Abstract
Hematopoietic stem cells (HSCs) are responsible for sustaining hematopoietic homeostasis and regeneration after injury for the entire lifespan of an organism through self-renewal, proliferation, differentiation, and mobilization. Their functions can be affected by reactive oxygen species (ROS) that are produced endogenously through cellular metabolism or after exposure to exogenous stress. At physiological levels, ROS function as signal molecules which can regulate a variety of cellular functions, including HSC proliferation, differentiation, and mobilization. However, an abnormal increase in ROS production occurs under various pathological conditions, which can inhibit HSC self-renewal and induce HSC senescence, resulting in premature exhaustion of HSCs and hematopoietic dysfunction. This review aims to provide a summary of a number of recent findings regarding the cellular sources of ROS in HSCs and the mechanisms of action whereby ROS induce HSC senescence. In particular, we highlight the roles of the p38 mitogen-activated protein kinase (p38)-p16Ink4a (p16) pathway in mediating ROS-induced HSC senescence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
More than 50 years ago, Denham Harman [1] proposed the free radical or oxidative stress theory of aging. He hypothesized that free radicals and/or reactive oxygen species (ROS) can be produced endogenously from normal cellular metabolic processes. In this theory, imbalances between ROS and antioxidants can lead to oxidative stress that damages various macromolecules. Accumulation of oxidatively damaged macromolecules with age can result in a progressive loss of cellular function, which eventually shortens the lifespan. Although it remains controversial whether age-associated increases in oxidative stress shorten the normal lifespan of an organism, a growing body of evidence demonstrates that increased production of ROS does play an important role in the development of various age-related diseases [2, 3]. However, the cellular mechanisms by which oxidative stress exerts its impacts on aging are not well defined, as not all cells in an organism are equally sensitive to oxidative stress, and oxidative damage to a cell can result in different consequences. This is best illustrated by several recent studies in the hematopoietic system, which demonstrate that an increase in oxidative stress can occur selectively in hematopoietic stem cells (HSCs) under various pathological conditions and after exposure to exogenous stress; HSCs are more sensitive to oxidative stress than their progeny, and oxidative damage to HSCs can lead to shortened lifespans in mice, at least in part via induction of HSC senescence and premature exhaustion [4–11]. The review below aims to provide more insight into the role of ROS in the induction of HSC senescence and the impact of HSC senescence on the hematopoietic system.
2 The hierarchy of the hematopoietic system and HSC niche
The hematopoietic system is organized in a hierarchical manner, in which the rare HSCs reside at the top of the hierarchy and have the ability to self-renew, proliferate, and differentiate into different lineages of peripheral blood cells though multipotent progenitors (MPPs) and hematopoietic progenitor cells (HPCs) [12, 13]. HSCs are quiescent under steady-state conditions, and serve as a reserve that protects the hematopoietic system from exhaustion under various stress conditions [14]. In contrast, MPPs and HPCs are proliferating cells with limited and no self-renewal ability, respectively. The proliferation and differentiation of MPPs and HPCs satisfy the needs of normal hematopoiesis, and also allow the hematopoietic system to react swiftly and effectively to meet demands for increased production of mature cells during hematopoietic crises, such as loss of blood, hemolysis, or infection. If MPPs and HPCs are depleted by an exogenous stressor, such as chemotherapy and/or IR, acute myelosuppression occurs. Under such circumstances, HSCs can undergo self-renewing proliferation and differentiation to repopulate MPPs and HPCs and restore homeostasis. However, if HSCs are injured or their self-renewing ability is impaired, long-term or permanent damage to the hematopoietic system occurs and bone marrow (BM) failure and death of the organism may occur [15].
