs. 16%), but not in the nilotinib group (42% vs. 56%). In this study protocol, since the accelerated phase was defined by hematological values despite the additional chromosomal abnormality (ACA), patients with ACA at the enrollment were acceptable as in CP. Two patients with ACA at the enrollment were treated with dasatinib. One patient had major route ACA (complex karyotype including trisomy 8), and the other had minor route ACA (loss of the Y chromosome).
Survival rate
The estimated 3-year OS rate after the enrollment was 100% (Fig. 1a), and the 3-year PFS and EFS rates were 98.7% (95% CI 91.1–99.8%; Fig. 1b) and 96.2% (95% CI 88.8–98.8%; Fig. 1c), respectively. Regarding disease progression during the observation period, only one patient, who had major route ACA at enrollment, progressed to the accelerated phase, but this patient was censored 1 month after progression because of loss to follow-up. Except for the patient, the estimated 3-year OS, PFS, and EFS rates were 100%, 100%, and 97.5% (95% CI 90.3–99.4%), respectively. Fourteen (17%) patients were censored within 3 years. Three patients died from colorectal cancer, aortic aneurysm, and heart failure due to atrial fibrillation during the follow-up period. However, no one died from CML-related causes.
Kaplan–Meier estimated survival rates after switching to second-line or subsequent tyrosine kinase inhibitors treatment. a The estimated 3-year overall survival in the intention-to-treat population was 100.0%. b The estimated 3-year progression-free survival and c event-free survival in the intention-to-treat population were 98.7% (95% CI 91.1–99.8%) and 96.2% (95% CI 88.8–98.8%)
Response to the second/subsequent TKIs therapy
Overall, the estimated probabilities of achieving CCyR and MMR were 89.3% (95% CI 81.4–94.8%; Fig. 2a) and 87.2% (95% CI 78.1–93.8%; Fig. 2b) during the follow-up period.
Subgroup analyses of the response rates are shown in Table 2. The cumulative incidence of CCyR and MMR was significantly higher among patients with CCyR than among patients without CCyR at enrollment (CCyR: 100% vs. 72.5%, P < 0.0001; MMR: 92.8% vs. 78.1%, P = 0.01). Sokal score at diagnosis had a significant effect on the achievement of CCyR (P < 0.01) but not of MMR. There was no difference in the achievement rates of CCyR or of MMR between the older (≥ 65 years old) and the younger (< 65 years old) groups. Regarding the type of TKIs, nilotinib achieved CCyR more frequently than dasatinib (92.3% vs. 83.8%, P = 0.04), while the proportions of the patients who achieved MMR were not different between these two groups (nilotinib 89.8% vs. dasatinib 83.0%, P = 0.11). Concerning the reason for discontinuation of imatinib, the cumulative achievement rates of CCyR and MMR were significantly higher in imatinib-intolerant patients than in imatinib-resistant patients (CCyR: 100% vs. 84.1%, P < 0.01; MMR: 96.8% vs. 82.3%, P = 0.02). In addition, in imatinib-resistant patients, the cumulative incidence of CCyR and MMR was not significantly different between the dasatinib group and the nilotinib group.
Mutation analysis
BCR-ABL mutation analysis was performed in 78 patients at enrollment. Nine types of BCR-ABL1 KD point mutations (L248V, G250E, E255K, T315I, N331S, M351T, F359I, L384M, and S385I) were detected in 9 (12%) patients, among which 8 mutations had shown resistance to the 1st-line TKI (Table 3). Six patients achieved MMR, and their mutations became undetectable after the 2nd-line TKI treatment. A case with T315I mutation was censored at 9 months because of loss to follow-up. Moreover, 6 types of BCR-ABL1 KD point mutations (E236G, Y353H, L384P, V422A, W423R, and C477T) newly emerged in 6 patients after enrolling in this study, and all of them achieved MMR without switching to the 3rd-line TKI (Table 4).
With regard to other mutations, BCR-ABL135INS, the insertion of 35 intronic nucleotides at the exon 8/9 splice junction that introduces a stop codon after 10 intron-encoded residues was detected in 16 patients (20%). This splicing variant was detected in seven patients at enrollment and in nine patients after enrollment. Fourteen of 16 patients (87.5%) with BCR-ABL135INS achieved MMR. In addition, the exon 7 deletion was detected in three patients during the follow-up period, in whom two patients achieved MMR. Overall, BCR-ABL1 KD point mutation at enrollment did not affect the response rates.
