Abstract
We investigated the relationship between serum copper and various prognostic factors, time to start treatment, and treatment response in patients with B-cell chronic lymphocytic leukemia (B-CLL) and related disorders. Fifty newly diagnosed CLL patients aged 36–70 years were included. Patients were studied for serum lactate dehydrogenase (LDH), serum copper, direct Coombs’ test, serum β2 microglobulin (β2M), immunophenotyping for diagnosis of B-CLL, evaluation of CD38 and zeta-associated protein (ZAP-70) expression, and fluorescence in situ hybridization technique for cytogenetic analysis. Fourteen of 50 patients had high serum copper level; they had a significant increase in LDH, serum β2M, incidence of positive Coombs’ test, CD38 and ZAP-70, incidence of 17p del, and a decrease in hemoglobin concentration, lymphocyte doubling time and time to start treatment with a lower treatment response rate. No significant difference was found with regard to Rai staging for CLL. These results indicate that serum copper level, a cheap and simple laboratory test, is of great value in CLL patients as it showed a significant association with some important adverse prognostic markers such as increased expression of ZAP-70 and CD38, shorter time to start treatment and poor response to treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
B-Chronic lymphocytic leukemia (CLL) is the most prevalent form of adult leukemia. B-CLL is characterized by the progressive accumulation of mature, monoclonal CD5+ CD19+ CD23+ B lymphocytes in the peripheral blood, lymph nodes, spleen and bone marrow. Typically, it has an indolent clinical course. However, a subset of patients experiences a more aggressive clinical course and worse prognosis [1]. Although the two major staging systems (Rai and Binet) have provided valuable information in addressing this clinical heterogeneity, they cannot be used to predict the individual risk of disease progression and survival in the early stages of CLL [2]. For this reason, several prognostic factors such as lymphocytes doubling time, serum levels of lactate dehydrogenase (LDH) and β2 microglobulin (β2M), CD38 expression, serum thymidine kinase, zeta-associated protein (ZAP-70), immunoglobulin heavy chain variable gene segment mutation status and the cytogenetic abnormalities have been added to the current staging system to differentiate prognostic subsets [3].
There is an increasing amount of available information about the possible coincidence and association of trace elements in the pathogenesis of malignant neoplasms. Copper is an essential trace element that participates in metabolic pathways involving cellular respiration, peptide biogenesis, connective tissue biosynthesis, and antioxidant defense. It is an acute phase reactant increasing in response to infection, injury and in chronic inflammatory conditions [4]. It serves as a cofactor in redox reactions of enzymes that carry out basic biological functions required for normal growth such as cytochrome c oxidase (involved in the mitochondrial electron transport chain) and superoxide dismutase involved in the detoxification of reactive oxygen species (ROS). This enzyme plays an important role in the protection of the organism against free radicals [5]. But excess of copper has been considered to be a potent oxidant, causing the generation of ROS in the organism and has a potential role in carcinogenesis. The interaction of ROS with DNA has been reported to result in fragmentation with the loss of bases and leads to strand breaks. This damage is normally fixed with the aid of specialized DNA repair enzymes present in the nucleus. However, if the repair process is not efficient, these DNA strand breaks can accumulate in mammalian cells leading to oncogenic transformation and/or cell killing, depending on the capacity of cellular repair processes to overcome them [6].
In this study, serum copper levels in patients with B-CLL in relation to clinical information and laboratory parameters including other known prognostic markers were examined.
Subjects and methods
This study included 50 newly diagnosed untreated B-CLL patients who were presented to Hematology Oncology Clinic, Zagazig University Hospitals in the period from June 2010 to May 2011. Informed consent was obtained in all cases; the study protocol was approved by the Ethical Committee of Faculty of Medicine, Zagazig University.
All members of the study were subjected to complete history taking, clinical examination, and laboratory investigations including complete blood picture (Sysmex Kx-21), direct Coombs’ test, serum LDH by Hitachi 902, serum β2M estimation by chemilumeniscence with Immulite and serum copper using Elitech kit. According to the serum copper level, patients were classified into two groups: normal serum copper group (normal range of serum copper is 70–150 μg/dL) and elevated serum copper group (serum copper >150 μg/dL).