Normally, the quiescent HSCs representing the long-term repopulating and the most primitive HSCs reside in the osteoblastic niche, which is adjacent to the endosteal bone surface [16–18]. The osteoblastic niche provides HSCs with a special environment that supports their self-renewal. This is likely achieved in part by extensive interactions between HSCs and the niche via a variety of soluble factors, such as Wnt, BMP, TPO, IL-3, and IL-6; various adhesion molecules, including CXCL12-CXCR4 and N-cadherin; and different signaling pathways, e.g. SCF/c-Kit, Jagged/Notch, and angiopoietin-1/Tie2 (Ang-1/Tie2). These intricate interactions promote HSC self-renewal not only by increasing HSC survival, but also by keeping them quiescent. Quiescent HSCs exhibit low metabolic rates and presumably produce less ROS capable of causing oxidative damage [19]. In addition, most endosteal osteoblastic niches are considered hypoxic, as they are relatively remote from blood flow [20–22]. It is estimated that the concentration of oxygen in these niches is below 1%. By residing in a hypoxic environment, quiescent HSCs show lower blood perfusion as determined by low Hoechst 33342 (Hoe) staining after dye injection, and express higher levels of HIF-1α [22–24]. Increased expression of HIF-1α alters the metabolism of HSCs by upregulating glycolysis while downregulating mitochondrial oxidative phosphorylation, leading to reduced production of ROS [23, 24]. By residing in a hypoxic environment and a quiescent state, HSCs are presumably subjected to a lower level of oxidative stress and thus better maintain their ability to self-renew. Indeed, it has been shown recently that Hoe-negative HSCs (CD41−CD48−CD150+Lin−Sca1+c-kit+ cells) from mouse BM cycle slowly and are capable of serial transplantation, whereas Hoe positive HSCs cycle more frequently and cannot reconstitute the recipients after secondary transplantation [22]. In addition, the low ROS producing population of HSCs (CD34−Lin−Sca1+c-kit+ cells) isolated from mouse BM exhibits greater self-renewal potential, and expresses higher levels of niche interacting molecules, such as calcium receptor, N-cadherin, and Notch1, than does the ROS high population [25]. The self-renewal ability of ROS high HSCs can be restored to a level similar to that of ROS low HSCs by treatment with the antioxidant N-acetyl-l-cysteine (NAC). Furthermore, the number of human SCID-repopulating cells expanded when human BM HSCs (Lin−CD34+CD38− cells) were cultured in a hypoxic environment, whereas the number of human SCID-repopulating cells reduced after the cells were cultured in normoxic conditions [26]. These findings suggest that the production of ROS plays an important role in regulation of HSC self-renewal.
3 Oxidative stress in HSCs
The first evidence to demonstrate that HSCs are sensitive to oxidative stress was found in ATM −/− mice (Fig. 1) [5]. ATM −/− mice exhibit progressive failure of hematopoietic function with aging. The failure is attributed primarily to HSC premature exhaustion resulting from an increased production of ROS, as treatment of ATM −/− mice with NAC can restore the function of HSCs and prevent the development of BM failure. However, the mechanism by which ATM regulates ROS production in HSCs remains to be elucidated. It was subsequently reported that triple-FoxO (FoxO1, FoxO3, and FoxO4) knockout mice also developed hematopoietic abnormalities [6]. The number of HSCs and their long-term repopulating activity were markedly reduced after the deletion of FoxOs. These defects were associated with increased production of ROS in HSCs (Fig. 1). Treatment of FoxOs knockout mice with NAC reversed the defects of HSCs and hematopoietic abnormalities. It appears that FoxO3 is the primary regulator of HSCs, because deletion of FoxO3 alone in mice can recapitulate most of the phenotypes observed in the triple-FoxO knockout mice [10, 11]. The molecular basis for FoxOs to regulate ROS production in HSCs is mainly attributed to their transcriptional regulation of the expression of superoxide dismutase and catalase [6]. In addition, there is a mechanistic link between ATM and FoxO3 in regulation of ROS production in HSCs as FoxO3 is essential for ATM expression [11].
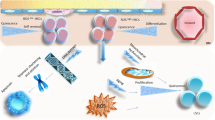
A hypothetic model for reactive oxygen species (ROS) to mediate the induction of hematopoietic stem cell (HSC) senescence under various pathophysiological conditions. Increased levels of ROS are produced by mitochondria and/or NADPH oxidases (NOXs) in HSCs when HSCs are exposed to stress, a high concentration of oxygen or after deletion of ATM, FoxOs, Bmi1, TSC1, and HIF1α. ROS can induce HSC senescence by activating the ATM-Chk2-p53-p21 pathway via induction of DNA double strand breaks (DSBs) and/or stimulating the p38 pathway. Both pathways converge at p16 for the induction of HSC senescence. In addition, activation of p53 can also induce the expression of various pro-apoptotic proteins to cause HSC apoptosis. ROS may induce HSC senescence and apoptosis in a dose-dependent manner. Both of them can be prevented by treatment with an antioxidant, such as N-acetyl-l-cysteine (NAC)
Increased production of ROS in association with HSC defect has been observed in several other pathological conditions, such as deletion of Bmi1 [27, 28], MDM2 [29], and tuberous sclerosis complex I (TSC1) [30], Fanconi anemia mutation [31], aging [4], and after exposure to genotoxic stress (Fig. 1) [7]. For example, we showed that exposure of mice to a sublethal dose of total body irradiation (TBI) induced a persistent increase in ROS production in HSCs [7]. The induction of chronic oxidative stress was associated with sustained increases in oxidative DNA damage in HSCs, inhibition of HSC clonogenic function, and induction of HSC senescence. Treatment of the irradiated mice with NAC after TBI significantly attenuated IR-induced inhibition of HSC clonogenic function and reduction of HSC long-term engraftment after transplantation. These findings provide the foremost direct evidence demonstrating that TBI induces long-term BM suppression, at least in part via induction of oxidative stress in HSCs.