Adverse events
AEs considered to be TKI-related are summarized in Table 5. As for hematologic AEs, anemia, neutropenia, and thrombocytopenia occurred in 24%, 9%, and 11% in all grades, and grade 3/4 AEs were observed in approximately 5% each.
The most commonly reported non-hematologic AEs were rash (17%), edema (12%), nausea (9%), and headache (9%). In addition to the AEs described in Table 5, the following AEs were observed in 1 patient each: pancreatitis, prolonged QT interval, bradycardia, palpitation, and bone pain in patients treated with nilotinib, and chronic kidney disease, gastrointestinal bleeding, bleeding, dyspnea, fatigue, and weight loss in patients treated with dasatinib. With regard to grade 3/4 non-hematologic toxicities, rash, headache, and chronic kidney disease were noted in only one patient each. Neither pulmonary hypertension nor peripheral artery occlusive disease was observed in this study.
The most commonly reported biochemical laboratory abnormalities were total bilirubin elevation (9%) and lipase elevation (5%). All but one of the patients with elevated bilirubin belonged to the nilotinib group.
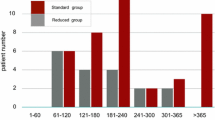
The onset of AEs is summarized in Fig. 3. Of the total 124 AEs, 65 (52%) were reported within 1 month after commencing the 2nd-line TKI, and new AEs rarely occurred 12 months after the 2nd-line TKI treatment. However, the influence of AEs on prescription was not obvious in this study.
The time-to-onset of adverse events. Times of the initial report of an adverse event (AE) after commencing a second/subsequent TKI are shown. The left bar indicates the proportion of patients with AEs of any grade, and the right bar indicates the proportion of patients with grade 3/4 AEs for each month
Discussion
This observational study revealed the “real-world” prognosis and responses to the 2nd-line and subsequent TKIs treatment in CML-CP patients with resistance and/or intolerance to the 1st-line TKI in Japan. Among the subjects of this study, no one died of a CML-related cause, and only one patient with major route ACA progressed to the accelerated phase. The probabilities of achieving CCyR and MMR were 89.3% and 87.2%, respectively.
In general, clinical trials for CML patients have excluded patients with major organ dysfunctions, especially cardiac disorder [11, 13], but there were no exclusion criteria for organ dysfunctions in this study. In fact, 23% of patients had comorbidity, including a small number of patients with heart disease. However, most of the patients had a good performance status (ECOG PS score 0 or 1). The median age was 54 years, which was comparable to that in the clinical trials [11, 13]. Regarding treatment response at enrollment, the proportion of patients with CHR was 96% in this study, which was much higher than that in the previous clinical trials [11, 13].
At enrollment, all patients were started on nilotinib or dasatinib, and no patient received bosutinib, which was approved in Japan after the close of the enrollment period of this study. Moreover, comparing the background of the patients treated with nilotinib and dasatinib, 25 of 31 patients (81%) with imatinib intolerance were started on nilotinib, and 30 of 49 patients (60%) with imatinib resistance were started on dasatinib. This difference was probably dependent on the expectancies and concerns of the Japanese hematologists as to the efficacy and safety of 2G-TKIs. The guidelines recommended that the choice of the TKI must take into account tolerability, safety, and patient characteristics, particularly comorbidities, because comorbidities are the major cause of death in CML patients and may be exacerbated by AEs of TKI [14, 15]. In general, dasatinib is associated with pleural effusion and, thus, should be avoided for patients with pulmonary diseases or uncontrolled hypertension. Nilotinib is associated with hyperglycemia and vascular events and, thus, should be avoided for patients with diabetes mellitus or vascular diseases. Actually, two patients with diabetes were started on dasatinib in this study.
The cumulative achievement rates of CCyR and MMR at 3 years after enrollment were 89.3% and 85.4%, and the 3-year OS and PFS were 100% and 98.7%, respectively, all of which were higher than those reported in the previous clinical trials. The response to TKI is the most important prognostic factor in CML-CP patients [14], and the response status at switching to a 2nd-line TKI has been shown to significantly affect the subsequent responses in patients with imatinib resistance or intolerance [5, 6]. The rate of CCyR at enrollment was much higher in this study than in previous clinical trials, which led to the very good prognosis observed in this study. As for the cause of the baseline responses in our study, we consider that the definition of resistance may not have been as strict in this study as compared with previous clinical trials [11, 13].
