Abstract
The TOPS trial evaluated high- (800 mg/day; n = 319) versus standard-dose (400 mg/day; n = 157) imatinib in patients newly diagnosed with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase. Patients had a minimum follow-up of 42 months or discontinued early. Major molecular response (MMR) rates were similar between arms at (51.6 vs 50.2 % for 400 and 800 mg/day, respectively; P = 0.77) and by (75.8 vs 79.0 %; P = 0.4807) 42 months. There were no differences in event-free survival (EFS), progression-free survival (PFS), or overall survival (OS) between arms. The estimated rates of PFS on treatment and OS at 42 months were significantly higher in patients with MMR at 6, 12, and 18 months compared with those without MMR. Adverse events were more frequent with high-dose imatinib. Patients with ≤1 treatment interruption (vs >1) and those able to maintain imatinib ≥600 mg/day (vs <600 mg/day) in the first year of treatment had faster and higher response rates, but no improvement in EFS or PFS. Adherence to prescribed dose without interruption may be more important than initiation of therapy with higher doses of imatinib. Achievement of MMR correlated with long-term clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) trial was designed to compare outcomes of patients with newly diagnosed, previously untreated Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP) on daily imatinib 400-mg (standard dose) versus 800-mg (high dose) therapy (registered at http://www.clinicaltrials.gov as NCT00124748). Previously published results of the TOPS trial demonstrated that major molecular response (MMR) rates at 12 months were similar for both dose cohorts, but that MMR and complete cytogenetic response (CCyR) occurred significantly earlier in patients treated with daily imatinib 800 mg versus standard dose [1]. The TOPS study was prematurely terminated in 2010 because no improvement was observed in the primary efficacy end point and long-term outcomes for the 800-mg treatment arm compared with the 400-mg treatment arm. The purpose of this report is to present the final analysis of data from this study, when all patients had a minimum follow-up of 42 months or discontinued early. This analysis includes the impact of imatinib dose intensity and sustained treatment interruptions on patient outcomes.
Materials and methods
Study design and treatments
The patient eligibility criteria and study design of TOPS have been described previously [1]. Briefly, patients newly diagnosed with Ph+ CML-CP were randomized 2:1 to imatinib 800 mg (400 mg twice daily) or 400 mg (once daily) at diagnosis, and patients were followed for at least 42 months or discontinued early. Treatment with study drug continued until disease progression or patient intolerance.
End points
The primary efficacy end point for this trial was the rate of MMR at 12 months, measured by real-time quantitative polymerase chain reaction (RQ-PCR) defined as a BCR-ABL/control gene mRNA ratio ≤0.1 %, which corresponds to a 3-log reduction in BCR-ABL mRNA transcripts compared with the standardized baseline established in IRIS [2].
Additional end points included rate of CCyR, time to MMR and CCyR, event-free survival (EFS) on treatment [time between randomization and death due to any cause, progression to accelerated phase/blast crisis (AP/BC), loss of major cytogenetic response (MCyR; Ph+ bone marrow cells >35 %), or loss of complete hematologic response (CHR) on treatment], progression-free survival (PFS) on treatment (time between randomization and death due to any cause or progression to AP/BC on treatment), PFS on study (time between randomization and death or progression to AP/BC on treatment or during follow-up after discontinuation of treatment), and overall survival (OS; time between randomization and death due to any cause on or off treatment). The rate of treatment failure was analyzed according to the modified European LeukemiaNet 2009 criteria [3].
Dose intensity and sustained treatment interruptions
In an exploratory analysis, the rates of MMR and CCyR and time to MMR, EFS, and PFS were analyzed by the number of sustained treatment interruptions (defined as zero dose for more than 5 consecutive days) and dose intensity (total amount of drug received divided by the number of days on treatment, including days of zero dose) up to the 12-month assessment.
