Abstract
To investigate the prognostic value of tumor-infiltrating T-cell density and programmed cell death ligand-1 (PD-L1) expression in diffuse large B cell lymphoma (DLBCL). One-hundred-twenty-five Chinese DLBCL patients were enrolled in our study and provided samples; 76 of all cases were treated with rituximab (R). Tumor tissues were immunostained and analyzed for CD3+ and CD8+ tumor-infiltrating T-cell density, tumoral PD-L1, and microenvironmental PD-L1 (mPD-L1). The density of CD3 was rated as high in 33.6% cases, while 64.0% of DLBCLs were classified as high CD8 density. Of all cases, 16.8% were PD-L1+. Of the remaining PD-L1–DLBCLs, 29.8% positively expressed mPD-L1. Both CD3 high density and CD8 high density were associated with mPD-L1 positivity (P = 0.001 and P = 0.0001). In multivariate analysis, independently, high CD3 density predicted better OS (P = 0.023), while CD8 high density and PD-L1 positivity were both associated with prolonged PFS (P = 0.013 and P = 0.036, respectively). Even in the subgroup treated with R, univariate analyses indicated that high CD3 density and PD-L1 positivity were associated with better OS (P = 0.041) and PFS (P = 0.033), respectively. The infiltrating densities of CD3+ T-cells, CD8+ T-cells, and PD-L1 expression are predictive of survival in DLBCLs, irrespective of R usage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B cell lymphomas (DLBCL) is the most common subtype of aggressive lymphoma with significant clinicopathological heterogeneity. In spite of international prognostic index (IPI) used to stratify the risk [1], the cell of origin (COO) classification with Hans’s immunohistochemical algorithm [2] was also widely used in DLBCL patients to identify two major subtypes of DLBCL with different prognosis, in which the germinal center B-cell-like (GCB) subtype of DLBCL carries a better prognosis.
However, the standard chemotherapy with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) has generally improved the survival of DLBCL patients, and thus obscured the survival difference between two subgroups classified by COO. But shortly after the treatment with R based immunochemotherapy, about 40% DLBCL patients still suffered relapse and death [3].
So, it is always a priority to develop new biomarkers independent with COO or R in the prognosis and targeted therapy research of DLBCL, especially for those relapsed and refractory (R&R) cases [3,4,5,6]. Recently, doule-expressor lymphoma (also known as Double-hit score lymphoma, DEL) [5] and expression of programmed cell death ligand-1 (PD-L1) [7, 8] were reported to be factors predicting worse prognosis in DLBCL.
Despite of those studies mainly focused on tumor cells, it has become increasingly recognized that the constitutional difference of tumor microenvironment cells, especially T cells, would also play critical roles in predicting survival in many kinds of cancers [9,10,11]. DLBCL with high density of CD8+ [12] or CD3+ infiltrating T cells [13] seemed to have improved outcome .Moreover, positive PD-L1 staining on background cells was reported to correlate with high density of CD3 and CD8 in hepatocellular carcinoma [14], thus further studies are needed to clarify similar relationship in DLBCL.
We collected the tumor tissues of some Chinese DLBCL patients. By reviewing expression of intratumoral CD3+ and CD8+ T cell density, and PD-L1 via immunostaining (either tumoral or microenvironmental cells), we explored their association with the other already well-recognized predictive factors in DLBCL and tried to prove the potential survival impacts of these three makers in DLBCL, according to the follow-up data and survival analyses.
Patients and methods
Patient selection and characteristics
This study was approved by the Ethical Committee of Peking University Cancer Hospital. Patients who were newly diagnosed as DLBCL at Peking University Cancer Hospital from Jun 21, 2002 to May 29, 2015 were enrolled. All cases have available clinical data and formalin fixed paraffin embedded tissue. Finally, 125 patients with chemotherapy as first line treatment were included in our study. Of them 76 cases were treated with R-CHOP/R-CHOP-like regimens, and the others were treated with CHOP/CHOP-like regimens without R. The details for clinical characteristics (including Age, Gender, Ann Arbor stage, B symptoms, Eastern Cooperative Oncology Group (ECOG) score, extranodal sites, normalized LDH ratio, bulky mass, IPI risk Group, treatment regimens) of these patients were listed on Table 1. The median follow-up time was 25.7 months (range 0.8–131.1).