4 Cellular sources of ROS in HSCs
Production of ROS is one of the by-products of mitochondrial respiration, and mitochondria have frequently been considered to be the main source of cellular-derived ROS (Fig. 1) [32]. It has been shown that cells, including HSCs, from Bmi1 −/− mice exhibit abnormal mitochondrial function resulting in increased production of ROS [9]. In addition, increased production of ROS in HSCs from TSC1 −/− mice has been attributed to the elevation of mitochondrial biogenesis and oxidative activities [30]. However, compared to their progeny, HSCs are dormant and have fewer mitochondria [33, 34]. It has also been shown that HSCs primarily utilize glycolysis rather than mitochondrial oxidative phosphorylation for ATP production [23], and it has yet to be determined whether mitochondria play a major role in contributing to the increased production of ROS in HSCs under various pathological conditions.
Recently, an increasing body of evidence demonstrates that cells can also actively produce ROS through a family of tightly regulated NADPH oxidases (NOXs) that are homologues of the phagocyte oxidase (Phox or NOX2) [35, 36]. ROS produced by NOXs participate in regulation of many cell functions and have been implicated in the pathogenesis of different diseases. Five different NOXs are expressed in different tissues or cells with distinctive functions and mechanisms of regulation in a tissue- or cell-specific manner [35, 36]. The expression of NOX1, 2 and 4 and various regulatory subunits of NOXs has been detected in human HSCs [33, 34]. It was estimated that NOX-mediated extra-mitochondrial oxygen consumption accounts for about half of the endogenous cell respiration in human HSCs [33]. Interestingly, our recent studies showed that NOX1, 2, and 4 are also expressed in mouse BM HSC-enriched Lin−Sca1+c-kit+ cells, whereas HPCs, Lin− cells and mononuclear cells from mouse BM express NOX1 and 2, but not NOX4, suggesting that the expression of NOX4 is downregulated upon HSC differentiation and that NOX4 may play an important role in regulation of HSC function [7]. More importantly, it was found that the expression of NOX4 was upregulated, whereas the expression of NOX1 and 2 was unchanged in HSCs after TBI. Because NOX4 is a constitutively active NOX and ROS production by NOX4 is regulated at the transcriptional level [35, 36], the finding that IR upregulates NOX4 in HSCs implies that NOX4 may primarily mediate the IR-induced increase in ROS production in HSCs. This is supported by the finding that DPI, but not apocynin, inhibits IR-induced elevation of ROS production in HSCs, because NOX4 is not sensitive to apocynin inhibition, whereas other NOXs are [7, 35–37]. However, additional experiments will be required to confirm the role of NOX4 in IR-induced chronic oxidative stress in HSCs utilizing a more specific approach, such as genetically knocking out NOX4 in HSCs. In addition, in a preliminary study, we observed that the increase in ROS production by HSCs with aging was also associated with upregulation of NOX4 (unpublished results) [4]. It has yet to be determined whether upregulation of NOX4 is a common mechanism for the induction of oxidative stress in HSCs not only by IR and aging, but also in other pathophysiological conditions associated with increased production of ROS by HSCs, such as deficiency in ATM, Bmi1, MDM2, TSC1, or FoxOs, and Fanconi anemia [5, 6, 9–11, 29–31].
In addition, cyclooxygenases and lipoxygenases can also produce ROS. It has recently been reported that HSCs from 12/15-lipoxygenase (12/15-LOX) deficient mice are highly deficient in hematopoietic reconstitution after transplantation due to a decrease in canonical Wnt signaling activated by 12/15-LOX-derived ROS and bioactive lipids [38]. However, it has yet to be determined whether 12/15-LOX and other lipoxygenase and cyclooxygenase contribute to increased production of ROS in HSCs under pathological conditions.