Safety analysis
Safety was analyzed for all patients who received at least 1 dose of study medication (safety population) by analyzing total adverse events (AEs), drug-related AEs, serious AEs, deaths, laboratory parameters (including hematology and chemistry), electrocardiograms, vital signs, physical examinations, and concomitant medications. Adverse events were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0.
Study conduct
This study was conducted in accordance with the Declaration of Helsinki. The ethics committee or institutional review board at each participating center was responsible for reviewing the study protocol. All participants gave written informed consent prior to study entry in accordance with institutional regulations.
Results
Patients and treatments
Patients (N = 476) were initially randomized to standard-dose imatinib (400 mg daily; n = 157) or high-dose imatinib (800 mg daily; n = 319). The current analysis is based on data collected until November 5, 2010. All patients in this analysis were followed for at least 42 months or had discontinued study treatment.
There were no clinically significant differences in baseline characteristics between the standard- and high-dose arms. At the time of the 36-month data cutoff, 114 of 157 (72.6 %) and 217 of 319 (68.0 %) patients randomized to standard- or high-dose imatinib, respectively, remained on study treatment. After this, discontinuation due to early termination of the study was required in 64.3 and 54.5 % of patients in the imatinib 400- and 800-mg/day arms, respectively.
Efficacy
Molecular response
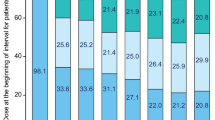
MMR was attained more rapidly in the high-dose arm (8.3 vs 10.0 months in the standard-dose arm), but MMR rates were not significantly different between the 2 arms from 9 to 42 months (Fig. 1). At 42 months, the rate of MMR in the 400-mg arm was 51.6 versus 50.2 % in the 800-mg arm (P = 0.77) in the intent-to-treat population. By month 42, the rate of MMR (including patients who achieved MMR at or before the 42-month time point) was 75.8 versus 79.0 % (P = 0.4807) in the standard- and high-dose arms, respectively.
Cytogenetic response
The median time from randomization to first CCyR for patients who achieved CCyR was similar (5.8 months in the 800-mg arm vs 5.9 months in the standard-dose arm), and the rates of CCyR were not significantly different between arms by 12, 24, 36, and 42 months. CCyR rates in the standard-dose and high-dose arms were 47.1 versus 57.1 %, respectively, by 6 months (P = 0.05), 66.9 versus 70.2 %, respectively, by 12 months (P = 0.46), 76.4 versus 76.8 %, respectively, by 24 months (P = 1.0), 79.0 versus 80.6 %, respectively, by 36 months (P = 0.72), and 80.3 versus 81.5 %, respectively, by 42 months (P = 0.80).
Dose management
Among patients with available PCR assessments at 12 months, median dose intensity up to month 12 was 400.0 mg/day in the standard-dose arm and 751.3 mg/day in the high-dose arm (Table 1). Patients in the high-dose arm had more treatment interruptions (65.4 %) lasting more than 5 days than patients in the standard-dose arm (33.1 %) in the first 12 months of treatment.
Impact of sustained treatment interruptions on patient outcomes
Among patients with available PCR assessments at 12 months, rates of MMR were significantly higher at 12, 24, 36, and 42 months among patients (400- and 800-mg/day arms combined) with 1 or no sustained treatment interruptions during the first 12 months compared with patients who had more than 1 interruption during the same period (Table 2). In addition, the Kaplan–Meier estimate of the median time to MMR was shorter among patients with 1 or no sustained treatment interruptions (P = 0.0003) (Fig. 2a). This was true for patients in both arms, although only 18 patients in the 400-mg arm had more than 1 treatment interruption (Table 1), and the difference was not significant.
