Abstract
Thrombophilia is frequently associated with venous thromboembolism (VTE) including cerebral venous sinus thrombosis (CVST). The possibility of thrombophilia was examined in 12 patients with CSVT diagnosed in the past 9 years. Thrombophilia due to abnormalities in antithrombin (AT), protein C (PC), or protein S (PS) or antiphospholipid syndrome (APS) was evaluated. Nine patients with abnormally decreased AT, PC or PS and one patient with APS were examined. Of the nine patients examined by a gene analysis of AT, PC, or PS, one had a congenital AT deficiency, one had a congenital PC deficiency, and two had congenital PS deficiencies including a novel mutant (Gly189Ala). AT, PC and PS levels were all decreased in one patient, PS level was decreased in three patients, and AT level was decreased in one patient at the onset of CVST, but these concentrations improved after treatment. CVST is frequently associated with thrombophilia and a transient decrease in AT, PC or PS may be a causal factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
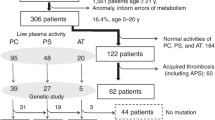
There are approximately 170,000 new cases of clinically recognized venous thromboembolism (VTE) in patients treated in short-stay hospitals in the United States each year [1]. While cerebral venous sinus thrombosis (CVST) is a rare disease with an estimated annual incidence of 3–4 cases per 2 million adults, and 7 cases per 1 million neonates, its precise incidence is not known because there have been only a few epidemiological studies [2–4]. The risk factors for CVST are tumors [5], cerebral infections or trauma [5, 6], oral contraceptive use [7], pregnancy and the peripartum period [8] and thrombophilia [4, 7, 9]. Thrombophilia is defined as patients having a high risk for thrombosis, which can be either inherited or acquired. The main acquired thrombophilia is due to the presence of antiphospholipid antibodies [10, 11], and congenital thrombophilia includes antithrombin (AT), protein C (PC) or protein S (PS) abnormalities [12–14] and Factor V {G1691A} and Factor II (G20210A) abnormalities [15, 16]. However, Factor V {G1691A} and Factor II (G20210A) abnormalities have never been reported in Japan [17, 18].
The overall annual incidence of VTE was 1.53% in patients with deficiencies of AT, PC and PS [19] and thrombophilia has also been reported to be a risk factor for CVST [20].
In this study, we examined in 12 patients with CVST for thrombophilia due to AT, PC, and PS abnormalities and anti-phospholipid antibodies, and the expression of novel PS mutant was determined.
Materials and methods
Twelve patients with CVST were diagnosed at Mie University Hospital from 1 January 2003 to 28 February 2011 (Table 1). The CVST was diagnosed by magnetic resonance imaging (MRI), magnetic resonance venography (MRV) or cerebral angiography (CAG). The study protocol was approved by the Human Ethics Review Committee of the Mie University School of Medicine and a signed consent form was obtained from each subject. This study has been faithfully carried out in accordance with the Declaration of Helsinki. Case 1 was pregnant, case 3 had severe inflammation, case 5 had iron deficient anemia, and case 6 had been taking a contraceptive drug. Case 1 was complicated with deep vein thrombosis (DVT) and case 11 was associated with mesenteric venous thrombosis (MVT). No patients suffered from recurrent either CVTS or any other types of VTE after the 1st episode of CSVT.
Measurement of AT, PC, PS and antiphospholipid antibody concentrations
Peripheral blood samples were collected in a 1/10 volume of 3.13% sodium citrate. The plasma free PS antigen concentration was measured by a monoclonal antibody-based enzyme-linked immunosorbent assay (ELISA) using an Asserachrom free PS kit (Diagnostica Stago, Asnières, France). The plasma PS and PC activity levels were measured by a clotting time method using a STA®-Staclot® Protein S and a STA®-Staclot® Protein C kit (Diagnostica Stago, respectively). The plasma PC antigen concentration was measured by a latex agglutination test using a LPIA-ACE PC (Mitsubishi Chemical Medience Corporation, Tokyo, Japan). The plasma AT activity was measured by a synthetic substrate assay using a Chromorate ATIII (C) kit (Mitsubishi Chemical Medience Corporation). An activity or antigen level of less than 70% in AT, PC and PS of the patients who did not receive warfarin treatment, was considered to be an abnormal decrease in AT, PC or PS. An abnormal decrease in PC or PS was defined as a >2-fold ratio of PS/PC or PC/PS activity in the patients who received warfarin treatment.
