Abstract
The international phase III DASISION trial demonstrated improved efficacy of dasatinib versus imatinib in treatment-naive patients with chronic myeloid leukemia in the chronic phase (CML-CP). We report efficacy and safety outcomes in a Japanese population from the final, 5-year follow-up of DASISION. At the end of the study, 77% (20/26) of dasatinib-treated and 61% (14/23) of imatinib-treated patients remained on initial therapy. Improved responses were observed in Japanese patients who received dasatinib versus imatinib (complete cytogenetic response: 96 vs 87%; major molecular response: 88 vs 74%; BCR-ABL1 ≤0.0032% International Scale [MR4.5]: 58 vs 52%). In patients who achieved BCR-ABL1 ≤10% at 3 months, 5-year progression-free survival and overall survival rates were high with dasatinib (96 and 96%) and imatinib (88 and 100%). The majority of adverse events were grade 1/2 in Japanese patients. Pleural effusion occurred more frequently in dasatinib-treated Japanese patients versus all patients (42 vs 28%), with no treatment discontinuations. Overall, in Japanese patients, dasatinib maintained its safety profile and had higher or comparable response and survival outcomes compared with imatinib or with all patients in DASISION. These findings demonstrate the long-term efficacy and tolerability of dasatinib and support frontline treatment of Japanese patients with CML-CP with dasatinib.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
BCR-ABL1 tyrosine kinase inhibitors (TKIs) can provide durable, positive responses for patients with chronic myeloid leukemia in chronic phase (CML-CP). Imatinib is a first-generation BCR-ABL1 TKI administered as a first-line medication to treat CML-CP with improved efficacy compared with interferon combination therapy [1,2,3]. Receiving the appropriate first-line treatment after a diagnosis of CML-CP is critical, as an early response could be predictive of long-term survival times [4,5,6,7,8]. Second-generation BCR-ABL1 TKIs, such as dasatinib, are effective first-line options for treatment of patients with CML-CP that can produce an early, deep response [8,9,10,11]. Recent data from the international phase III Dasatinib Versus Imatinib Study In Treatment-Naïve Chronic Myeloid Leukemia (DASISION) trial demonstrated the clinical efficacy and consistent, tolerable safety profile of dasatinib in newly diagnosed patients with CML-CP after a minimum of 5 years of follow-up [9].
The course a disease takes over time and the most appropriate treatment can vary based on a patient’s physical characteristics and geographical location. The annual incidence of CML, from a survey of patients in Asian countries (China, Hong Kong, Japan, the Philippines, Singapore, South Korea, Taiwan, and Thailand) between November 2008 and April 2009, was found to range from 0.4 to 1.0 per 100,000 people [12]. This is lower than the incidence of 1.8 per 100,000 people in the USA (based on statistics collected from 2009–2013) [13]. Information on the global incidence of CML is sparse, but CML is responsible for approximately 20% of all leukemia cases, which had a total, estimated worldwide incidence of 4.7 per 100,000 people (approximately 350,000 cases) in 2012 [14]. Patients in Asian countries are also typically diagnosed at a younger age (median 45 years) [12] compared with patients in the USA (median 64 years) [13]. A significant association between age at CML diagnosis and geographical region was described in a population-based study from the registry for the International Agency for Research on Cancer [15]. This study reported that age-standardized incidence rates for CML were lowest in Africa and Asia compared to other regions and lowest yet in Asian females. A similarity between patients in Asia and the USA is that more men than women are diagnosed with CML [12, 13]. There are some indications that the incidence of select adverse events (AEs), such as grade 3/4 neutropenia and thrombocytopenia, may be higher in Asian populations due to age and pharmacokinetics; however, larger studies are required to determine statistical differences [16].
Imatinib is commonly prescribed as a first-line therapy for patients with CML in Japan, but issues with imatinib intolerance suggest that use of a second-generation BCR-ABL1 TKI may be preferable [17, 18]. Japanese patients with CML may be likely to benefit from first-line treatment with dasatinib; however, there are limited data available demonstrating its efficacy specifically in this population. The D-First study (N = 52) was designed to investigate the efficacy and safety of dasatinib and explore factors affecting the outcomes of dasatinib-treated Japanese patients with newly diagnosed CML-CP [19]. Results included that dasatinib produced early, deep responses in Japanese patients, as BCR-ABL1 ≤10% by 3 months or BCR-ABL1 ≤1% by 6 months was reached by 90% of patients and major molecular response (MMR) by 12 months was reached by 75% of patients. While D-First supplied some early treatment results, analysis of the Japanese population from the multi-region DASISION study can be used to examine the long-term efficacy and safety profile of dasatinib as a first-line treatment for Japanese patients with CML-CP [9]. Analysis of this population after 2 years of treatment concluded that the safety and efficacy of dasatinib in Japanese patients was comparable to that observed in the total DASISION patient population [20].
