Abstract
Direct oral anticoagulants (DOACs) have been approved to treat and prevent thrombotic events. However, they are not yet labeled for use in patients with active cancers. Myeloproliferative neoplasms (MPNs) are clonal chronic disorders with a high incidence of thrombotic events, for which low-dose aspirin (LDA) is the standard drug treatment. We analyzed efficacy and safety of DOACs prescription in patients treated for MPNs. An MPN database, the OBENE registry, was established at our institution. We collected biological and clinical data from diagnosis to last follow-up for every patient included in this study. Thrombotic and hemorrhagic events and hematologic evolutions were categorized as major events in the database. Of the 760 MPN patients in the OBENE registry, 25 (3.3%) were treated with a DOAC. Median follow-up duration was 2.1 years (0.12–4.3 years). The reasons for prescribing DOACs were atrial fibrillation and thrombotic events for 13 and 12 patients, respectively. We only observed one thrombotic event (4%) and three major hemorrhagic events (12%). A case–control study did not detect a significant difference in thrombotic or hemorrhagic events in patients treated with LDA and DOACs. These preliminary results suggest that DOACs may be highly efficient and safe for use in MPN patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apixaban and rivaroxaban are specific factor Xa inhibitors in the direct oral anticoagulant (DOAC) family. They are approved to treat venous thromboembolic (VTE) events and to prevent post-surgery deep vein thrombosis (DVT) and arterial thrombosis (AT) in patients with atrial fibrillation (AF) [1,2,3]. However, they are not yet labeled for use in patients with active cancers.
Essential thrombocythemia (ET) and polycythemia vera (PV) are myeloproliferative neoplasms (MPNs). They are clonal chronic disorders that develop after a clonal mutation is generated. This provokes the proliferation of myeloid cells in bone marrow and the accumulation of mature cells in blood, which induces hyperviscosity. Thrombosis is the most frequent complication observed in MPN patients (among 15–30% of patients throughout follow-up). In most series, the arterial/venous thrombosis ratio is 2/3–1/3 [4, 5]. Low-dose aspirin (LDA) has been the standard therapy for MPN patients since the publication of the ECLAP study [6].
Due to the therapeutic indications of DOACs across multiple MPN clinical scenarios, it is evident that the rate of prescription of these drugs will constantly increase over time. Currently, no experience with DOAC use in MPN patients has been published. Here, we report our experience with DOAC prescription in an MPN population.
Methods
After institutional review board approval, an MPN database was established at our Institution: the OBENE registry (Observatoire brestois des néoplasies myéloprolifératives) (NCT02897297). At our institution, MPN diagnoses were made based on PVSG or WHO criteria throughout the years [7, 8]. Prior to enrollment in the current study, all patients signed informed consent. Biological and clinical data were collected from diagnosis to last follow-up (dead or alive). Only proven thromboses were recorded in the registry. The following were defined as major hemorrhagic events: intracranial bleed, retroperitoneal bleed, overt hemorrhage associated with a decrease in hemoglobin ≥20 g/l and overt hemorrhage requiring a blood transfusion of two units or more. All other hemorrhages were classified as minor [9].
We performed a case–control study (following a 1:1 pattern) by matching patients from the OBENE registry by age, sex and type of MPN to control for major confounders.
Results
Among our 760 patients included in our cohort, 25 (3.3%) were treated with a DOAC (17 ET and 8 PV), and 12 patients were females. The median age at the time of DOAC prescription was 75.4 years. Nineteen (76%) patients carried cardiovascular risk factors (hypertension (84%), hypercholesterolemia (52.6%), diabetes mellitus (15.8%) or tobacco (10.5%). Also, nine patients had a history of thrombosis: five arterial events, two venous events and two mixed events. So, according to these elements, 92% of the patients could be considered at high-risk for thromboses.
The hemograms at the time of MPN diagnosis, showed median values of leukocytes and platelets at 10.1 and 668 giga/l, respectively. Twenty-one patients had thrombocythemia and twelve had leukocytosis.