As reported in the previous clinical trials [5, 6], subgroup analysis showed that reasons for discontinuation of the 1st-line TKI were significantly associated with the subsequent achievement of CCyR and MMR with the 2nd-line TKI. The Sokal score was also significantly associated with probability of achieving CCyR, but not of achieving an MMR in this study. The latter score was developed in the chemotherapy era, but several studies have demonstrated that that it is also predictive for clinical responses to TKIs [16,17,18]. However, we could not calculate the EUTOS score, because did not collect the data of basophils at diagnosis in our study. Regarding the type of TKIs, CCyR was more frequently achieved in patients treated with nilotinib than those treated with dasatinib. However, there was no significant difference in the rates of MMR achievement between the two groups. There have been no data directly comparing nilotinib and dasatinib as 2nd-line TKIs in CML-CP. In this study, because the baseline characteristics of the patients were considerably different between the two groups, especially in terms of imatinib resistance or intolerance and frequency of ACA, we could not conclude which TKI was better as a 2nd-line TKI from our results. Also, there was no difference in treatment responses by age groups. However, the percentage of patients who required a dose reduction or a switch to a 3rd-line TKI was higher in elderly than in younger patients.
Among the KD mutations detected at the time of registration, L248V, G250E, E255K, T315I, N331S, M351T, F359I, and L384M were identified in clinical samples from patients with imatinib resistance in previously published papers [19,20,21]. To the best of our knowledge, S385I was not previously reported. F359I was detected in only one patient, who showed suboptimal response to imatinib and nilotinib, and dasatinib could overcome this mutation [22]. Conversely, six mutations detected after enrollment were new mutations not previously reported in patients with imatinib resistance. All of the six patients with these mutations achieved MMR, suggesting that these mutations did not affect treatment responses to 2G-TKIs.
BCR-ABL135INS is a 35-bp insertion between ABL kinase domain exons 8 and 9, resulting in a frameshift leading to the addition of 10 intron-encoded residues and truncation of 653 residues [23, 24]. Previous reports suggested that BCR-ABL135INS has no kinase activity. Although it escapes from TKI binding due to the conformational change [24], it does not contribute to TKI resistance [25]. Basically, cells expressing BCR-ABL135INS are not excluded by TKI treatment, but do not proliferate due to the lack of BCR-ABL kinase activity, and thus remain for a long time. In a recent study using deep sequencing analysis, BCR-ABL135INS was detected in all patients (n = 37) who showed suboptimal response to the frontline imatinib. BCR-ABL135INS was persistently detected in 34 of the 37 patients after switching to nilotinib, but 24 of 34 patients achieved MMR [26]. In our results, BCR-ABL135INS was detected in 16 patients (20%) at enrollment or during the course, among whom 14 patients achieved MMR, also suggesting that BCR-ABL135INS does not confer absolute resistance to 2G TKI. Moreover, exon 7 deletion caused by an alternative splicing was detected in 3 patients during the follow-up period, and it has previously been reported that this mutation, while frequent, does not influence TKI responses [27].
The frequency of hematological and non-hematological AEs was lower in this study compared with previous clinical trials. Previous studies demonstrated that grade 3/4 hematological AEs were observed in 10% or more of patients treated with either nilotinib or dasatinib [11, 28], but in this study, the percentage was lower. Regarding non-hematological AEs, rash, nausea, headache, and diarrhea were common regardless of the types of TKI. Bilirubin elevation and hyperglycemia were frequently observed in patients treated with nilotinib, and pleural effusion was frequent in patients treated with dasatinib, as reported. However, the incidences were lower in our results than in previous clinical trials [11, 28]. As for the reasons for these differences, patterns of care and treatment adherence may differ between clinical trial and clinical practice. However, the treatment outcome of this study was not inferior to those of reported clinical trials. Thus, the lower incidence of AEs in this study could not be explained by the poor adherence to TKIs. This observational study started 1 year after the introduction of 2G-TKIs to clinical practice. Therefore, it may be plausible that the hematologists in the community had already become familiarized with the handling of AEs related to 2G-TKIs by the time of the start of this study.