In this report, we present efficacy and safety results from the Japanese population of patients in DASISION after 5 years of follow-up by comparing the dasatinib and imatinib treatment arms within the Japanese population and between Japanese patients and the total global population.
Materials and methods
Study design and patients
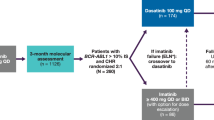
The DASISION (NCT00481247) trial was a phase 3, open-label, multinational, randomized trial of adults newly diagnosed with CML-CP and with final results of a 5-year follow-up [9, 21]. The trial design, eligibility criteria, and methodology have been previously described [21]. Patients must have been at least 18 years of age and diagnosed with Philadelphia chromosome-positive (Ph+) CML-CP within 3 months of enrollment to be considered eligible. Prior to randomization, all patients provided written, informed consent in accordance with the Declaration of Helsinki. Enrolled patients were stratified by EURO (Hasford) risk score [21, 22] and then randomized to receive either 100 mg dasatinib once a day (QD) or 400 mg imatinib QD [21]. Allowable reasons for discontinuation of treatment were disease progression [loss of complete hematologic response, increase in Ph+ cells by ≥30% from patient’s nadir, doubling of white blood cell count to >20.0 × 109/L, transformation to accelerated/blast phase (AP/BP) CML, or death], investigator-determined unacceptable toxicity, pregnancy, and withdrawal of consent. Dose interruptions and/or reductions were allowed for management of AEs, and dose escalations were permitted for patients with a suboptimal response (based on the European LeukemiaNet 2006 definition) [23]. Results from Japanese patients enrolled into DASISION were part of an exploratory analysis to compare treatment with dasatinib with imatinib in Japanese patients and with the total patient population, which includes the Japanese subpopulation.
Efficacy and safety assessments
Data from efficacy and safety analyses are presented after a 5-year follow-up (database lock: March 24, 2014) of the DASISION trial. A complete cytogenetic response (CCyR) was defined as no Ph+ metaphases in ≥20 bone marrow metaphases. Molecular analyses were assessed by quantitative polymerase chain reaction (qPCR) measured on the International Scale (IS). A 3-log, 4-log, or 4.5-log drop in BCR-ABL1 transcript levels from baseline represents BCR-ABL1 (IS) transcript levels of ≤0.1% (MMR), ≤0.01% (MR4.0), and ≤0.0032% (MR4.5), respectively. Based on recommendations at the time of study development, disease progression for this protocol was defined as a loss of complete hematologic response, increase in Ph+ cells by ≥30% from patient’s nadir, doubling of white blood cell count to >20.0 × 109/L, transformation to accelerated/blast phase (AP/BP) CML, or death. A sensitivity analysis of progression-free survival was performed to include patients who discontinued treatment and experienced progression or died on treatment or during follow-up. Survival assessments were conducted for up to 5 years in patients who agreed to follow-up.
Mutational analysis of BCR-ABL1 was required following discontinuation, and samples were obtained within 45 days before or after discontinuation. All molecular and mutational assessments were performed at a centralized laboratory (Molecular MD, Portland, OR, USA). AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 and evaluated throughout the study. Safety assessments were made in all patients who received at least one dose of the study drug.
Statistical analyses
The 95% confidence intervals (CIs) for response rates were calculated using the Clopper and Pearson method, and efficacy was analyzed based on the intention-to-treat principle. Results from qPCR were analyzed using SDS software (Applied BioSystems, Foster City, CA, USA). Patients who received at least one dose of study drug were included in the safety analysis. Comparison of response rates was performed at various time points for post hoc analyses; therefore, P values are descriptive and unadjusted for multiple comparisons. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Patient disposition
As previously reported, a total of 519 patients newly diagnosed with CML-CP from 108 centers and 26 countries were enrolled into DASISION and randomized to receive either dasatinib 100 mg QD (n = 259) or imatinib 400 mg QD (n = 260) for a minimum of 5 years [9, 21]. Of these patients, 49 (9%) were Japanese and were assigned to, and received, dasatinib (n = 26) or imatinib (n = 23). Baseline characteristics have been previously reported and were well balanced [9, 19]. The most common reason for discontinuation across Japanese and all patients was intolerance, whether patients were taking dasatinib (19 and 16%) or imatinib (13 and 7%) (Table 1) [9]. For Japanese patients, none treated with dasatinib and 3 (13%) treated with imatinib discontinued due to progression or treatment failure, compared with 11% of all treated patients on dasatinib and 14% of all treated patients on imatinib [9]. By the end of the 5-year study, 20 (77%) and 14 (61%) Japanese patients were on their initial therapy of dasatinib and imatinib, respectively, compared with 61 and 63%, respectively, in the total DASISION population.