Tissue microarrays
Formalin-fixed, paraffin-embedded tissue blocks of 125 DLBCL were subjected to the construction of tissue microarray (TMA), and representative tumor areas were selected and marked on hematoxylin–eosin stained slides. TMA were constructed with a computer driven semi-automated instrument (Alphelys, Plaisir, France), dual cores of 1 mm diameter from each tumor sample were arrayed into the recipient blocks.
Immunohistochemical analysis
TMA blocks were cut to 4-µm thick sections and applied to immunohistochemical staining (IHC), all IHCs were performed using a BenchMark ULTRA automated IHC staining instrument (Tucson, VENTANA-Roche, USA) with an ultra-VIEW kit (Tucson, VENTANA-Roche, USA). Information for the antibody panels is provided in supplementary Table S1, appropriate internal and external controls were used as positive and negative controls for each IHC biomarker.
Epstein-Barr virus in situ hybridization
4-µm thick tissue sections were hybridized with a biotin-labeled nucleic acid probe (Pan Path, Budel, Netherlands) complement to EBV-encoded RNA (EBER1/2) to determine the existence of EBV. The DLBCL specimens with EBV infection would be indicated by the presence of signals in the nuclear of lymphoma cells.
Image scanning and semi-quantitative analysis
The TMA slides after immunostaining and EBER1/2 hybridization were scanned with whole-slide-images, using an Aperio XT Scanscope (Leica), two pathologists (Yunfei Shi and Yumei Lai) independently viewed digital slides using ImageScope Software (Leica). Both CD20 and PAX5 stained slides were reviewed to confirm the TMA cores with high proportion of representative DLBCL tumor cells. The relative positive proportion of targeted cells were determined by average of three different scanned tumor areas in both duplicated TMA core for each sample.
CD3+ T-cell density and CD8+ T-cell density were calculated by the percentage of CD3+ T-cells or CD8+ T-cells to total cellularity in each representative tumor region. According to the results of survival analysis, the degree of CD3+ T-cell density was classified into two categories: ≤ 25% as low and > 25% as high density. The cut-off values for CD8+ T-cell was ≤ 10% as low and > 10% as high density (Cut-off set up details can be found in the supplementary method section). To keep in accordance with literature, PD-L1 staining on tumor cells was considered positive in cases with moderate (2+) or strong (3+) cytoplasm reaction and the percentage of the positive tumor cells were set as above 30% [6], while for microenvironmental PD-L1 (mPD-L1) the positivity was defined when the PD-L1 positive nonmalignant stromal cells represented 20% or more of the total tissue cellularity [6].
Other IHC makers or algorisms (included COO, MYC, BCL2, P53, Ki67, CD30, EBER) were evaluated in the following way: COO immunophenotype was classified as GCB or non-GCB and determined by Hans’ algorithm, and a uniform cut-off of 30% was chosen on the expression of CD10, BCL6, and MUM1 [2]. The positive cut-off value for DEL were assigned as ≥ 40% for MYC (nuclear) and ≥ 70% for BCL2 (cytoplasmic) [3], while cut-off value for high expression of sole MYC or sole BCL2 was ≥ 40% and > 30%, respectively [12, 15]. The expression level of Ki67 was rated as ‘high’ if > 80% neoplastic cells were nuclear-positive [8]. The cut-off value for high level P53 expression was ≥ 30% as reported in the literature [16]. CD30 were considered as positive if > 5% tumor cells showed positive staining on cell membrane. Finally, EBER1/2 were judged as ‘positive’ if > 5% tumor cells showed nuclear signal.