Kaplan–Meier estimates of time to first major molecular response among patients with polymerase chain reaction assessments at 12 months with sustained treatment interruptions longer than 5 days (a 400-mg/day and 800-mg/day arms combined) or according to median dose intensity in the first 12 months of therapy (b 800-mg/day arm). The estimated rate of major molecular response at 48 months in patients with 2 or more sustained treatment interruptions was 88.7 % (95 % CI, 77.5–95.8 %) versus 93.3 % among patients with ≤1 interruption (95 % CI, 89.6–96.0 %). The estimated rate of major molecular response at 48 months in patients with a median dose intensity <600 mg/day was 91.1 % (95 % CI, 85.7–95.1 %) versus 93.2 % among patients with a median dose intensity ≥600 mg/day (95 % CI, 87.9–96.7 %)
Rates of CCyR were also significantly higher at 12 and 36 months and numerically higher at 24 and 42 months among patients (400- and 800-mg/day arms combined) with 1 or no sustained treatment interruptions during the first 12 months compared with patients who had more than 1 interruption during the same period (Table 2). Because follow-up data were collected annually once patients achieved CCyR, fewer 42-month data were available compared with the other time points. No significant differences in PFS rates on treatment or EFS rates were observed between patients with 1 or no sustained treatment interruptions versus those with more than 1 interruption (Table 2).
Impact of dose intensity on patient outcomes
Only 2 patients in the standard-dose arm maintained dose intensity of ≥600 mg/day in the first 12 months of therapy (Table 1). Thus, all analyses in this section were performed on patients randomized to the imatinib 800-mg/day arm. Patients receiving high-dose imatinib with available PCR assessments at 12 months who maintained an average dose intensity of at least 600 mg/day during the first 12 months of therapy had significantly higher MMR rates at 12, 24, 36, and 42 months compared with those with dose intensity <600 mg/day (Table 2). Additionally, the Kaplan–Meier estimate of the median time to achieve MMR was significantly shorter (P = 0.0002) (Fig. 2b) for patients on high-dose imatinib who maintained a dose intensity of at least 600 mg/day. Patients on high-dose imatinib with available PCR assessments at 12 months who maintained an average dose intensity of at least 600 mg/day during the first 12 months of therapy also had higher CCyR rates at 12, 24, 36, and 42 months compared with those with dose intensity <600 mg/day, although these differences were significant only at 12 and 36 months (Table 2). No significant differences in PFS on treatment or EFS were observed between patients achieving dose intensities <600 mg/day or ≥ 600 mg/day (Table 2).
Long-term outcomes
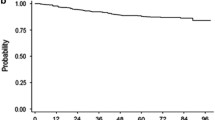
The analyses of EFS on treatment, PFS on treatment and on study, and OS showed no significant differences for the standard- versus high-dose arms (P = 0.40, P = 0.46, P = 0.86, and P = 0.90, respectively) (Fig. 3). Treatment failure was also comparable between arms (11.5 and 7.8 % of patients experienced treatment failure in the 400- and 800-mg/day arms, respectively). Table 3 provides a summary of events.
Kaplan–Meier estimates of event-free survival (a on treatment), progression-free survival (b on treatment), and overall survival (c on and off treatment) (intent-to-treat population). Estimated event-free survival, progression-free survival, and overall survival at 48 months were 92.3 % (95 % CI, 86.5–95.7 %) and 93.6 % (95 % CI, 89.9–96.0 %), 94.4 % (95 % CI, 89.0–97.1 %) and 95.8 % (95 % CI, 92.5–97.7 %), and 94.0 % (95 % CI, 88.7–96.8 %) and 93.4 % (95 % CI, 89.7–95.8 %) in the imatinib 400- and 800-mg/day arms, respectively
Landmark analyses of PFS on treatment and OS showed significant differences between patients with and without MMR at 6, 12, and 18 months. The estimated rates of PFS on treatment and OS at 42 months were higher in patients with MMR at 6 months compared with those without MMR at those landmarks (PFS, 99.2 vs 95.3 %; P = 0.04; OS, 99.2 vs 94.1 %; P = 0.007), at 12 months (PFS, 99.5 vs 94.1 %; P = 0.002; OS, 98.5 vs 94.7 %; P = 0.007), and at 18 months (PFS, 99.1 vs 96.9 %; P = 0.04; OS, 98.7 vs 95.6 %; P = 0.01).