Exposure to either study drug was evaluated in the terms of average daily dose, duration of treatment, and the number of dose modifications (reductions/interruptions) that occurred (Table 2). Japanese patients received a median average daily dose of 88 mg (range 56–100 mg) of dasatinib or 378 mg (range 279–456 mg) of imatinib. These values were lower than what was received by all study patients, with a median average daily dose of 99 mg (range 21–139 mg) of dasatinib or of 400 mg (range 125–741 mg) of imatinib [9].
Japanese patients were treated for a median duration of approximately 61 months in both arms. When time for dose interruptions was not being considered, 11 (42%) and 12 (52%) patients received dasatinib or imatinib treatment, respectively, for greater than 60 months (Table 2). More patients on dasatinib had dose interruptions compared with imatinib in both the Japanese population and all patients, and more patients had interruptions in the Japanese population versus the total population in both the dasatinib (92 vs 69%) and imatinib (74 vs 52%) arms. The same trend was observed for dose reductions of dasatinib (73 and 37%) and imatinib (57 and 17%) in Japanese and all patients. The first dose interruptions/reductions in the Japanese population were largely due to nonhematologic and hematologic toxicities for both dasatinib and imatinib. Toxicities were the most common reason for dose interruptions/reductions across all patients in DASISION as well, although to a lesser degree than in Japanese patients. No dose escalations of dasatinib were required for Japanese patients, compared with escalations in 17% of imatinib-treated Japanese patients. Fewer dose escalations occurred in both treatment arms of the Japanese population compared with all patients treated with dasatinib (9%) or imatinib (23%).
Efficacy
CCyR rates at any time were numerically higher for patients who received dasatinib versus imatinib across Japanese patients (96 vs 87%) and all patients (88 vs 84%) (Table 3). Also, in both treatment arms, the CCyR rates were higher in Japanese patients compared with the total population for both study drugs.
As observed with all patients in DASISION [9], the cumulative MMR rates were higher for dasatinib- versus imatinib-treated Japanese patients (Fig. 1; Table 3). Cumulative MR4.5 and MR4.0 rates were also higher for Japanese patients who received dasatinib versus imatinib. Cumulative MMR rates were higher for Japanese patients compared with the total population in both treatment arms (Table 3). By 5 years, 89% of Japanese patients and 76% of all patients who received dasatinib, and 74% of Japanese patients and 64% of all patients treated with imatinib, achieved MMR. Overall, after 5 years, molecular response rates at any time were numerically higher in Japanese patients versus all patients for both dasatinib (MMR: 88 vs 76%; MR4.0: 65 vs 54%; MR4.5: 58 vs 44%) and imatinib (MMR: 74 vs 64%; MR4.0: 61 vs 45%; MR4.5: 52 vs 34%) (Table 3).
Molecular response over time. The cumulative incidence of major molecular response (MMR) (a, b) and MR4.5 (c, d) over the course of 5 years of treatment for Japanese patients and all patients. Responses from the dasatinib arm are shown with a solid line and those from the imatinib arm with a dashed line. P values for comparing molecular response between dasatinib- and imatinib-treated patients by 60 months are 0.2657 (Japanese patients) and 0.0022 (all patients) for MMR and 0.9644 (Japanese patients) and 0.0251 (all patients) for MR4.5. The rate of response, calculated every 12 months, is displayed in the table associated with each graph
In DASISION, patients were stratified according to EURO risk score prior to randomization [21, 22] and MMR was analyzed according to these prognostic scores. All 12 Japanese, dasatinib-treated patients with a low EURO risk score achieved MMR, compared with 60% (6 of 10) of imatinib-treated, low-risk patients (Table 3). This was comparable to results observed in all low-risk patients, where 90% (77 of 86) treated with dasatinib and 69% (60 of 87) treated with imatinib achieved MMR. A slightly higher percentage of intermediate-risk Japanese patients treated with imatinib achieved MMR (91%) compared with those treated with dasatinib (85%). These values were greater than MMR achieved by all intermediate-risk patients treated with imatinib (65%) or dasatinib (71%) from the total population. Only 3 patients in the Japanese population were considered high risk. One high-risk patient treated with dasatinib and 1 of 2 treated with imatinib did not achieve MMR; however, comparisons based on risk scores are difficult due to the low number of Japanese patients per risk score group.