The dilute Russell’s viper venom time (DRVVT) was measured by a clotting time method using a Gradipore LA test (Gradipore, Sydney, Australia). Anti-caldiolipin-β2 glycoprotein I (ACL-β2GPI) antibodies were measured with an ELISA kit (Yamasa Co, Tokyo, Japan).
Gene analysis of AT, PC and PS
Genomic DNA was prepared from peripheral blood leukocytes using a QIAamp DNA Blood Mini Kit (QIAGEN) according to the manufacturer’s instructions. Each exon and exon/intron boundary of the gene was amplified from genomic DNA using the polymerase chain reaction (PCR) as described previously. The PCR products were directly sequenced using a Big-Dye Terminator Cycle Sequencing Kit and a 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
The transient and stable expression levels of recombinant Protein S and quantification of the expression level of PS were carried out as in a previous report [21]. The initiating Methionine was set to +1 and the amino acid residue of PS was numbered.
Statistical analysis
The differences in PS expression between mutant and wild type patients were determined using the unpaired t test. A value of p < 0.05 was considered to be significant.
Results
Decreased activity of AT was observed in 3 of the 12 patients (cases 1, 3, and 12) with CVST, decreased activity or antigen of PC was observed in 5 of the 12 patients (cases 3, 6, 7, 9, and 11), and decreased activity or antigen of PS was observed in 7 of the 12 patients (cases 2, 3, 4, 5, 6, 7, and 11), but 2 patients (cases 7 and 11) were already being treated with warfarin (Table 1). Case 8 was positive for both DRVVT and the ACL-β2GPI antibody, and was diagnosed as having antiphospholipid syndrome (APS). A gene analysis for AT, PC and PS was carried out, and 4 patients were diagnosed as having congenital thrombophilia: two with PS genetic abnormalities (cases 2 and 11), one with a PC genetic abnormality (case 9) and one with a AT genetic abnormality (case 1) (Table 2). Case 1 had a heterozygous Pro429Leu (AT Budapest) mutation of the AT gene. The pedigree of the family under investigation is shown in Fig. 1a. The proband developed CVST and DVT when she was pregnant. Her mother had the same mutation of AT and also had CVST. Her brother had no mutation, but the proband’s child had same mutation. Case 2 had two heterozygous PS mutations (Asp79Tyr and Thr630Ile). Case 9 had a heterozygous Glu153 (458-460delAGG) mutation of PC. The pedigree of the family under investigation is shown Fig. 1b. The proband developed CVST at the age of 47 years, and her daughter was detected to have the same PC mutation. Therefore, prophylaxis for VTE could be performed for the daughter during her pregnancy. Case 11 was a compound heterozygous mutation of the PS gene, including a novel mutation (Gly189Ala) and the PS Tokushima mutation (Lys196Glu). A PCR cloning strategy revealed that the Gly189Ala and the Lys196Glu mutations were in different alleles. This novel mutation was not detected in 100 healthy volunteers. Figure 2a shows the transient expression of wild type and mutant recombinant PS (Gly189Ala) in COS7 cells. The concentration of PS in the conditioned media from the different mutations was measured. No significant differences were observed between the mutant and wild type levels of antigen. Figure 2b shows the stable expression of wild type and mutant recombinant PS in baby hamster kidney (BHK) cells. The APC cofactor activity was significantly lower for the mutant PS (33.9 ± 5.8%) than for the wild type (100%, p < 0.001).