At the time of the prescription of DOAC, 21 (84%) of the patients were under cytoreductive drugs: hydroxycarbamide (n = 13), pegylated interferon or piprobroman (n = 3, each) and anagrelide (n = 2).
Seven (28%) patients were treated with a DOAC before MPN diagnosis, and 18 (72%) received their drug after MPN diagnosis: 14 after treatment with LDA (n = 14), 3 after treatment with vitamin K antagonists (n = 3) and 1 without previous drug treatment. Patient characteristics are shown in Table 1.
Apixaban and rivaroxaban were prescribed for 9 and 16 patients, respectively. The median follow-up duration for the study cohort was 2.1 years (0.12–4.3 years). The reasons for prescribing DOACs were the following: AF for 13 patients and thrombotic events for 12 patients. The thrombotic events included eight VTE events (4 pulmonary embolisms and 4 deep vein thromboses, most commonly as first VTE events) and four strokes (all patients also experienced AF). Among the 13 patients who changed from LDA to DOAC, the reasons were a first episode of AF (n = 9) or a first episode of thrombosis or a recurrence (n = 4). All the patients had a normal renal function at the time of the prescription of DOAC (i.e. clearance of the creatinine >60 ml/min) which did not induce an adaptation of the dose.
DOACs was stopped in six (24%) patients (two due to side effects, two due to physician discretion, one due to post-infectious acute renal insufficiency and one due to a planned high-risk surgery). Seven (28%) deaths were recorded: two resulting from profound asthenia, two from stroke, one from cancer, one from trauma and one from pneumopathy.
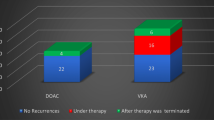
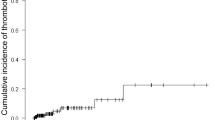
In terms of efficacy, only one patient (4%) experienced a thrombotic event under DOAC (stroke). Another patient experienced stroke fifteen days after DOAC therapy discontinuation, prior to a planned surgery.
Regarding hemorrhages, three patients (12%) experienced major events, all of which were provoked: two due to surgery and one due to traumatic brain injury. Two additional patients experienced minor hemorrhages.
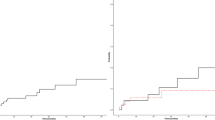
We performed a case–control study to compare the outcomes of patients treated with DOACs and LDA (control). Interestingly, the control group experienced two new thromboses and 3 major hemorrhages and 6 deaths during the same follow-up period as the DOAC group. No differences in outcomes were observed between the two groups (Table 2).
Discussion
To the best of our knowledge, this is the first specific report of DOAC prescription in MPN patients. Here, we report the impact of DOACs in 25 MPN patients with ET or PV with a pro-thrombotic profile describing them at high-risk of thromboses in accordance with ELN criteria: a median age over 60 years old, the presence of cardiovascular risk factors (mostly high blood pressure) in 76% and atrial fibrillation in 72%. Patients with such pro-thrombotic profiles require drug therapies to manage the risk of arterial and venous thromboses [10]. In this cohort, we only observed one thrombotic event during DOAC use and a low rate of major hemorrhage.
The impact of DOACs in the prevention of ischemic events in cases of AF (like most of the patients in our cohort) has been well established. A meta-analysis collecting the data of 96 826 patients included in trials, clearly showed significant advantages of DOACs versus LDA (alone or in association) in term of reduction of arterial thrombotic events (−60%) and death (−10%) [11].
The most concerning clinical risks in MPN patients are hyperviscosity and thrombosis. The recommended therapeutic strategy for high-risk MPN patients is prescription of LDA in conjunction with cytoreductive drugs. This strategy is based on the results of the ECLAP phase 3 study of PV patients, which showed that LDA reduced the risk of nonfatal myocardial infarction, nonfatal stroke and death from cardiovascular causes by 60% compared to placebo [6, 10].
Two authors have suggested that DOACs could be useful alternatives for the prevention of thrombotic phenomena in the MPN population, as they do not require anticoagulation monitoring (a major drawback of VKA therapy) and induce less bleeding than VKA therapy [12].