As previously reported, a higher percentage of all dasatinib-treated patients achieved BCR-ABL1 ≤10% at 3 months compared with all imatinib-treated patients (Table 3) [9, 20]. Similarly, 100% of Japanese patients treated with dasatinib versus 74% treated with imatinib achieved BCR-ABL1 ≤10% at 3 months [20]. Regardless of achievement of BCR-ABL1 ≤10% or >10% at 3 months, the 5-year overall survival (OS) was high in both the dasatinib-treated Japanese patients (96%) and imatinib-treated Japanese patients (100%). These OS rates are comparable to the 5-year OS rates for dasatinib- and imatinib-treated patients in the total population who achieved BCR-ABL1 ≤10% at 3 months (94 and 95%, respectively); however, achievement of BCR-ABL1 ≤10% at 3 months in Japanese patients was not predictive of improved OS as was observed in the total population of DASISION [9].
Analysis of progression-free survival (PFS), which includes patients who discontinued and progressed or died during treatment/follow-up, was also conducted based on BCR-ABL1 levels at 3 months. The 5-year PFS for the Japanese dasatinib-treated patients (of which all achieved BCR-ABL1 ≤10% at 3 months) was 96%. Five-year PFS rates were 88 versus 100% for imatinib-treated patients with BCR-ABL1 ≤10% versus >10% at 3 months in the Japanese population. PFS for dasatinib-treated Japanese patients was higher compared with PFS in all dasatinib-treated patients (96 vs 89%) [9]. Comparable PFS rates were observed between Japanese and all patients who received imatinib and achieved BCR-ABL1 ≤10% at 3 months (88 vs 93%). BCR-ABL1 levels ≤10% at 3 months were not predictive of improved PFS in Japanese patients as they were for the total population.
Mutational analysis
Patients who discontinued DASISION underwent mutational analysis to determine whether any mutations in the BCR-ABL1 gene were present. Samples from 45 patients from the Japanese population were analyzed for mutations. The majority of samples analyzed from dasatinib-treated patients did not produce results (20 of 24), and the four samples that did amplify did not identify any mutations. Fourteen of the 21 samples analyzed from imatinib-treated patients also did not produce results; however, mutations were identified in two of the seven samples that did amplify. The two mutations identified in Japanese patients in the imatinib arm were D276G and F359I, and both occurred in patients with disease progression.
Safety
For nonhematologic AEs (any grade) that occurred in at least 10% of Japanese patients and were related to either study drug treatment, after 5 years, the majority (16 of 22) occurred more often in imatinib-treated Japanese patients compared with those who received dasatinib (Table 4; Fig. 2). There were six AEs that occurred at a higher frequency in Japanese patients who received dasatinib: pleural effusion (42%), constipation, stomatitis, headache, and palpitations (each in 15% of patients), and hemorrhage (12%).
The most common nonhematologic AEs identified were similar between the Japanese population and all patients (Table 4). These AEs typically occurred at a higher or comparable frequency in Japanese patients versus all patients, with the exception of headache (0 vs 11%), muscle spasms (9 vs 21%), and conjunctivitis (0 and <1%) in imatinib-treated patients and diarrhea (15 vs 22%), vomiting (4 vs 5%), muscle spasms (4 vs 5%), arthralgia (0 vs 6%), and back pain (0 vs 3%) occurring more often in dasatinib-treated patients in the total population.
Serious, grade 3/4, nonhematologic AEs were low in both Japanese and all patients. For Japanese patients, one case each of serious, grade 3/4 pleural effusion, pulmonary hypertension, and altered consciousness occurred in the dasatinib arm and grade 3/4 subdural hematoma was reported in one patient in the imatinib arm. Across all study patients there were five (2%) cases of grade 3/4 pleural effusion and two (1%) cases of grade 3/4 pulmonary hypertension in the dasatinib arm and no additional cases of subdural hematoma.