The transient and stable expression of wild type and mutant recombinant PS in COS7 or BHK cells. a Transient expression in COS7 cells The determination of the concentration of PS in the conditioned media from genes containing different expressions using the ELISA method. The mean value of the wild type PS was defined as 100%. The bars represent the mean ± SD of six transfection experiments per each gene. The mutant and wild type expression levels were compared using unpaired t test. There was no significant difference. Wild type: 100.00 ± 24.26%, Gly189Ala: 113.70 ± 20.25%. b Stable expression in BHK cells. The APC cofactor activity was calculated using the clotting time and comparing it to the same concentration (100 ng/ml) of the different expressions levels. The mean value of wild type PS was defined to be 100%. The bars represent the mean ± SD of four experiments per gene. The mutant and wild type APC cofactor activity (100 ng/ml) was compared using unpaired t test. Wild: type; 100.00 ± 7.20%, Gly189Ala: 33.92 ± 5.81%
With regard to the onset of CVST, AT, PC and PS were all decreased in case 3, who had multiple organ failure, PS was decreased in cases 4, 5 and 6 and AT was decreased in case 12. After treatment for the CVST, these concentrations were improved, suggesting a transient AT, PC or PS deficiency. In addition, Case 7 had an abnormality in both PS and PC, but this patient was treated with warfarin. The ratio of PC/PS antigen was less than 2.0 and neither PC nor PS activity was measured. Therefore, case 7 was excluded from the gene analysis.
Discussion
CSVT is an uncommon condition with many clinical manifestations, so many cases remain clinically undetected. The incidence of severe thrombophilia due to AT, PC or PS deficiency and APS in CVST was reported to be 9% [4]. The odds ratio of the risk for CVST was reported to be 7.06 in patients with AT deficiency, 8.76 for PC deficiency, 3.20 for PS deficiency and 6.95 in patients with APS [20]. Although this study was of a small number of patients, the incidence of congenital thrombophilia was present in about 33.3% (4/12) of cases, thus suggesting that thrombophilia might be associated with CVST more frequently than was suggested in a previous report [4]. Because, a genetic analysis was not done in all cases with CVST of large-scale study [14].
In case 1, a patient with congenital AT deficiency had a heterozygous AT Pro429Leu (AT Budapest) mutation. This mutation is reported to lead to a decrease of heparin binding capability and protease inhibitor capability of the protein [22]. Case 2 had both PS Asp79Tyr and PS Thr630Ile mutations [21]. Asp79Tyr was previously reported as Asp38Tyr according to the previously established nomenclature system [21]. Asp79Tyr is a Type I PS deficiency (quantitative deficiency) that is characterized by a decrease in both the PS antigen levels and the PS activity [21]. We thought that the PS Asp79Tyr mutation was likely the cause of the decrease in PS, because it was previously reported that the PS Thr630Ile mutation does not influence the expression of PS [21]. Case 9 had a heterozygous PC Glu153del (458-460delAGG) mutation [23]. She experienced no complication during her two deliveries, but her daughter developed a hypercoagulable state during her pregnancy and was treated with low-dose heparin. In case 11, the patient had a PS Tokushima type II mutation characterized by a normal total and free PS antigen level, but a decrease in APC cofactor activity. This mutation is present in about 2% of the Japanese population [24–28]. The PS Gly189Ala is a novel mutation that we identified which was not a polymorphism in an analysis of 100 healthy volunteers [29]. The expression of the protein from this mutant is similar to the wild type protein, but the activity level is lower in comparison to wild type, thus suggesting this mutant to be a type II PS abnormality.
No mutations in AT, PC or PS were observed in the other 5 patients who did not receive warfarin treatment (Cases 3–6 and 12), although they had low AT, PC or PS at the onset of CVST. A decreased AT level was reported in patients with disseminated intravascular coagulation (DIC) [30] and liver diseases [31], decreased PC and PS levels were reported in patients treated with warfarin [32], and decreased PS was reported in pregnant females [33]. In case 3 who was in MOF status, the decreases in AT, PC and PS might have been caused by liver failure. The down regulation of the PS gene expression by 17β-estradiol, which increases in concentration in the late stages of pregnancy, has also been reported [34]. However, the decreased AT, PC or PS might be an outcome from thrombosis, rather than the cause of thrombosis in these patients. The mechanism(s) responsible for a decreased AT, PC or PS activity should be examined in future studies of various types of thrombosis, including CVST.