Statistical analysis
Pearson correlation χ2 test or Fisher exact test were performed for the relation between CD3, CD8, PD-L1 and all other IHC variables and clinical parameters. The outcomes measured by overall survival(OS), defined as the time from diagnosis to death from any cause, with surviving patients censored at last follow-up, and progression-free survival(PFS), defined as the time from diagnosis to failure of treatment, including partial response (PR), relapse after complete response (CR) or death from any cause. The univariate Kaplan–Meier method was used to estimate OS/PFS survival, and all survival curves were compared using the log-rank test. Cox proportional hazards regression models that were age-and sex-adjusted and multivariate-adjusted were both used to evaluate the proposed independent prognostic factors. And only P values (2-sided) < 0.05 were considered with statistically significant difference. All statistical analyses were performed using SPSS version 17.0 (IBM, Corporation, Armonk, NY, USA).
Results
IHC characteristics of CD3, CD8 and PD-L1 in DLBCL
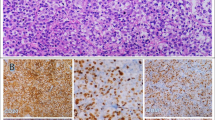
The density of infiltrating CD3+ T-cells in TMA slides presented a wide spectrum (Fig. 1a, b), from rarely scattered (0.8%) to densely distributed (60.0%), with a median density of 20.0%. The cellular density percentage of CD8+ cytotoxic T-cells ranged between 0.5–47.5%, with a median value of 12.5% (Fig. 1c, d). According to the immunol scoring parameters for CD3/CD8 defined cut-off value in methods section, there were 42 cases with CD3- high density and 83 cases with CD3-low density. While for IHC staining of CD8+ T cells, 80 cases were rated as CD8-high density and other 45 cases were rated as CD8-low density.
Representative images of CD3, CD8 and programmed cell death ligand 1 (PD-L1) immunostains in diffuse large B cell lymphoma (DLBCL). Images a and image b indicated high (a) and low (b) density of CD3 expression, whereas images c, d showed high (c) and low (d) density of CD8 expression, all images from a to d focused the non-neoplastic lymphocytes. In image E, PD-L1 was strongly immunostained in tumor cells on the membrane and partial cytoplasm, however, for the case showed in image f, PD-L1 was mainly immunostained in the non-tumor cells (also called mPD-L1), but with little staining in tumor cells. All expressions of biomarkers were shown by HRP-DAB immunostaining
21 out of 125 (16.8%) cases showed PD-L1 positivity on lymphoma cells (Fig. 1e), and for those tumoral PD-L1 negative ones, 31 (29.8%) of all samples showed mPD-L1 expression in the non-lymphoma cells (Fig. 1f). The percentages of PD-L1 expression in malignant cells (both ratios to total tumor cells or total cellularity) and nonmalignant cells (ratios to total tumor cellularity) were all summarized in Fig. 2.
The percentages of PD-L1 expressed by malignant and nonmalignant cells in tumor areas of the 125 DLBCL cases. The red line indicates the percentage of PD-L1+ tumor cells among all malignant cells. The blue bars showed the percentages of PD-L1+ malignant cells to total tissue cellularity, whereas the green bars indicated the PD-L1+ nonmalignant cells among total tissue cellularity. The PD-L1 positivity threshold was above 30% of all lymphoma cells
The association of CD3+ T-cell density, CD8+ T-cell density and PD-L1 with other reported prognostic factors
The high density of CD3+ T-cell was significantly correlated with high density of CD8+ T-cell (P < 0.001). Neither CD3+ nor CD8+ T-cell density level showed statistically significant association with PD-L1 expression (P = 0.136 and P = 0.076). However, both CD3 and CD8 high were associated with mPD-L1 positivity (P = 0.001 and P = 0.0001, Table 2).
Their statistical correlation with those already reported pathological prognostic parameters were also evaluated, respectively (Table 2). CD3-high was found only correlated with low level of Ki67 (P = 0.026). There were 41/83 (49.4%) cases with Ki67 at ‘high’ level in CD3-low density subgroup, while only 12/42 (28.6%) in CD3-high density subgroup. CD8-high was only correlated with BCL2 positivity (P = 0.039).