The incidence of drug-related pleural effusion was numerically higher in Japanese patients treated with dasatinib (42%) compared with those treated with imatinib (4%) and also higher than all patients treated with dasatinib (28%) (Table 5) [9]. The median duration of pleural effusion was similar in the Japanese population and all patients receiving dasatinib, 331 and 283 days (range 7–2009 days), respectively. Only one Japanese patient experienced grade 3 pleural effusion, which was after 274 weeks of treatment with dasatinib. Grade 1/2 cases of pleural effusion in dasatinib-treated Japanese patients had a median time to first occurrence of 69 weeks (range 5–222 weeks) after treatment initiation. For all dasatinib-treated patients, grade 1/2 pleural effusions occurred after a median of 114 weeks (range 4–299 weeks) and grade 3 pleural effusions occurred after a median 175 weeks (range 114–274 weeks). There was one Japanese patient who was treated with imatinib who had a case of grade 2 pleural effusion 4.5 years after the first dose.
Of the 11 dasatinib-treated Japanese patients who experienced pleural effusion, eight (73%) patients were treated with a dose interruption. Comparatively, 45 (62%) dasatinib-treated patients in the total population underwent a dose interruption for treatment of pleural effusion [9]. Dose reductions occurred at a slightly higher frequency in Japanese patients (55%) compared with all patients (41%) in the dasatinib arm. While 15 (6%) dasatinib-treated patients overall discontinued treatment due to pleural effusion, no Japanese patients discontinued treatment in order to treat this AE. Therapeutic interventions, such as corticosteroids (9 vs 32%) and therapeutic thoracentesis (9 vs 12%), occurred less frequently in Japanese patients versus the total population of patients. Diuretics were used most often and to a similar degree in Japanese and all patients (46 and 47%) [9].
There were no cases of arterial ischemic events in the Japanese subpopulation, regardless of treatment. A low incidence of arterial ischemic events occurred in patients who received dasatinib (5%) and imatinib (2%) in the total population [9].
The occurrence of drug-related grade 3/4 hematologic AEs was also assessed. Neutropenia occurred to a similar degree in Japanese patients treated with dasatinib or imatinib, 15 and 13%, and anemia occurred in 4 and 0% of dasatinib- and imatinib-treated Japanese patients, respectively (Table 4; Fig. 2). Thrombocytopenia occurred in 4% of Japanese patients on dasatinib and 9% on imatinib, all of which were grade 3/4. Comparison of drug-related grade 3/4 hematologic AEs between Japanese and all patients revealed a comparable incidence of anemia (dasatinib: 4 vs 2%; imatinib: 0 vs 1%) and neutropenia (dasatinib: 15 vs 16%; imatinib: 13 vs 12%) regardless of treatment arm. Thrombocytopenia occurred at a lower frequency in dasatinib-treated Japanese versus all patients (dasatinib: 4 vs 16%) and at the same frequency in Japanese and all imatinib-treated patients (9% each).
A total of five of 26 (19%) dasatinib-treated Japanese patients discontinued therapy due to study drug toxicity (Table 1). Of these patients, one each discontinued due to pulmonary hypertension, platelet count, pneumothorax, prolonged QT interval, and elevated creatine phosphokinase. The three of 23 (13%) Japanese imatinib-treated patients who discontinued due to toxicity each did so due to thrombocytopenia, neutropenia, or hypophosphatemia. These results are comparable to the 16 and 7% of dasatinib- and imatinib-treated patients in the total population who discontinued due to intolerance (recurrent grade ≥3 hematologic or grade ≥2 nonhematologic toxicity). Out of the entire Japanese population, there was one death over 2 years after the last dose of dasatinib was received. This patient discontinued dasatinib after approximately 5.5 months in the study, and no BCR-ABL1 mutations were identified at this time. Treatments subsequent to study therapy included imatinib, nilotinib, methotrexate/prednisolone, multi-agent chemotherapy, and hydroxyurea. This patient progressed to accelerated/blast phase while being treated with post-study therapy and ultimately died from progressive disease.