Safety
The majority of drug-related AEs in both dose cohorts were mild to moderate in intensity (Table 4). Serious AEs were reported in 42 (26.8 %) and 121 (38.3 %) patients in the 400- and 800-mg/day arms, respectively. Grade 3/4 AEs were more common in the high-dose arm. Overall, the AE profiles observed in both arms were comparable with what was observed in the 12-month analysis [1].
Discussion
Compared with standard-dose imatinib, initial therapy with imatinib 800 mg/day was not associated with higher rates of MMR at 12 months or at later time points in the TOPS study, and grade 3/4 toxicities and SAEs both occurred more frequently in patients on imatinib 800 mg/day. These results support the use of imatinib at the standard dose in newly diagnosed CML-CP and are consistent with the majority of studies in the literature, which show a lack of significant improvement in response rates and an increase in toxicity with higher doses of imatinib in patients with CML-CP [4–8].
In the phase 2 RIGHT trial (Rationale and Insight for Gleevec High-Dose Therapy; N = 115, treatment with imatinib 800 mg/day was associated with higher rates of cytogenetic and molecular responses in patients with newly diagnosed CML-CP compared with data from IRIS. However, all patients had 1 or more AEs, and 74 patients (64 %) required a dose adjustment or interruption of therapy [4]. Data from the phase 3 SPIRIT trial (STI571 Prospective Randomized Trial; N = 636) demonstrated similar rates of CCyR at 12 months with imatinib 600 mg/day compared with imatinib 400 mg/day (P = 0.005) in patients with newly diagnosed CML-CP, despite a significantly higher CCyR rate at 6 months with the higher imatinib dose, and there was no improvement in progression rate between the 2 groups by 24 months. Any-grade AEs were also more frequent with the higher dose of imatinib [5]. Similarly, in the European LeukemiaNet study of initial therapy with imatinib 800 versus 400 mg/day in patients with high Sokal risk CML-CP (N = 216), there was no difference in either cytogenetic or molecular response rates or in events between the arms at 1 year. In addition, there were higher rates of AEs and SAEs in patients treated with the higher imatinib dose, many of whom discontinued due to toxicity [6].
Results from TOPS demonstrating no advantage in survival with high-dose imatinib are also consistent with prior publications showing no improvement in clinical outcomes with the higher dose over the already good survival outcomes achieved with standard-dose imatinib, including outcomes analyzed according to imatinib dose intensity [6–8]. For instance, in the phase 2 GIMEMA study (Italian Group for Hematologic Malignancies of the Adult) evaluating imatinib 800 mg/day in patients with newly diagnosed CML-CP and intermediate Sokal risk (N = 78), the rate of OS was 96 % after a median follow-up of 24 months [7], which is similar to that in IRIS [9]. In the European LeukemiaNet study, there were no differences in OS, PFS, failure-free survival, or EFS at 36 months with imatinib 800 mg/day compared with standard-dose imatinib [6]. The single-arm TIDEL-I study (Therapeutic Intensification in De Novo Leukaemia; N = 103) showed that despite a statistically significantly higher MMR rate at 24 months in patients who maintained an average daily dose of imatinib 600 mg compared with patients averaging less than 600 mg (77 versus 53 %, respectively; P = 0.016), the actuarial rates of OS and PFS at 1 or 2 years were comparable with those in IRIS [8, 9]. Compared with TIDEL-I, patient outcomes appeared to be better in TIDEL-II, a study in which patients who failed to meet molecular milestones were able to switch to the more potent BCR-ABL tyrosine kinase inhibitor (TKI) nilotinib [10].