Two recent studies showed the impact of VKA therapy in MPN populations. Hernandez-Boluda JC et al. showed a 2.8-fold reduction in the risk of thrombotic recurrence with VKA therapy in a population of 150 patients with PV or ET. Furthermore, the rate of hemorrhage was not increased with VKA therapy, even when a VKA and LDA were administered concurrently [13]. De Stefano V et al. observed a reduction of the recurrence rate of thrombosis in 206 MPN patients treated with VKAs (4.7 versus 8.9 events/100 patient-years, p = 0.03). Furthermore, discontinuation of VKA therapy was shown to increase the risk of new thrombosis (5.3 versus 12.8 events/100 patient-years, p = 0.08) [14].
The proportion of patients prescribed DOACs in the OBENE cohort was very low (3.3%), and the 2-year follow-up period was relatively short; however, this was unavoidable due to the delay in marketing authorization after the drugs became available in 2011.
A recent retrospective study of 437 MPN patients conducted in Germany identified only 8 patients treated with rivaroxaban (1.8%). The data analysis revealed an odds ratio of major bleeding for patients on rivaroxaban of 1.61 (non-significant), which is much lower than those for patients on VKA therapy (1.97), double platelet inhibition (3.05) and heparin (5.64, the only drug with a significant odds ratio for major bleeding) [15].
MPN patients have a very high rate of thromboses. Therefore, they require antithrombotic drugs to reduce the number of thrombotic events they experience: LDA is the preferred treatment, but VKAs and DOACs can also be useful. We admit that our results are preliminary. Despite the limited 2-year follow-up period for DOAC use, the results of this study indicate that DOAC therapy may be efficient and safe in MPN patients. We are currently preparing a protocol for a prospective study that will compare LDA to DOAC therapy in high-risk MPN patients.
References
Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510.
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17.
Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. New Engl J Med. 2009;361:2342–52.
Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120:5128–33.
Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–32.
Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, et al. European Collaboration on Low-Dose Aspirin in Polycythemia Vera Investigators. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:1114–24.
Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. WHO classification of tumours: tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. p. 35–41.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51.
Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, et al. United Kingdom Medical Research Council Primary Thrombocythemia 1 Study. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353:33–45.
Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelpia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:761–70.
Tawfik A, Bielecki JM, Krahn M, Dorian P, Hoch JS, Boon H, et al. Systematic review and network meta-analysis of stroke prevention treatments in patients with atrial fibrillation. Clin Pharmacol. 2016;8:93–107.
Karali V, Panayiotidis P. Novel oral anticoagulants in the management of polycythemia vera and essential thrombocythemia. Cardiovasc Hematol Ag Med Chem. 2014;12:26–8.
Hernandez-Boluda JC, Arellano-Rodrigo E, Cervantes F, Alvarez-Larrán A, Gómez M, Barba P, et al. Grupo Español de Enfermedades Mieloproliferativas Filadelfia Negativas (GEMFIN). Oral anticoagulation to prevent thrombosis recurrence in polycythemia vera and essential thrombocythemia. Ann Hematol. 2015;94:911–8.
De Stefano V, Ruggeri M, Cervantes F, Alvarez-Larrán A, Iurlo A, Randi ML, et al. High rate of recurrent venous thromboembolism in patients with myeloproliferative neoplasms and effect of prophylaxis with vitamin K antagonists. Leukemia. 2016;30:2032–8.
Kaifie A, Kirschner M, Wolf D, Maintz C, Hänel M, Gattermann N, et al. Study Alliance Leukemia (SAL). Bleeding, thrombosis, and anticoagulation in myeloproliferative neoplasms (MPN): analysis from the German SAL-MPN-registry. J Hematol Oncol. 2016;9:18.
Acknowledgements
The authors wanted to thank all the patients who have accepted to be included in the OBENE database and the nurses who participated to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in this work, and received no funding for it.
About this article
Cite this article
Ianotto, JC., Couturier, MA., Galinat, H. et al. Administration of direct oral anticoagulants in patients with myeloproliferative neoplasms. Int J Hematol 106, 517–521 (2017). https://doi.org/10.1007/s12185-017-2282-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2282-5