Discussion
After 5 years of follow-up, the Japanese patients from the DASISION trial continued to have deep and long-term responses. CCyR rates and MMR rates at any time were comparable between the 2- and 5-year reports for the Japanese subpopulation, whether patients received dasatinib or imatinib. MMR was consistently higher for Japanese patients treated with dasatinib compared with imatinib throughout the study and by 5 years (89 and 74%, respectively). Rates of MR4.0 and MR4.5 increased over time for both treatment arms of the Japanese population [20], and more dasatinib- versus imatinib-treated patients achieved MR4.0 (65 vs 61%) and MR4.5 (58 vs 52%). PFS and OS rates based on BCR-ABL1 ≤10% at 3 months remained high at 5 years.
More Japanese patients achieved MMR, MR4.0, and MR4.5 by 5 years than by 2 years in both treatment arms [20]. The rates of MMR and MR4.5 at any time were higher with dasatinib versus imatinib in the Japanese population, but the difference in these rates became less distinct over time. After 48 months, the MR4.5 rates in Japanese patients were comparable between the imatinib and dasatinib arms, and this was maintained through the 5-year end of study time point. The difference in MMR rates over time between the two treatment arms of Japanese patients decreased after 48 months, but MMR was consistently higher for patients on dasatinib versus imatinib.
A limitation of this study is the small sample size of the Japanese population. Because of the lower patient numbers in the two arms of this subpopulation, this study was not powered for statistical analysis comparing dasatinib- and imatinib-treated Japanese patients. Although this can make comparison of results between the two arms difficult, we speculate that one possible explanation for the difference in MMR rates over time in dasatinib- and imatinib-treated Japanese patients is the number of dose modifications received by patients in each arm. More Japanese patients received dose interruptions and reductions on dasatinib (92 and 73%) than imatinib (74 and 57%), which were largely due to toxicities in both arms. Dose modifications appeared to resolve most of these AEs to a tolerable level, as only five (19%) and three (13%) patients discontinued dasatinib or imatinib, respectively, due to a study drug-related AE.
A significantly higher MMR rate was reported for dasatinib- versus imatinib-treated patients in the total population from DASISION [9]. Molecular responses were numerically higher for both arms of the Japanese population compared with their respective arms in the total population. For example, MR4.0 and MR4.5 were achieved by 11–14% more dasatinib-treated and 16–18% more imatinib-treated Japanese patients than all patients, respectively, at any time over the 5-year study. The reason why molecular responses for both treatment arms were higher in the Japanese population versus all patients is unclear; however, the small number of patients in the Japanese subpopulation may explain these differences. Another possibility is that there were fewer high-risk patients (determined by EURO risk score) in the Japanese population versus the total population (6 vs 19%) [20], affording a greater likelihood for the Japanese subpopulation to achieve MMR compared to all patients.
The different treatment modifications while on study that occurred between the Japanese population and all patients should be considered as one potential explanation for the noted differences in molecular response results between these two populations. For example, the increased number of patients with dose escalations in the total population of DASISION may be indicative of a lack of early molecular response, which has been shown to be related to positive long-term outcomes [4,5,6,7,8]. All dasatinib-treated and 74% of imatinib-treated Japanese patients achieved an early molecular response of BCR-ABL1 ≤10% at 3 months, whereas this milestone was reached by fewer dasatinib-treated (84%) and imatinib-treated (64%) patients in the total study population [9].
While both the Japanese subpopulation and total population had dose modifications, primarily to manage AEs, more Japanese patients required dose interruptions (84%) and reductions (65%) compared with all patients (60 and 27%), regardless of study drug. In contrast, twice as many patients in the total population versus the Japanese population required a dose escalation (16 vs 8%). The varying degree of dose modifications between the two populations resulted in an overall lower median average daily dose for Japanese patients versus all patients, whether they received dasatinib (88 vs 99 mg/day) or imatinib (378 vs 400 mg/day).
The safety profile of dasatinib remained consistent with previous reports, with no new AEs identified. AEs of special interest occurred in a greater percentage of Japanese patients than all patients; however, the majority of these events were grade 1/2. The only drug-related nonhematologic grade 3 events to occur in Japanese patients were an altered state of consciousness, pericardial effusion, pulmonary hypertension (diagnosed with 2D echocardiography), and pleural effusion (one each) in the dasatinib arm, and diarrhea (two cases) and convulsions and subdural hematoma (one each) in the imatinib arm. In the Japanese population, there were no nonhematologic events higher than grade 3 other than one case of grade 4 prolonged QT interval.