Immunophenotyping was done for diagnosis of B-CLL performed on a FACScan (Becton-Dickinson, San Jose, CA). A panel of monoclonal antibodies labeled with fluorescein isothiocyanate (FITC) and phycoerythrin (PE) were used as follow: CD20, CD5, CD19, CD3, CD22, Κ/λ, CD7, CD23, CD79b, and FMC7. Estimation of surface CD38 and cytoplasmic ZAP-70 expression was done using monoclonal antibodies [7]. Anti-CD38-PE (DAKO, CA, USA) and anti-ZAP-70-FITC (Becton-Dickinson, San Jose, CA, Bioscience) with an intrastain kit (Dako) were provided with a permeabilizing agent. Specific isotypic control for FITC, PE and PC-5 (negative control) conjugated monoclonal antibody was used. Lymphocytes were selected in the forward scatter versus side scatter dot blot and additionally gated as CD19/CD5 positive cells. Results were expressed as a percentage of gated cells showing positive expression over the corresponding isotypic control with cut-off ≥20 % for ZAP-70 positive and ≥30 % for CD38 positive.
Cytogenetic analysis
Cytogenetic analysis was performed on peripheral blood using fluorescence in situ hybridization (FISH) technique, using a locus specific identifier DNA probe (LSI) Kit, Vysis (Abbott Park, Ill, USA).
Indications to start treatment
Indications to start treatment included development of systemic symptoms, bulky lymphadenopathy, increasing organomegaly, high white blood cell count with a lymphocyte doubling time <12 months, the development of anemia or thrombocytopenia due to bone marrow infiltration and repeated infection. Patients <55 years received fludarabine/cyclophosphamide, while those >55 years received chlorambucile with or without prednisolone. Failure of response was defined as stable (did not achieve any remission) or progressive disease, and overall response was defined as complete and partial remission of the disease.
The patients were followed-up for 24 months to detect time to start first therapy and to evaluate the disease outcome. After 6 months of starting therapy, patients were reevaluated to assess their response according to National Cancer Institute-sponsored group guidelines.
Statistics analysis
Analysis of data was performed with statistical package for social science computer program (SPSS Inc., version 16.0, Chicago, IL). Numerical data were expressed as mean ± standard deviation and range. Qualitative data were expressed as frequency and percentage. Fisher’s exact test was used to examine the relation between qualitative variables. For quantitative data (normally distributed), comparison between two groups was done using student t test (P < 0.05; significant). Associations between potential predictors and serum copper were evaluated by Cox proportional hazards models. Kaplan–Meier method was used to estimate the time to start treatment, and comparison between groups was done using the log-rank test. Time to start treatment was estimated from the time of diagnosis to the time of starting first treatment. Comparison of data was done for levels obtained at diagnosis.
Results
The clinical, laboratory, and cytogenetic data of all patients with B-CLL are summarized in Table 1. There were 28 males and 22 females. High serum copper levels were found in 14 patients and normal serum copper levels were found in 36 cases. No statistically significant difference with regard to age or sex was found between patients with high serum copper levels and patients with normal serum copper levels. There was statistically significant decrease in hemoglobin (Hb) concentration and lymphocyte doubling time, and statistically significant increase in serum LDH, serum β2M, positive Coombs’ test, CD38, ZAP-70 expression and incidence of 17p del (as a sole anomaly plus 11q del (6 patients) in patients with elevated serum copper levels compared to patients with low serum copper levels, while there was no statistical significant difference regarding absolute lymphocytic count (ALC), Rai staging (stages I, II and III versus stages IV and V), organomegaly and lymphadenopathy.
We included the parameters which showed significant difference between high and normal serum copper groups in cox regression hazards analysis which revealed that β2M, CD38 and Zap-70 are the only predictors that add significance to the model; (Table 2).