5 Mechanisms of action of ROS
ROS can regulate HSC function in a concentration-dependent manner. It is well documented that ROS at physiological concentrations can function as signal molecules that regulate a variety of cellular functions. Low and moderate levels of ROS appear to be required for HSC proliferation, differentiation, and mobilization [38–41]. For example, it was reported recently that HSCs from AKT1/2 double knockout mice exhibit a defect in long-term hematopoietic reconstitution after transplantation [39]. The defect is attributable to the reduced production of ROS, as moderate elevation of ROS in HSCs by incubation of the cells from the knockout mice with low doses of the pro-oxidant l-buthionine-S,R-sulfoximine (BSO) increased their clonogenicity. This is in agreement with another recent observation that ROS-dependent proliferation of HSCs also plays an important role in the early steps of hematopoietic reconstitution after HSC transplantation [40].
However, high levels of ROS are toxic to HSCs. This has been demonstrated both in vitro and in vivo [4–7, 9–11, 29, 30]. As discussed above, deletion of ATM and FoxOs and exposure to TBI cause an increase in ROS production in HSCs, which in turn leads to HSC senescence and premature exhaustion [5–7, 10, 11]. In addition, incubation of HSCs with BSO, which induces oxidative stress by depleting intracellular reduced glutathione, resulted in a dramatic reduction in HSC clonogenicity [4, 39]. The mechanisms of action by which ROS induces HSC damage are emerging from several recent studies (Fig. 1). A moderate increase in ROS production after deletion of ATM and FoxOs can disrupt HSC quiescence by stimulating HSC cycling, which comprises the ability of HSCs to self-renewal and leads to HSC premature exhaustion [4–6, 10, 11]. Further increases in ROS production can induce HSC senescence and/or apoptosis. The induction of HSC senescence/apoptosis may result from an increase in oxidative DNA damage. As shown in our recent studies and those of others, HSCs from TBI mice not only produced higher levels of ROS, but also accumulated more oxidative DNA damage and double strand breaks (DSBs) analyzed by 8-hydroxy-2′-deoxyguanosine (8-OH-dG) immunostaining and γH2AX foci assay, respectively [7, 42]. It is well established that DSBs can initiate DNA damage response by sequential activation of ATM, Chk2, and p53 [43, 44]. Activation of p53 induces p53 downstream targets, including the cell cycle inhibitors p21Cip1/Waf1 (p21) and p16Ink4a (p16), and pro-apoptotic proteins Puma and Bax. p21 and p16 inhibit cyclin-dependent kinase (CDK) 2 and CDK4/6, respectively, to induce cell cycle arrest and senescence; whereas Puma and Bax can disrupt mitochondrial integrity to induce apoptosis. Alternatively, ROS can activate p38 mitogen-activated protein kinase (p38), which in turn induces HSC senescence by upregulating p16 and/or Arf as discussed below (Fig. 1) [4].
6 HSC senescence
In the early 1960s, Hayflick and Moorhead [45] demonstrated that normal human diploid fibroblasts (HDFs) have finite growth potential. The intrinsic replicative lifespan of a cell appears to be determined by telomere length [46]. Without the expression of telomerase, telomeric sequences shorten each time when DNA replicates. When the telomeres reach a critically short length (~4 kb) after a certain number of cell doublings, known as the Hayflick limit, cells stop dividing and are irreversibly arrested at the G1 phase, entering replicative senescence [46, 47]. The occurrence of replicative senescence has been demonstrated for most cell types. Exceptions include embryonic stem cells and the majority of tumor-derived cell lines [46, 47]. This is because these cells express high levels of telomerase. A moderate level of telomerase activity is detectable in HSCs [48–51]. This activity is needed to maintain the normal function of HSCs, as a deficiency in telomerase activity can lead to telomere shortening and reduction in HSC transplantation ability, as seen in late generations of telomerase RNA component (TERC) null mice [51]. In addition, the development of aplastic anemia or marrow failure has been observed in patients with telomerase deficiency, due to mutations in telomerase reverse transcriptase (TERT) or TERC [52]. However, overexpression of TERT in HSCs maintains the length of HSC telomeres, but fails to extend HSC lifespan in a serial BM transplantation setting [53]. It is, therefore, the subject of intense debate whether HSCs are characterized by finite cell replicative ability and undergo replicative senescence after extensive proliferation [54, 55].