Pleural effusion occurred more often in dasatinib- versus imatinib-treated patients; however, only one of these events was higher than grade 1/2, reported as grade 3 pleural effusion. The mechanism behind a higher incidence of pleural effusion in Japanese patients is unknown. Effective management of these low-grade cases of pleural effusion through dose modifications was likely a key contributor to the lack of discontinuation in Japanese patients who experienced a pleural effusion event. Discontinuation rates due to dasatinib-related pleural effusion were low in the total patient population of DASISION (6%) and were also effectively managed [9]. Despite the majority of pleural effusion cases being low grade, there is the potential for these events to develop into a serious condition; therefore, patients should be closely monitored to identify these events early and receive the appropriate treatment(s). One patient discontinued dasatinib due to pulmonary hypertension. Echocardiograms, to monitor for pulmonary hypertension, were not routinely performed on study for patients with pleural effusion; however, they were conducted at baseline, at 3 months, and then if clinically indicated (as determined by the investigator).
The occurrence of arterial ischemic events can be a concern with some of the second-generation BCR-ABL1 TKIs [24,25,26]. While a low incidence of cardiovascular ischemic events was reported in all patients from DASISION [9], there were no arterial ischemic events reported in either treatment arm for the Japanese population.
Overall, dasatinib maintained a high, long-term efficacy profile after 5 years of follow-up in Japanese patients newly diagnosed with CML-CP from DASISION. Although no new AEs were identified and no patients experienced an arterial ischemic event, the occurrence of pleural effusion in the dasatinib arm was higher in Japanese patients than the total DASISION population; therefore, it should continue to be monitored carefully. Of note, more patients in the Japanese population achieved MR4 and MR4.5 compared with the total population. With its ability to generate an early and deep response, that is sustained, dasatinib should be considered an effective first-line option for Japanese and all patients newly diagnosed with CML-CP.
References
Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17.
Gleevec® (imatinib) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp.; 2015. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/gleevec_tabs.pdf. Accessed 1 Nov 2016.
O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004.
Alvarado Y, Kantarjian H, O’Brien S, Faderl S, Borthakur G, Burger J, et al. Significance of suboptimal response to imatinib, as defined by the European LeukemiaNet, in the long-term outcome of patients with early chronic myeloid leukemia in chronic phase. Cancer. 2009;115:3709–18.
Branford S, Kim DW, Soverini S, Haque A, Shou Y, Woodman RC, et al. Initial molecular response at 3 months may predict both response and event-free survival at 24 months in imatinib-resistant or -intolerant patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase treated with nilotinib. J Clin Oncol. 2012;30:4323–9.
Hanfstein B, Müller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia. 2012;26:2096–102.
Hughes TP, Hochhaus A, Branford S, Müller MC, Kaeda JS, Foroni L, et al. IRIS investigators. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood. 2010;116(19):3758–65.
Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boqué C, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2014;123:494–500.
Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333–40.
Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54.
Sprycel® (dasatinib) [package insert]. Princeton, NJ: Bristol-Myers Squibb Co.; 2015. https://packageinserts.bms.com/pi/pi_sprycel.pdf. Accessed 1 Nov 2016.
Kim DW, Banavali SD, Bunworasate U, Goh YT, Ganly P, Huang H, et al. Chronic myeloid leukemia in the Asia-Pacific region: current practice, challenges and opportunities in the targeted therapy era. Leuk Res. 2010;34:1459–71.
Efficace F, Baccarani M, Breccia M, Alimena G, Rosti G, Cottone F, et al. GIMEMA. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood. 2011;118:4554–60.
World Health Organization Union for International Cancer Control. 2014 Review of Cancer Medicines: Chronic Myeloid Leukemia—Executive Summary; 2014. http://www.who.int/selection_medicines/committees/expert/20/applications/CML.pdf?ua=1. Accessed October 10, 2016.
Mendizabal AM, Younes N, Levine PH. Geographic and income variations in age at diagnosis and incidence of chronic myeloid leukemia. Int J Hematol. 2016;103:70–8.
Chuah CT, Nakamae H, Shen ZX, Bradley-Garelik MB, Kim DW. Efficacy and safety of dasatinib versus imatinib in the East Asian subpopulation of the DASISION trial of newly diagnosed chronic myeloid leukemia in chronic phase. Leuk Lymphoma. 2014;55:2093–100.
Ng KP, Hillmer AM, Chuah CT, Juan WC, Ko TK, Teo AS, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18(4):521–8.