“APS is one of the important causes of thrombosis [35], and APS is often observed in cases of cerebral infarction [36]. Most cerebral infarctions are considered to be due to arterial thrombosis, and these patients are usually treated with antiplatelet drugs, such as aspirin. CVST is usually recommended to be treated with warfarin. The differential diagnosis of CVST in patients with APS from other types of thrombosis due to arterial thrombosis is therefore important.”
The frequency of thrombophilia is higher in CVST than in DVT, because CVST does not occur as frequently after surgery as DVT.
References
Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933–8.
Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791–8.
deVeber G, Andrew M, Adams C, Bjornson B, Booth F, Buckley DJ, et al., Canadian Pediatric Ischemic Stroke Study Group. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–23.
Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121:2740–6.
Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F, ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664–70.
Raizer JJ, DeAngelis LM. Cerebral sinus thrombosis diagnosed by MRI and MR venography in cancer patients. Neurology. 2000;54:1222–6.
Martinelli I, Sacchi E, Landi G, Taioli E, Duca F, Mannucci PM. High risk of cerebral-vein thrombosis in carriers of a prothrombin-gene mutation and in users of oral contraceptives. N Engl J Med. 1998;338:1793–7.
Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31:1274–82.
Martinelli I, Battaglioli T, Pedotti P, Cattaneo M, Mannucci PM. Hyperhomocysteinemia in cerebral vein thrombosis. Blood. 2003;102:1363–6.
Simchen MJ, Goldstein G, Lubetsky A, Strauss T, Schiff E, Kenet G. Factor v Leiden and antiphospholipid antibodies in either mothers or infants increase the risk for perinatal arterial ischemic stroke. Stroke. 2009;40:65–70.
Berkun Y, Padeh S, Barash J, Uziel Y, Harel L, Mukamel M, et al. Antiphospholipid syndrome and recurrent thrombosis in children. Arthritis Rheum. 2006;55:850–5.
Kottke-Marchant K, Duncan A. Antithrombin deficiency: issues in laboratory diagnosis. Arch Pathol Lab Med. 2002;126:1326–36.
Dahlbäck B. The protein C anticoagulant system: inherited defects as basis for venous thrombosis. Thromb Res. 1995;77:1–43.
Beauchamp NJ, Dykes AC, Parikh N, Campbell Tait R, Daly ME. The prevalence of, and molecular defects underlying, inherited protein S deficiency in the general population. Br J Haematol. 2004;125:647–54.
Segers K, Dahlbäck B, Nicolaes GA. Coagulation factor V and thrombophilia: background and mechanisms. Thromb Haemost. 2007;98:530–42.
Vicente V, González-Conejero R, Rivera J, Corral J. The prothrombin gene variant 20210A in venous and arterial thromboembolism. Haematologica. 1999;84:356–62.
Takamiya O, Ishida F, Kodaira H, et al. APC-resistance and Mnl I genotype (Gln 506) of coagulation factor V are rare in Japanese population. Thromb Haemost. 1995;74:996.
Miyata T, Kimura R, Kokubo Y, Sakata T. Genetic risk factors for deep vein thrombosis among Japanese: importance of protein S K196E mutation. Int J Hematol. 2006;83:217–23.
Mahmoodi BK, Brouwer JL, Ten Kate MK, Lijfering WM, Veeger NJ, Mulder AB, et al. A prospective cohort study on the absolute risks of venous thromboembolism and predictive value of screening asymptomatic relatives of patients with hereditary deficiencies of protein S, protein C or antithrombin. J Thromb Haemost. 2010;8:1193–200.
Kenet G, Lütkhoff LK, Albisetti M, Bernard T, Bonduel M, Brandao L, et al. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta-analysis of observational studies. Circulation. 2010;121:1838–47.