Therapy
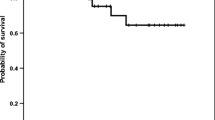
Forty-two patients (84 %) received treatment during the follow-up period. Six patients did not need any therapy for CLL and two patients were lost from follow-up. There was a significant negative correlation between serum copper level and time of first treatment (P < 0.001) (Fig. 1). The Kaplan–Meier estimate of the time to start treatment was significantly shorter in patients with high serum copper compared to those with normal level (median 6, 15.9 months, P < 0.05) (Fig. 2). Twenty-six of treated patients (61.9 %) showed overall response and 16 patients (38.1 %) revealed failure of response. There was statistically significant decrease in the first time to start treatment in patients with elevated serum copper levels compared to patients with low serum copper levels and statistically significant increase in failure of response in patients with elevated serum copper levels (odds ratio = 26.4, confidence interval = 4.4–157.9, and P < 0.001); Table 3. The results of treatment response were analyzed with selected clinical, cytogenetic, and laboratory data other than serum copper level. There were statistically significant increases in serum LDH, serum β2M, positive Coombs’ test, CD38 expression, ZAP-70 expression, lymphocytes doubling time and incidence of 17p del in group with failure of response compared to overall response; (Table 4).
Discussion
Chronic lymphocytic leukemia was traditionally considered as a disease of elderly patients. The duration and outcome of the disease are, however, highly variable. Currently, the clinical staging systems (Rai, Binet) are the basis for classification. However, they are unable to predict disease progression especially in the early stages. The novel treatment options would necessitate the precise identification of patients with unfavorable prognosis who are at the highest risk for early progression and who would gain the most benefit from early administration of more aggressive, targeted treatment. For this reason, the value of seeking out prognostic factors of CLL has increased [3].
Patients with CLL may present with a markedly elevated ALC which can be considered as a simple, reliable reflection of the tumor mass; its value is independent of other factors such as clinical stage or degree of bone marrow infiltration. The ALC in our patients has no significant difference with regard to serum copper level. It should not be used as the sole indicator for treatment, but should be included as a part of the total clinical picture [8].
In our study, serum LDH levels and β2M were found to be increased in B-CLL patients (normal reference range of LDH is 100–500 IU/L [9] ). They are markers of tumor burden. We have found a significant positive correlation between them and serum copper levels also; they are significantly increased in patients with high serum copper levels. Beguin et al. [10] reported higher serum copper in patients than controls and correlated with serum LDH. Dohner et al. [11] have found serum LDH level together with age, clinical staging, white blood cell count and the presence or absence of 11q del, 17p del can give significant prognostic information. Keating et al. [12] have found serum β2M to be the strongest predictor of 5-year survival on multivariate analysis.
CD38 signaling may play a role in increased proliferation and decreased apoptosis through activation of ZAP-70 and BCL [13]. In this study, there was significant increase in CD38 expression in patients with high serum copper levels than those with normal levels and patients having CD38 expression had 6.3 times to have high serum copper levels than patients without CD38 expression. We have found comparable results of positive CD38 expression (34 %) in B-CLL with Schroers et al. [14] (29 %), Dürig et al. [15] (42 %), but the later defined such expression as ≥20 % that may explain the difference. Damle et al. [16] have found patients with unmutated immunoglobulin V gene displayed higher percentages of CD38 positive B-CLL cells than those with mutated V gene.
ZAP-70 is a key signaling molecule for T lymphocytes and natural killer cells. ZAP-70 is not expressed in normal B lymphocytes, but it is associated with increased intracellular signaling via the immunoglobulin receptor in B-CLL cells. We have found significant increase in ZAP-70 expression in patients with high serum copper levels than those with normal levels and patients having ZAP-70 expression had 5.0 times to have high serum copper levels than patients without ZAP-70 expression. We have found the incidence of positive ZAP-70 expression 26 % in B-CLL, while Schroers et al. [14] have found an incidence 47 %, but our work was on newly diagnosed cases, but their patients were retrospectively enrolled and not newly diagnosed cases.