Here, we also observed that, whereas patients who maintained an average dose intensity of ≥600 mg/day during the first 12 months had significantly higher rates of MMR at later time points compared with patients with dose intensity <600 mg/day, this did not translate into improved long-term outcomes. There was no significant relationship between dose intensity or treatment interruptions and EFS or PFS rates at 42 months in the larger, randomized TOPS study; these findings are also similar to the lack of improvement in long-term outcomes seen with imatinib dose escalation in the German CML IV study [11]. In those patients (N = 1014), initial treatment with imatinib 800 mg/day was associated with higher rates of MMR compared with imatinib 400 mg/day, although the difference in MMR rates between the 2 groups decreased markedly at later time points. Importantly, the rates of OS, PFS, deaths, and progression events were not significantly different between these 2 treatment arms [11], although deeper molecular responses did appear to be achieved faster with higher dose-imatinib [12].
The TOPS study highlights the importance of limiting sustained treatment interruptions for patients on TKI therapy. After 42 months on therapy, only 11 % of patients randomized to high-dose imatinib remained on that dose, indicating that imatinib 800 mg/day cannot be tolerated by most patients. Patients on high-dose imatinib were also approximately twice as likely to have treatment interruptions lasting more than 5 days. Treatment interruptions of more than 5 days during the first 12 months of treatment were associated with reduced responses. Higher rates of molecular and cytogenetic responses and shorter time to MMR were seen in patients with 1 or no sustained treatment interruptions. Together, these data suggest that high-dose imatinib is not associated with superior long-term clinical outcomes and is poorly tolerated compared with standard-dose imatinib, and that continuous BCR-ABL inhibition is important for achieving optimal outcomes on therapy with a TKI. In addition, sustained interruption of TKI treatment should be avoided, especially during the first year of treatment.
These results are consistent with recent studies of compliance with TKI therapy, which have demonstrated that lack of adherence to the assigned dose of imatinib is associated with lower rates of response [13, 14]. The ADAGIO study (Adherence Assessment with Glivec: Indicators and Outcomes) demonstrated that nonadherence to daily imatinib therapy is associated with suboptimal response and poorer outcomes [13]. Furthermore, in a study conducted at the Hammersmith Hospital in London, adherence to more than 90 % of assigned dose was the most important factor for attainment of MMR and deeper molecular responses beyond MMR [14].
Importantly, landmark analyses suggested that achievement of MMR at 12 months on therapy is predictive of better EFS and PFS rates. These results are consistent with other studies showing that achievement of MMR correlates with a decreased risk of progression to AP/BC [11, 15–18]. European LeukemiaNet and National Comprehensive Cancer Network each recommend switching to a different TKI over imatinib dose escalation for patients who do not achieve optimal responses on first-line imatinib, such as MMR at 12 months [19, 20]. Recent data from randomized studies established the superiority of the second-generation TKIs nilotinib [21–24] and dasatinib [25–] over imatinib in the induction of cytogenetic and molecular responses, and significantly decreased progression to AP/BC with nilotinib versus imatinib, in the treatment of newly diagnosed Ph+ CML-CP. In the case of nilotinib, the decreased progression rates and improved rates of complete molecular response (BCR-ABL/control gene mRNA ratio ≤0.01 and ≤0.0032 %) were statistically significant and fewer CML-related deaths were observed versus imatinib at 1, 2, 3, and 4 years [21–24]. These findings suggest that the future of CML therapy may be with more powerful agents that provide deeper levels of response and have better tolerability profiles.
References
Cortes JE, Baccarani M, Guilhot F, Druker BJ, Branford S, Kim DW, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol. 2010;28:424–30.
Branford S, Fletcher L, Cross NC, Muller MC, Hochhaus A, Kim DW, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112:3330–8.
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51.
Cortes JE, Kantarjian HM, Goldberg SL, Powell BL, Giles FJ, Wetzler M, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. J Clin Oncol. 2009;27:4754–9.
Preudhomme C, Guilhot J, Nicolini FE, Guerci-Bresler A, Rigal-Huguet F, Maloisel F, et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010;363:2511–21.