In the clinical trial setting, the safety profile was similar across age groups, except for the higher hyperglycemia rates in elderly patients treated with nilotinib and higher fluid retention rates in those treated with dasatinib. Moreover, discontinuation rates were similar between the older and younger patients treated with either nilotinib or dasatinib [29]. Our results demonstrate that the frequency of hematological AEs tended to be higher in patients 65 years old or older compared with the younger patients and that the percentage of patients who decreased the dose of TKI or moved to a 3rd-line TKI was higher in the elderly. Breccia et al. showed that elderly patients with a high Charlson comorbidity index (CCI) score were at a greater risk of developing grade 3/4 hematologic toxicity, leading to the frequent dose reduction and/or suspension in a retrospective study of 2nd-line dasatinib [30]. In accord with this result, our results also suggest that elderly patients had a higher risk of developing AEs than younger patients in clinical practice. Regarding the timing, most of the AEs occurred in the first few months after switching to the 2nd-line TKI. This result indicates that frequent monitoring is necessary in the first several months after switching to a 2nd-line TKI.
In conclusion, our data indicate that CML-CP patients with resistance and/or intolerance to a 1st-line TKI had an extremely good outcome by a 2nd-line and/or subsequent TKI in Japan. These results should provide a valuable resource for understanding the real-life treatment outcomes of CML patients.
References
Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–27.
Milojkovic D, Apperley J. Mechanisms of resistance to imatinib and second-generation tyrosine inhibitors in chronic myeloid leukemia. Clin Cancer Res. 2009;15:7519–27.
Quintas-Cardama A, Kantarjian HM, Cortes JE. Mechanisms of primary and secondary resistance to imatinib in chronic myeloid leukemia. Cancer Control. 2009;16:122–31.
Cortes JE, Kantarjian HM, Brummendorf TH, Kim DW, Turkina AG, Shen ZX, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567–76.
Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz J, Larson RA, Gattermann N, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117:1141–5.
Shah NP, Kim DW, Kantarjian H, Rousselot P, Llacer PE, Enrico A, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95:232–40.
Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164:536–45.
Kizaki M, Okamoto S, Tauchi T, Tanaka H, Tanimoto M, Inokuchi K, et al. Current and future perspectives on the TARGET system: the registration system for Glivec established by the JSH. Int J Hematol. 2008;88:409–17.
Tauchi T, Kizaki M, Okamoto S, Tanaka H, Tanimoto M, Inokuchi K, et al. Seven-year follow-up of patients receiving imatinib for the treatment of newly diagnosed chronic myelogenous leukemia by the TARGET system. Leuk Res. 2011;35:585–90.
Kizaki M, Takahashi N, Iriyama N, Okamoto S, Ono T, Usui N, et al. Efficacy and safety of tyrosine kinase inhibitors for newly diagnosed chronic-phase chronic myeloid leukemia over a 5-year period: results from the Japanese registry obtained by the New TARGET system. Int J Hematol. 2019;109:426–39.
Shah NP, Kantarjian HM, Kim DW, Rea D, Dorlhiac-Llacer PE, Milone JH, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–12.
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51.
Kantarjian HM, Giles F, Gattermann N, Bhalla K, Alimena G, Palandri F, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–6.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84.
Hochhaus A, Saussele S, Rosti G, Mahon FX, Janssen JJWM, Hjorth-Hansen H, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv41–iv51.
Kuntegowdanahalli LC, Kanakasetty GB, Thanky AH, Dasappa L, Jacob LA, Mallekavu SB, et al. Prognostic and predictive implications of Sokal, Euro and EUTOS scores in chronic myeloid leukaemia in the imatinib era-experience from a tertiary oncology centre in Southern India. Ecancermedicalscience. 2016;10:679.
Marin D, Ibrahim AR, Goldman JM. European Treatment and Outcome Study (EUTOS) score for chronic myeloid leukemia still requires more confirmation. J Clin Oncol. 2011;29:3944–5.
Yamamoto E, Fujisawa S, Hagihara M, Tanaka M, Fujimaki K, Kishimoto K, et al. European Treatment and Outcome Study score does not predict imatinib treatment response and outcome in chronic myeloid leukemia patients. Cancer Sci. 2014;105:105–9.
Jabbour E, Kantarjian HM, Jones D, Shan J, O'Brien S, Reddy N, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood. 2009;113:2154–60.
Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118:1208–15.
Redaelli S, Piazza R, Rostagno R, Magistroni V, Perini P, Marega M, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009;27:469–71.
Baranska M, Lewandowski K, Gniot M, Iwola M, Lewandowska M, Komarnicki M. Dasatinib treatment can overcome imatinib and nilotinib resistance in CML patient carrying F359I mutation of BCR-ABL oncogene. J Appl Genet. 2008;49:201–3.
Laudadio J, Deininger MW, Mauro MJ, Druker BJ, Press RD. An intron-derived insertion/truncation mutation in the BCR-ABL kinase domain in chronic myeloid leukemia patients undergoing kinase inhibitor therapy. J Mol Diagn. 2008;10:177–80.
Lee TS, Ma W, Zhang X, Giles F, Cortes J, Kantarjian H, Albitar M. BCR-ABL alternative splicing as a common mechanism for imatinib resistance: evidence from molecular dynamics simulations. Mol Cancer Ther. 2008;7:3834–41.
O'Hare T, Zabriskie MS, Eide CA, Agarwal A, Adrian LT, You H, et al. The BCR-ABL35INS insertion/truncation mutant is kinase-inactive and does not contribute to tyrosine kinase inhibitor resistance in chronic myeloid leukemia. Blood. 2011;118:5250–4.
Yuda J, Miyamoto T, Odawara J, Ohkawa Y, Semba Y, Hayashi M, et al. Persistent detection of alternatively spliced BCR-ABL variant results in a failure to achieve deep molecular response. Cancer Sci. 2017;108:2204–12.
Gaillard JB, Arnould C, Bravo S, Donadio D, Exbrayat C, Jourdan E, et al. Exon 7 deletion in the bcr-abl gene is frequent in chronic myeloid leukemia patients and is not correlated with resistance against imatinib. Mol Cancer Ther. 2010;9:3083–9.
Giles FJ, le Coutre PD, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107–12.
Gugliotta G, Castagnetti F, Apolinari M, Pirondi S, Cavo M, Baccarani M, Rosti G. First-line treatment of newly diagnosed elderly patients with chronic myeloid leukemia: current and emerging strategies. Drugs. 2014;74:627–43.
Breccia M, Latagliata R, Stagno F, Luciano L, Gozzini A, Castagnetti F, et al. Charlson comorbidity index and adult comorbidity evaluation-27 scores might predict treatment compliance and development of pleural effusions in elderly patients with chronic myeloid leukemia treated with second-line dasatinib. Haematologica. 2011;96:1457–61.
Acknowledgements
This study was supported by research funding from Novartis Pharmaceuticals and Bristol-Myers Squibb to JSH. The authors would like to thank all study participants and their families, and the study investigators at participating study sites. We also thank the New TARGET data center (EPS Co.) for managing and monitoring the study. Finally, we would like to thank the following institutes participating in this study: Chugoku Central Hospital, Ehime Prefectural Central Hospital, Fukuoka University Hospital, Hamamatsu University Hospital, Hirosaki National Hospital, Hyogo Cancer Center, Japanese Red Cross Kyoto Daini Hospital, Japanese Red Cross Shizuoka Hospital, JCHO Kobe Central Hospital, Kanazawa Medical University Hospital, Kumamoto Shinto General Hospital, Kumamoto University Hospital, Kyoto City Hospital, Kyoto Kuramaguchi Medical Center, Mie University Hospital, Nagasaki University Hospital, Nagoya City East Medical Center, National Hospital Organization Kyushu Cancer Center, National Hospital Organization Tokyo Medical Center, Nippon Medical School Hospital, NTT Medical Center Tokyo, Oita Prefectural Hospital, Osaka City University Hospital, Osaka University Hospital, Saiseikai Noe Hospital, Sanraku Hospital, Sapporo Hokushin Hospital, Shinshu University Hospital, Tenshi Hospital, The Jikei University Hospital, Tohoku University Hospital, Tosei General Hospital, Toyama Red Cross Hospital, Tsukuba University Hospital, University Hospital Kyoto Prefectural University of Medicine, Yokohama Minami Kyousai Hospital.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