Ikejiri M, Tsuji A, Wada H, Sakamoto Y, Nishioka J, Ota S, et al. Analysis three abnormal Protein S genes in a patient with pulmonary embolism. Thromb Res. 2010;125:529–32.
Sas G, Blaskó G, Bánhegyi D, Jákó J, Pálos LA. Abnormal antithrombin III (antithrombin III “Budapest”) as a cause of a familial thrombophilia. Thromb Diath Haemorrh. 1974;32:105–15.
Takahashi T, Shinohara K, Nawata R, Wakiyama M, Hamasaki N. A novel mutation of the protein C gene with a frameshift deletion of 3 base pair F ((3380)AGG) in exon 6 in type 1 deficiency associated with arterial and venous thrombosis. Am J Hematol. 1999;62:260–1.
Hayashi T, Nishioka J, Shigekiyo T, Saito S, Suzuki K. Protein S Tokushima: abnormal molecule with a substitution of Glu for Lys-155 in the second epidermal growth factor-like domain of protein S. Blood. 1994;83:683–90.
Hayashi T, Nishioka J, Suzuki K. Molecular mechanism of the dysfunction of protein S(Tokushima) (Lys155 → Glu) for the regulation of the blood coagulation system. Biochim Biophys Acta. 1995;1272:159–67.
Kimura R, Honda S, Kawasaki T, Tsuji H, Madoiwa S, Sakata Y, et al. Protein S-K196E mutation as a genetic risk factor for deep vein thrombosis in Japanese patients. Blood. 2006;107:1737–8.
Yamazaki T, Sugiura I, et al. A phenotypically neutral dimorphism of protein S: the substitution of Lys155 by Glu in the second EGF domain predicted by an A to G base exchange in the gene. Thromb Res. 1993;70:395–403.
Kinoshita S, Iida H, et al. Protein S and protein C gene mutations in Japanese deep vein thrombosis patients. Clin Biochem. 2005;38:908–15.
Shindo A, Ikejiri M, Ii Y, Nakatani K, Wada H, Nobori T, et al. A novel protein S gene mutation combined with protein S Tokushima mutation in a patient with superior sagittal sinus thrombosis. J Neurol. 2012;259:178–9.
Aibiki M, Fukuoka N, Nishiyama T, Maekawa S, Shirakawa Y. Differences in antithrombin III activities by administration method in critical patients with disseminated intravascular coagulation: a pharmacokinetic study. Shock. 2007;28:141–7.
Wada H, Usui M, Sakuragawa N. Hemostatic abnormalities and liver diseases. Semin Thromb Hemost. 2008;34:772–8.
Pabinger-Fasching I. Warfarin-reversal: results of a phase III study with pasteurised, nanofiltrated prothrombin complex concentrate. Thromb Res. 2008;122:S19–22.
Trigg DE, Wood MG, Kouides PA, Kadir RA. Hormonal influences on hemostasis in women. Semin Thromb Hemost. 2011;37:77–86.
Suzuki A, Sanda N, Miyawaki Y, Fujimori Y, Yamada T, Takagi A, et al. Down-regulation of PROS1 gene expression by 17beta-estradiol via estrogen receptor alpha (ERalpha)-Sp1 interaction recruiting receptor- interacting protein 140 and the corepressor-HDAC3 complex. J Biol Chem. 2010;285:13444–53.
Tripodi A, de Groot PG, Pengo V. Antiphospholipid syndrome: laboratory detection, mechanisms of action and treatment. J Intern Med. 2011;270:110–22.
Muscal E, Brey RL. Antiphospholipid syndrome and the brain in pediatric and adult patients. Lupus. 2010;19:406–11.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Blood Coagulation Abnormalities from the Ministry of Health, Labor and Welfare of Japan.
Conflict of interest
Authors have no direct or indirect conflict of interest in this manuscript
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ikejiri, M., Shindo, A., Ii, Y. et al. Frequent association of thrombophilia in cerebral venous sinus thrombosis. Int J Hematol 95, 257–262 (2012). https://doi.org/10.1007/s12185-012-1006-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-012-1006-0