For PD-L1, it was only found related to COO, and in the PD-L1 positive cases, 85.7% (18/21) was from non-GCB, but in the 104 PD-L1 negative cases, the non-GCB accommodated 59.6% (62/104). However, mPD-L1 did not correlate with any pathological biomarkers in our analysis.
As to those clinical prognostic variables (Table 3), both CD3-low and CD8-low density showed association with more than one extra nodal sites involvement (P = 0.045 for CD3 and P = 0.028 for CD8) and ‘high’ score of IPI (scored 3–5, P = 0.029 for CD3 and P = 0.030 for CD8). Besides, CD3-low density also had a significant association with patient death (P = 0.029). PD-L1 expression in tumor cells showed negative correlation with relapse (P = 0.041) and mPD-L1did not correlated with any clinical risk factors. Details are shown in Table 3.
CD3+ T-cell density,CD8+ T-cell density, and PD-L1 expression associated with clinical outcomes
To investigate the prognostic impact of CD3+ T-cell density, CD8+ T-cell density, and expressions of PD-L1 or mPD-L1, a univariate survival analysis was done in combination with those well recognized clinicopathological factors, and therapeutic regimens with or without rituximab (R) as well. All variables were listed in Tables 2 and 3.
By univariate analysis of OS, CD3-low density and CD8-low density predicted worse OS (P = 0.023, P = 0.048, Fig. 3a, b). Patients with DEL (P = 0.012), Ann Arbor stage (III/IV, P = 0.003), extranodal sites > 1 (P = 0.003), normalized LDH ratio > 1 (P = 0.029), IPI high-score (3–5, P = 0.001) also had a lower probability of OS.
CD3 density, CD8 density and PD-L1 expression associated with clinical outcomes. Univariate (Kaplan–Meier estimations) with the log rank test for overall survival (OS) and progression-free survival (PFS) in DLBCL patients. Patients with CD3 high density (> 25% of total cellularity) (a) and CD8 high density (> 10% of total cellularity, b) in tumor microenvironment exhibited prolonged (OS). Patients with CD8 high density also showed better prognosis in terms of PFS (c), and those patients with PD-L1 positively expression in tumor cells tended to show prolonged PFS with statistical significance (d). Log-rank P values and number of cases analyzed were all provided
While for the univariate analysis of PFS,those cases with CD8-low density (P = 0.013, Fig. 3c) or PD-L1 negativity (P = 0.036, Fig. 3d) had shorter PFS time. Patients with DEL (P = 0.002), BCL2 positivity (P = 0.02), Ann Arbor stage (III/IV, P = 0.0001), extranodal sites > 1 (P = 0.0002), LDH ratio > 1 (P = 0.029), IPI high-score (3–5, P = 0.0004) and treatment with R also significantly correlated with lower PFS rates (P = 0.008).
With regard to age- and sex-adjusted analyses of OS, CD, 3-low density (P = 0.043), in addition with DEL (P = 0.008), Ann Arbor stage (III/IV, P = 0.006), extranodal sites > 1 (P = 0.011), LDH ratio > 1 (P = 0.042) and IPI high-score (3–5, P = 0.010), were all identified as adverse-impact prognostic variables (Table 4). For PFS analysis adjusted by age and sex, CD8-low density (P = 0.012), PD-L1 negativity (P = 0.046), existence of DEL (P = 0.002), BCL2 positivity (P = 0.020), Ann Arbor stage III/IV (P = 0.0002), extranodal sites > 1 (P = 0.0006), normalized LDH ratio > 1 (P = 0.031), IPI high-score (3–5, P = 0.002), and treatment without R (P = 0.0113) were all identified as risk factors (Table 5).
Cox regression analyses were performed for the survival, all clinical and pathological parameters associated with OS and PFS in the univariate analysis described above together with age and sex included in the model. Since treatment with R improved the outcome of DLBCL it was forced to join the OS multivariate analysis, and “Backward Stepwise (Conditional LR)” method was adapted. Only CD3+ T-cell density (P = 0.023), Ann Arbor stage (P = 0.010) and treatment (P = 0.015) were still independently significant for the OS of DLBCL (Table 5). While CD8+ T-cell density (P = 0.025), PD-L1 expression (P = 0.041), DEL (P = 0.024), Ann Arbor stage (P = 0.00001) and treatment (P = 0.0002) remained independent predictors for PFS.