With regard to cytogenetic analysis, we found a significant increase in the incidence of 17p del (as a sole anomalies plus 11q del) in patients group with high serum copper than those with normal levels; we cannot compare our results with others as, to the best of our knowledge, no pervious study discussed the relation of serum copper level with cytogenetic finding in CLL patients.
Several pathophysiological mechanisms have been proposed to explain the relationship between elevated serum copper levels and progression of hematological malignancies. Primarily, it has been suggested that copper promotes angiogenesis [17]. Second, excess of copper has been considered to be a potent oxidant, causing the generation of ROS. It is well known that cancer cells are under increased and persistent oxidative stress, due to elevated levels of intracellular ROS generation [2]. Also, it has a role in tumor cell invasion and metastasis due to a loss of integrity of target tissue cell to cell junctions as a result of E-cadherin down-regulation which is normally regulated by a complex network involving the copper-dependent lysyl oxidase like proteins [18].
The precise mechanisms responsible for the elevation in serum copper levels are still unclear and require further evaluation in the future. The elevation of ceruloplasmin and copper in cancer patients has often been considered as part of a nonspecific acute phase reaction, but this statement has been challenged by others [19] as it was not usually correlated with erythrocyte sedimentation rate and C-reactive protein.
We have found significant increases of serum LDH, serum β2M, positive Coombs’ test, CD 38, ZAP-70, 17p del, failure of response to therapy; and decreased Hb concentration, lymphocytes doubling time, and time to start first therapy in patient with high serum copper levels compared to those with normal levels, also there was a significant negative correlated with time of first treatment, while no significant difference according to Rai staging, this is similar to that reported for the first time by Kaiafa et al. [20] who found an association between high serum copper levels and several adverse prognostic markers in CLL, such as increased expression of ZAP-70 and CD38, along with elevated percentage of unmutated V immunoglobulin gene.
Regarding response to therapy, patients with failure of response showed a statistically significant increase in serum copper, β2M, LDH, the incidence of positive Coombs’ test, LDT, CD38 expression, and ZAP-70 expression, but no significant results were obtained with regard to Rai staging or cytogenetic finding (except for 17p del) as compared to those with overall response. Zwiebel and Cheson [21] reported that patients with poor response to therapy with fludarabine had significant increased serum β2M and the incidence of 11q and 17p del. They recommended the use of these biological factors to predict the chemosensitivity of B-CLL patients and to indicate when more novel or polychemotherapeutic approaches are needed.
From all the previous statistical relation, it is likewise that increased serum copper levels correlate with tumor burden, rapid progression, early started therapy with bad response to it even in the cases classified as low and intermediate in clinical staging as five patients included in Rai stage I, II, and III had poor response to therapy, so these clinical staging cannot be used to predict the risk of disease progression.
There was association between several adverse prognostic markers in CLL, such as increased expression of ZAP-70, CD38 [13, 14, 16, 22] elevated percentage of unmutated immunoglobulin V gene [16, 23], but their measures are expensive and technically not easy like measuring serum copper. So, serum copper determination could be of great importance.
Conclusion
We conclude that serum copper level which is a cheap and simply measured laboratory parameter was positively correlated with most of the important prognostic markers of CLL. Also, its high level can predict a shorter time to start treatment with poor response and rapid disease progression in patients with newly diagnosed B-CLL and related disorders, especially in patients in early clinical stages as some of them were at risk of early disease progression and whether these patients would have a better disease outcome if treatment was started early in the course of the disease rather than waiting for disease progression. So the current challenge is the identification of CLL patients at high risk, at time of diagnosis, for therapy–tailoring.
References
Rawstron AC, Green MJ, Kuzmiccki A, Kennedy B, Fenton JA, Evans PA, et al. Monoclonal B lymphocytes with the characteristics of “indolent” chronic lymphocytic leukemia are present in 3.5 % of adults with normal blood counts. Blood. 2002;100:635–42.
Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Eng J Med. 1995;333:1052–9.