Ohnishi K, Nakaseko C, Takeuchi J, Fujisawa S, Nagai T, Yamazaki H, et al. Japan Adult Leukemia Study Group. Long-term outcome following imatinib therapy for chronic myelogenous leukemia, with assessment of dosage and blood levels: the JALSG CML202 study. Cancer Sci. 2012;103:1071–8.
Iriyama N, Fujisawa S, Yoshida C, Wakita H, Chiba S, Okamoto S, et al. Early cytotoxic lymphocyte expansion contributes to a deep molecular response to dasatinib in patients with newly diagnosed chronic myeloid leukemia in the chronic phase: results of the D-first study. Am J Hematol. 2015;90:819–24.
Fujisawa S, Nakamae H, Ogura M, Ishizawa K, Taniwaki M, Utsunomiya A, et al. Efficacy and safety of dasatinib versus imatinib in Japanese patients with newly diagnosed chronic-phase chronic myeloid leukemia (CML-CP): subset analysis of the DASISION trial with 2-year follow-up. Int J Hematol. 2014;99:141–53.
Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–70.
Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. Writing Committee for the Collaborative CML Prognostic Factors Project Group. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. J Natl Cancer Inst. 1998;90:850–8.
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51.
Gainor JF, Chabner BA. Ponatinib: accelerated disapproval. Oncologist. 2015;20:847–8.
Quintás-Cardama A, Kantarjian H, Cortes J. Nilotinib-associated vascular events. Clin Lymphoma Myeloma Leuk. 2012;12:337–40.
Saglio G, le Coutre P, Cortes J, Mayer J, Rowlings PA, Mahon F-X, et al. The observed and expected incidence of cardiovascular ischemic events in dasatinib-treated patients across a clinical trial program [abstract]. Blood. 2014;124:4534.
Acknowledgements
The authors would like to thank all participating study sites for this Bristol-Myers Squibb (BMS)-sponsored analysis. Professional medical writing and editorial assistance was provided by Kelly M. Fahrbach, PhD, of StemScientific, an Ashfield Company, part of UDG Healthcare plc, funded by BMS. The authors did not receive financial compensation from BMS for authoring this manuscript.
Author contributions
All authors provided feedback and guidance on the analysis and interpretation of the results, critically reviewed and provided revisions to the manuscript, and approved the final draft for submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hirohisa Nakamae has worked as a consultant for, received honoraria from, received research funding and travel expense reimbursement from, and served on speakers’ bureaus for Bristol-Myers Squibb and Novartis. Shin Fujisawa received research funding from Pfizer. Michinori Ogura has received research funding from Celltrion and SymBio Pharmaceuticals, has received honoraria from AstraZeneca, Janssen, and Takeda, and acted as a consultant for AstraZeneca, Celltrion, Meiji-Seika Pharma, and Mundipharma. Toshiki Uchida has received honoraria from Janssen Pharmaceuticals. Yasushi Onishi has received research funding from Bristol-Myers Squibb KK, and Novartis. Masafumi Taniwaki has received research funding from Chugai Pharmaceutical, Eisai, Kyowa Hakko Kirin, Pfizer, and Toyama Chemical. Atae Utsunomiya has served as a consultant to and received honoraria from Bristol-Myers Squibb KK and Kyowa Kakko Kirin Co., Ltd. Kosei Matsue has received honoraria from Celgene. Yasushi Takamatsu has received honoraria from Celgene, Kyowa Hakko Kirin, and Taisho Toyama Pharmaceutical. Kensuke Usuki has received research funding from Astellas Pharma, Fujimoto Pharmaceutical, Otsuka Pharmaceutical, Sumitomo Dainippon Pharma. Mitsune Tanimoto has received research funding from Astellas Pharma, Chugai Pharmaceutical, Kyowa Hakko Kirin, Nippon Shinyaku, Novartis, Otsuka Pharmaceutical, and Pfizer. Yoji Ishida declares no conflict of interest. Kazuteru Ohashi declares no conflict of interest. Li Li is an employee of Bristol-Myers Squibb. Masafumi Miyoshi is an employee of Bristol-Myers Squibb KK.
About this article
Cite this article
Nakamae, H., Fujisawa, S., Ogura, M. et al. Dasatinib versus imatinib in Japanese patients with newly diagnosed chronic phase chronic myeloid leukemia: a subanalysis of the DASISION 5-year final report. Int J Hematol 105, 792–804 (2017). https://doi.org/10.1007/s12185-017-2208-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2208-2