Baccarani M, Rosti G, Castagnetti F, Haznedaroglu I, Porkka K, Abruzzese E, et al. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet study. Blood. 2009;113:4497–504.
Castagnetti F, Palandri F, Amabile M, Testoni N, Luatti S, Soverini S, et al. Results of high-dose imatinib mesylate in intermediate Sokal risk chronic myeloid leukemia patients in early chronic phase: a phase 2 trial of the GIMEMA CML working party. Blood. 2009;113:3428–34.
Hughes TP, Branford S, White DL, Reynolds J, Koelmeyer R, Seymour JF, et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic-phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood. 2008;112:3965–73.
O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004.
Yeung DT, Osborn MP, White DL, Branford S, Kornhauser M, Slader C, et al. Early switch to nilotinib does not overcome the adverse outcome for CML patients failing to achieve early molecular response on imatinib, despite excellent overall outcomes in the TIDEL-II trial. Blood (ASH annual meeting abstracts). 2012;(abstract 3771).
Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Muller MC, Pletsch N, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-{alpha} in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29:1634–42.
Hehlmann R, Muller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–23.
Noens L, van Lierde MA, De Bock R, Verhoef G, Zachee P, Berneman Z, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113:5401–11.
Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–8.
Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–32.
Hughes TP, Hochhaus A, Branford S, Muller MC, Kaeda JS, Foroni L, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the international randomized study of interferon versus STI571 (IRIS). Blood. 2010;116:3758–65.
Press RD, Love Z, Tronnes AA, Yang R, Tran T, Mongoue-Tchokote S, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response (CCR) predict the duration of CCR in imatinib mesylate-treated patients with CML. Blood. 2006;107:4250–6.
Kantarjian H, O’Brien S, Shan J, Huang X, Garcia-Manero G, Faderl S, et al. Cytogenetic and molecular responses and outcome in chronic myelogenous leukemia: need for new response definitions? Cancer. 2008;112:837–45.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84.
National Comprehensive Cancer Network. Chronic myeloid leukemia. v3. 2014.
Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9.
Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12:841–51.
Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–203.
Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood 2014;123:1353–60.
Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–70.
Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2012;119:1123–9.
Acknowledgments
This work was supported by research funding from Novartis Pharmaceuticals Corporation. We thank Erinn Goldman, Ph.D. (ArticulateScience, LLC), for medical editorial assistance with this manuscript. We would also like to thank all investigators and patients who participated in the TOPS trial.
Conflict of interest
Dr. Baccarani acted as a consultant for Novartis and Bristol-Myers Squibb Company, Pfizer, and Ariad. Dr. Druker is a principal investigator on Novartis and Bristol-Myers Squibb clinical trials. His institution receives payment from these companies to cover patient costs, nurse/data manager salaries, and institutional overhead. He does not receive salary, and his laboratory does not receive funds. Dr. Branford received honoraria from Novartis and Bristol-Myers Squibb. Dr. Kim received research funding, honoraria and acted as a consultant for Novartis, Wyeth, and Bristol-Myers Squibb, received honoraria for Ilyang, and is a clinical trial lead for Pfizer. Dr. Pane has nothing to disclose. Dr. Mongay, Dr. Mone, and Christine-Elke Ortmann are Novartis employees and Dr. Mone holds Novartis stock. Dr. Kantarjian has nothing to disclose. Dr. Hughes received research funding/honoraria from Novartis and Bristol-Myers Squibb and consultancy from Ariad. Dr. Cortes received research funding and acted as a consultant for Bristol-Myers Squibb, Novartis, BMS, and Pfizer. Dr. Guilhot received research funding/honoraria from Novartis.
Author information
Authors and Affiliations
Consortia
Corresponding author
About this article
Cite this article
Baccarani, M., Druker, B.J., Branford, S. et al. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int J Hematol 99, 616–624 (2014). https://doi.org/10.1007/s12185-014-1566-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-014-1566-2