Zenz T, Hling SF, Mertens D, Dohner H, Stilgenbauer S. Moving from prognostic to predictive factors in chronic lymphocytic leukaemia (CLL). Best Pract Res Clin Haematol. 2010;23:71–84.
Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–85.
Turnlund JR. Human whole-body copper metabolism. Am J Clin Nutr. 1998;67(Suppl):960S–4S.
Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. 2009;35:38–46.
Bryant PE. Use of restriction endonucleases to study relationships between DNA double-strand breaks, chromosomal aberrations and other end-points in mammalian cells. Int J Radiat Biol. 1988;54:869–90.
Cheson B, Bennett J, Grever M. National Cancer Institute-sponsored working group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–7.
Kornberg A, Polliack A. Serum lactic dehydrogenase (LDH) levels in acute leukemia: marked elevations in lymphoblastic leukemia. Blood. 1980;56:351–5.
Beguin Y, Brasseur F, Weber G, Bury J, Delbrouck JM, Roelandts I, et al. Observations of serum trace elements in chronic lymphocytic leukemia. Cancer. 1987;60:1842–6.
Dohner H, Stilgenbauer S, Benner A. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:910–6.
Alatrash G, Albitar M, O’Brien S, Wang X, Manshouri T, Faderl S, Ferrajoli A, Burger J, Garcia-Manero G, Kantarjian HM, Lerner S, Keating MJ, Wierda WG. Circulating CD52 and CD20 levels at end of treatment predict for progression and survival in patients with chronic lymphocytic leukaemia treated with fludarabine, cyclophosphamide and rituximab (FCR). Br J Haematol. 2010;148:386–93.
Chen L, Widhopf G, Huynh L, Rassenti L, Rai KR, Weiss A, et al. Expression of ZAP-70 is associated with increased B-cell receptor signalling in chronic lymphocytic leukemia. Blood. 2002;100:4609–14.
Schroers R, Griesinger F, Trumper L, Haase D, Lulle B, Klein-Hitpass L. Combined analysis of ZAP-70 and CD38 expression as a predictor of disease progression in B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:750–8.
Dürig J, Naschar M, Schmücker U, Renzing-Köhler K, Hölter T, Hüttmann A, et al. CD38 expression is an important prognostic marker in chronic lymphocytic leukaemia. Leukemia. 2002;16:30–5.
Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–7.
Tam CS, Shanafelt TD, Wierda WG, Abruzzo LV, Van Dyke DL, O’Brien S, et al. De novo deletion 17p13.1 chronic lymphocytic leukemia shows significant clinical heterogeneity: the M.D. Anderson and Mayo Clinic experience. Blood. 2009;114:957–64.
Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, et al. Concurrent up-regulation of BCL-XL and BCL2 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–10.
Linder MC, Moor JR, Wright K. Ceruloplasmin assays in diagnosis and treatment of human lung, breast, and gastrointestinal cancers. J Natl Cancer Inst. 1981;67:263–75.
Kaiafa G, Saouli Z, Diamantidis M, Kontoninas Z, Voulgaridou V, Raptaki M, et al. Copper levels in patients with hematological malignancies. Eur J Intern Med. 2012;23:738–41.
Zwiebel JA, Cheson BD. Chronic lymphocytic leukemia staging and prognostic factor. Semin Oncol. 1998;25:42–59.
Ibrahim S, Keating M, Do KA, O’Brien S, Huh YO, Jilani I, et al. CD38 expression as an important prognostic factor in B-cell. Blood. 2001;98:181–6.
Hamblin TJ, Orchard JA, Gardiner A, Oscier DG, Davis Z, Stevenson FK. Immunoglobulin V genes and CD38 expression in CLL. Blood. 2002;99:1023–9.
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Labib, H.A., Hassanein, M. & Etewa, R.L. Serum copper is a simple but valuable prognostic marker in B-cell chronic lymphocytic leukemia. Int J Hematol 100, 575–581 (2014). https://doi.org/10.1007/s12185-014-1686-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-014-1686-8