The impacts of CD3, CD8 and PD-L1 on outcomes classified by treatment with or without rituximab
As treatment “with R” would independently improve the prognosis in multivariate analysis, so we further discussed the impacts of CD3+ T-cell density, CD8+ T-cell density and tumoral PD-L1 expression on clinical outcomes in two different treatment subgroups (with R and without R) by univariate analyses. In the treatment subgroup treated with R, CD3-high density indicated better outcome for OS (P = 0.041, Fig. 4a), and PD-L1 positive patients also showed higher rate of PFS (P = 0.033, Fig. 4b). On the contrary,
CD3 density, CD8 density and PD-L1 expression could predict clinical outcomes in patients with or without rituximab(R) in their treatment. In patients treated with R, Kaplan Meier plots with log rank test showed prolonged overall survival (OS) in patients with CD3 high density (> 25% of total cellularity) (a), and patients with PD-L1 positivity (b) on tumor cells exhibited prolonged progression-free survival (PFS). However, in patients without R-CHOP/R-CHOP-like therapy, those cases with CD8 high density (> 10% of total cellularity) showed better prognosis in terms of both OS (c) and PFS (d). Log-rank P values and number of cases analyzed were all provided
in subgroup treated without R, only patients with CD8-high density would have both longer OS (P = 0.016, Fig. 4c) and PFS (P = 0.011, Fig. 4d), further details are presented in supplementary Table S2.
Discussion
Our study demonstrated that the densities of infiltrating T-cells expressed CD3 and CD8 were variable in DLBCL, and those cases with either high density of CD3+ or CD8+ T-cells correlate with lower IPI score. In our study, patients with CD3-high predicted improved OS, while CD8-high correlated with better PFS, suggesting densities of infiltrating CD3+ or CD8+ T-cells might be used as important predictive factors in clinicopathological practice.
Coutinho, et al. also reported that patients with high density of CD3 and FoxP3 would have better outcome [13]. However, FoxP3, which generally represents regulatory T-cells [17], showed a controversial and negative prognostic impact in some functional studies [18,19,20], and CD4+ T-cells density was never considered as a predictive biomarker in DLBCL and other cancers [10, 13, 14, 21]. It may be due to the heterogeneity of CD4+ T-cells, which include different Th and Treg subsets [17], and different subsets even have opposite effect on tumor growth [21]. Therefore, we did not involve CD4 nor FoxP3 in our study.
With respect to results from Rajnaiet al [12], higher number of tumor-infiltrating CD8+ T-cells was an independent favorable prognostic marker for the survival in primary bone DLBCL. Increased infiltrate of CD3+ and CD8+ T cells might reflect activation of host anti-tumor immunity [14]. Cytotoxic CD8+ T cells were found associated with a Th1-oriented immune reaction strongly correlate with good clinical course in most studied cancer types [22]. These might partially explain our finding that high density of infiltrating T-cells (either CD3+ or CD8+) independently improved patients’ survival.
We also confirmed PD-L1 expressed variably on either tumor cells or non-neoplastic cells in DLBCL, and those patients with PD-L1 positivity on tumor cells tended to be non-GCB phenotype, which is also in concordance with previously reported results [6, 8, 23]. It was mPD-L1 rather than tumoral PD-L1 which was associated with the high densities of CD3+ and CD8+ T-cells in DLBCL. This was also mentioned in the studies of HCC [14]. The expression of mPD-L1 came from nonmalignant cells, including macrophages, dendritic cells and lymphocytes [6, 14, 23]. This finding might also explain the immunosuppressive mechanism: both tumor escape from host immune surveillance and inhibition of activated T-cells.