M. Sakurai reports personal fees from Bristol-Myers Squibb, Takeda Pharmaceutical, Eisai Pharmaceuticals and Nippon Shinyaku, outside the submitted work; S. Okamoto reports grants and personal fees from Otsuka Pharmaceutical, Novartis Pharmaceuticals, Bristol-Myers Squibb, Astellas Pharma, Kyowa Hakko Kirin and Chugai Pharmaceutical, and personal fees from Pfizer, outside the submitted work; I. Matsumura reports grants and personal fees from Bristol-Myers Squibb, Novartis Pharmaceuticals and Otsuka Pharmaceutical, and personal fees from Pfizer, during the conduct of the study; grants and personal fees from Nippon Shinyaku, Celgene, Pfizer, Ono Pharmaceutical, Shionogi and Takeda Pharmaceutical, grants from Kyowa Hakko Kirin, Sumitomo Dainippon Pharma, Teijin Pharma, Boehringer Ingelheim, Sanofi, Chugai Pharmaceutical, Eisai Pharmaceuticals, MSD, Asahi Kasei Pharma, Astellas Pharma, Japan Blood Products Organization, Nihon Pharmaceutical, Daiichi Sankyo, and personal fees from AbbVie GK, outside the submitted work;. N. Takahashi reports grants and personal fees from Novartis Pharmaceuticals, Pfizer and Otsuka Pharmaceutical, grants from Kyowa Hakko Kirin, Astellas Pharma, Chugai Pharmaceutical, Asahi Kasei Pharma, Ono Pharmaceutical and Eisai Pharmaceuticals, and personal fees from Bristol-Myers Squibb, outside the submitted work; T. Kawaguchi reports grants and personal fees from MSD, and personal fees from Pfizer, Alexion Pharma and Novartis Pharmaceuticals, outside the submitted work; R. Suzuki reports personal fees from Bristol-Meyer Squib, Novartis Pharmaceuticals, Kyowa Hakko Kirin, Chugai Pharmaceutical, Shionogi, Takeda Pharmaceutical, Meiji Seika Pharma, MSD, Otsuka Pharmaceutical, Sawai, Celgene, Sumitomo Dainippon Pharma, Eisai Pharmaceuticals, Alexion Pharma, Sanofi, Gilead Sciences, Abbvie Inc., Mundi Pharma, Jazz Pharma, Ono Pharmaceutical and Janssen Pharmaceuticals, outside the submitted work; K. Yamamoto reports grants and personal fees from Astra-Zeneca, Celgene, Chugai Pharmaceutical, Eisai Pharmaceuticals, MSD, Novartis Pharmaceuticals, Ono Pharmaceutical, Takeda Pharmaceutical, Zenyaku, Abbvie Inc., Mundi Pharma and Nippon Shinyaku, grants from ARIAD, Bayer, Gilead Sciences, Solasia Pharma, SymBio and Incyte, and personal fees from Bristol-Myers Squibb, Kyowa Hakko Kirin, Meiji Seika Pharma, Mochida, Otsuka Pharmaceutical, Pfizer, Sumitomo Dainippon Pharma, Boehringer Ingelheim, HUYA/IQVIA Services Japan, Janssen and Stemline Therapeutics, outside the submitted work; M. Kizaki reports grants from Kyowa Hakko Kirin, Chugai Pharmaceutical and Pfizer, and personal fees from Bristol-Myers Squibb, Celgene, Nippon Shinyaku, Takeda Pharmaceutical and Ono Pharmaceutical, outside the submitted work; T. Naoe reports personal fees from Astellas Pharma, Nippon Shinyaku, Bristol-Myers Squibb, Pfizer, Otsuka Pharmaceutical, FujiFilm and Eisai Pharmaceuticals, outside the submitted work; K. Akashi reports grants and personal fees from Bristol-Myers Squibb, Astellas Pharma, Janssen Pharmaceuticals and Kyowa Hakko Kirin, grants from Canon and Otsuka Pharmaceutical, and personal fees from Novartis Pharmaceuticals, Abbvie Inc., Eisai Pharmaceuticals, Celgene and Chugai Pharmaceutical, outside the submitted work; the other authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sakurai, M., Okamoto, S., Matsumura, I. et al. Treatment outcomes of chronic-phase chronic myeloid leukemia with resistance and/or intolerance to a 1st-line tyrosine kinase inhibitor in Japan: the results of the New TARGET study 2nd-line. Int J Hematol 111, 812–825 (2020). https://doi.org/10.1007/s12185-020-02843-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-02843-8