In our study, patients with tumoral PD-L1 positivity tended to have longer PFS time. Although PD-L1 correlated with worse survival in studies of gastric cancer, renal cell carcinoma and squamous cell carcinoma at head and neck [24,25,26], it was also correlated with better survivals in studies about small cell lung cancer, and ovarian cancer [27, 28], the predictivity remained controversial in both melanoma [29] and non-small cell lung cancer [30, 31].In the DLBCL studies by Kiyasu et al. and Xing et al. [6, 8], patients with tumoral PD-L1 expressions had worse overall survival. To identify patients with worse outcome, their thresholds for PD-L1 positivity was > 30% tumor cells [6, 8].Our study adopted the same threshold and demonstrated that cases with PD-L1 positivity on lymphoma cells did not significantly affect OS, but did have significant longer PFS time. This result was never mentioned in previous PD-L1 studies of DLBCL. Another study by Kwon et al. [23] reported that patients with strong PD-L1 expression (> 10% tumor cells) tended to show prolonged OS but without significant difference. We also noted that unlike these DLBCL studies, the PD-L1-positive patients had a trend toward better overall survival in NK/T cell lymphoma [32]. It should be pointed out that the IHC of PD-L1 expression mentioned above were performed with different clones of PD-L1 antibody, including E1L3N [8, 23] and PD-L128-8 [6], and we used the FDA approved SP142 clone [33]. More recent and prospective studies confirmed that all these antibodies showed excellent concordance when applied in scoring tumor cell positivity [34, 35].
We did not find a correlation that mPD-L1 expression can also be utilized as prognostic makers for DLBCL patients, which might be caused by the poor concordance for scoring background immune cells by pathologists, when stained with different clones of antibody [35].
As DLBCL patient treated with R or without R were both included in our study, although multivariate analysis confirmed the usage of R significantly and independently affected both OS and PFS as previous studies [36], CD3+ T-cell density still independently affect OS, CD8+ T-cell density and PD-L1 still independently affect PFS, irrespective of the usage of rituximab. When we clarified the patients into two groups with “R” and without “R”, from univariate analyses, in the subgroup treated “with R”, CD3-high DLBCL cases had better OS, and patients with PD-L1 positivity also had favorable PFS. While in subgroup treated “without R”, only patients with CD8-high density will have both better OS and PFS, indicating that CD3 and PD-L1 would affect the survival of DLBCL in this “Rituximab Era”. We did not go further with multivariate analysis because of the limited patient sample scales in the classified subgroups.
In conclusion, our study demonstrated that the potential predictive impacts of microenvironmental T-cell densities in DLBCL, and we further identified PD-L1 expression on tumor cells correlated with prolonged PFS. These findings might contribute to the explorations of new prognostic biomarkers in DLBCL. It will also be helpful to future therapeutic strategies in DLBCL, including either activators for stimulator of interferon genes (STING) or inhibitors to immune checkpoints.
References
Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837 – 42.
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275 – 82.
Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460–7.
Meyer PN, Fu K, Greiner TC, Smith LM, Delabie J, Gascoyne RD, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29:200–7.
Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–9.
Kiyasu J, Miyoshi H, Hirata A, Arakawa F, Ichikawa A, Niino D, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126:2193–201.
Boussiotis VA. Cell-specific PD-L1 expression in DLBCL. Blood. 2015;126:2171–2.
Xing W, Dresser K, Zhang R, Evens AM, Yu H, Woda BA, et al. PD-L1 expression in EBV-negative diffuse large B-cell lymphoma: clinicopathologic features and prognostic implications. Oncotarget. 2016;7:59976–86.
Becht E, Giraldo NA, Germain C, de Reynies A, Laurent-Puig P, Zucman-Rossi J, et al. Immune contexture, immunoscore, and malignant cell molecular subgroups for prognostic and theranostic classifications of cancers. Adv Immunol. 2016;130:95–190.
Galon J, Fox BA, Bifulco CB, Masucci G, Rau T, Botti G, et al. Immunoscore and Immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. J Transl Med. 2016;14:273.
Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pages F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–82.
Rajnai H, Heyning FH, Koens L, Sebestyen A, Andrikovics H, Hogendoorn PC, et al. The density of CD8 + T-cell infiltration and expression of BCL2 predicts outcome of primary diffuse large B-cell lymphoma of bone. Virchows Arch. 2014;464:229–39.
Coutinho R, Clear AJ, Mazzola E, Owen A, Greaves P, Wilson A, et al. Revisiting the immune microenvironment of diffuse large B-cell lymphoma using a tissue microarray and immunohistochemistry: robust semi-automated analysis reveals CD3 and FoxP3 as potential predictors of response to R-CHOP. Haematologica. 2015;100:363–9.
Gabrielson A, Wu Y, Wang H, Jiang J, Kallakury B, Gatalica Z, et al. Intratumoral CD3 and CD8 T-cell densities associated with relapse-free survival in HCC. Cancer Immunol Res. 2016;4:419 – 30.
Horn H, Ziepert M, Becher C, Barth TF, Bernd HW, Feller AC, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253–63.
Schiefer AI, Kornauth C, Simonitsch-Klupp I, Skrabs C, Masel EK, Streubel B, et al. Impact of single or combined genomic alterations of TP53, MYC, and BCL2 on survival of patients with diffuse large B-cell lymphomas: a retrospective cohort study. Medicine (Baltimore). 2015;94:e2388.
Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14:517 – 34.
Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+ CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–46.
Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin’s lymphoma. Cancer Res. 2006;66:10145–52.
Galani IE, Wendel M, Stojanovic A, Jesiak M, Muller MM, Schellack C, et al. Regulatory T cells control macrophage accumulation and activation in lymphoma. Int J Cancer. 2010;127:1131–40.
Xerri L, Huet S, Venstrom JM, Szafer-Glusman E, Fabiani B, Canioni D, et al. Rituximab treatment circumvents the prognostic impact of tumor-infiltrating T-cells in follicular lymphoma patients. Hum Pathol. 2017;64:128 – 36.
Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306.
Kwon D, Kim S, Kim PJ, Go H, Nam SJ, Paik JH, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. 2016;68:1079–89.
Li Z, Lai Y, Sun L, Zhang X, Liu R, Feng G, et al. PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Hum Pathol. 2016;55:182–9.
Iacovelli R, Nole F, Verri E, Renne G, Paglino C, Santoni M, et al. Prognostic role of PD-L1 expression in renal cell carcinoma. A systematic review and meta-analysis. Target Oncol. 2016;11:143–8.
Muller T, Braun M, Dietrich D, Aktekin S, Hoft S, Kristiansen G, et al. PD-L1: a novel prognostic biomarker in head and neck squamous cell carcinoma. LID. https://doi.org/10.18632/oncotarget.17547
Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, Tokito T, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol. 2015;10:426 – 30.
Webb JR, Milne K, Kroeger DR, Nelson BH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol. 2016;141:293–302.
Gadiot J, Hooijkaas AI, Kaiser AD, van Tinteren H, van Boven H, Blank C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer. 2011;117:2192 – 201.
Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015;41:450–6.
Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Investig. 2014;94:107 – 16.
Jo JC, Kim M, Choi Y, Kim HJ, Kim JE, Chae SW, et al. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2017;96:25–31.
Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thoracic Oncol. 2017;12:208–22.
Gaule P, Smithy JW, Toki M, et al. A Quantitative Comparison of Antibodies to Programmed Cell Death 1 Ligand 1. JAMA Oncol. 2017;3:256–9.
Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol. 2017;3:1051–8.
Held G, Poschel V, Pfreundschuh M. Rituximab for the treatment of diffuse large B-cell lymphomas. Expert Rev Anticancer Ther. 2006;6:1175–86.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Shi, Y., Deng, L., Song, Y. et al. CD3+/CD8+ T-cell density and tumoral PD-L1 predict survival irrespective of rituximab treatment in Chinese diffuse large B-cell lymphoma patients. Int J Hematol 108, 254–266 (2018). https://doi.org/10.1007/s12185-018-2466-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-018-2466-7