In addition, many types of human and animal cells undergo senescence after exposure to oxidative and genotoxic stress (including IR) in a species-independent manner [56]. This also occurs when cells are subjected to an oncogenic stress and/or aberrant activation of the p38 pathway [56]. The senescence induced by oxidative, genotoxic, and oncogenic stress is also referred to as premature senescence in order to differentiate it from replicative senescence [47, 56]. This is because cells undergoing stress-induced premature senescence have a shortened intrinsic replicative lifespan without significant erosion in telomeres. However, cells undergoing premature senescence are morphologically indistinguishable from replicatively senescent cells, and exhibit many of the characteristics ascribed to replicatively senescent cells [46, 47, 56]. These changes include an enlarged and flattened appearance, increased senescence-associated β-galactosidase (SA-β-gal) activity, and elevated expression of p16 [46, 47, 56]. Moreover, premature and replicative senescence share common induction pathways [46, 47, 56]. Therefore, senescence has been frequently used as a general term to describe cells undergoing either premature or replicative senescence.
The first evidence that HSCs can undergo senescence was observed in Bmi1 −/− mice. It was found that mice lacking the Bmi1 gene developed progressive BM hypoplasia and die early (<2 months) after birth [27, 57]. Although Bmi1 −/− mice had a normal pool of fetal liver HSCs, transplantation of their fetal liver HSCs to a lethally irradiated recipient resulted only in a transient reconstitution of the hematopoietic system [27, 57]. This suggests that the mutant fetal liver HSCs have the ability to proliferate and differentiate into HPCs enabling transient reconstitution of the BM, but cannot self-renew and generate HSCs to ensure long-term hematopoietic engraftment. Deficiency in self-renewal was also found in neural and leukemia stem cells lacking Bmi1, indicating that Bmi1 is a general regulator of stem cell self-renewal [27, 57, 58]. Bmi1 is a member of the Polycomb group of transcriptional repressors. Its downstream targets include the gene products of the Ink4a/Arf locus, e.g. p16 and Arf. HSCs from Bmi1 −/− mice express increased levels of p16 and Arf [27, 57, 58]. Enforced expression of p16 and Arf in HSCs induces cell cycle arrest and apoptosis, respectively, whereas p16 knockout partially restores the ability of Bmi1 −/− stem cells to self-renew [27, 57, 58]. In addition, Bmi1 also regulates mitochondrial production of ROS [9]. HSCs from Bmi1 −/− mice produce increased levels of ROS, which can also contribute to the induction of p16 and HSC senescence, probably through the p38 pathways. These findings demonstrate that Bmi1 promotes HSC self-renewal at least in part by inhibiting ROS production and p16 expression to prevent the cells from undergoing premature senescence [9, 27, 57, 58]. Similarly, it has been reported that ATM −/− mice have a defect in HSC self-renewal and exhibit a progressive decline in HSCs with age [4, 5]. This defect can be corrected by downregulation of p16 expression with Bmi1 or suppression of the p16-Rb pathways by human papillomavirus (HPV) protein E7. However, overexpression of TERT in ATM −/− HSCs did not correct the deficiency [5]. These findings indicate that ATM regulates HSC self-renewal via a telomere-independent mechanism, probably by inhibiting ROS production and p16 expression to prevent HSCs from undergoing premature senescence. Induction of HSC senescence also occurs after exposure to IR [7, 59]. Senescent HSCs induced by IR produce elevated levels of ROS, express increased levels of SA-β-gal and p16, and exhibit diminished clonogenicity [7, 59]. All these findings suggest that induction of HSC senescence may have a common etiology, e.g. increased production of ROS and expression of p16 (Fig. 1). Interestingly, a shortening of the intrinsic replicative capacity of HSCs or loss of HSC self-renewal in the conditions discussed above (including IR) does not affect HSC differentiation to generate various HPCs and more mature progeny prior to their final exhaustion [5, 7, 27, 59]. Moreover, HPCs from irradiated, Bmi1 −/− or ATM −/− mice showed neither abnormalities nor did they exhibit signs of senescence. These findings indicate that hematopoietic cell senescence mainly occurs at the level of HSCs.
7 Role of p16 in induction of HSC senescence
The Ink4a-Arf locus encodes two tumor suppressors, p16 and Arf [60, 61]. The transcripts for these proteins have different first exons (α for p16 and β for Arf), but share exons 2 and 3. However, there is no amino acid sequence similarity between these two proteins due to the use of alternative reading frames for their translation. p16 is a potent CDK4/6 inhibitor. By inhibiting CDK4/6 activity, p16 causes Rb hypophosphorylation and suppresses the expression of E2F-dependent genes, resulting in restriction of G1/S cell cycle progression and formation of senescence-associated heterochromatic foci (SAHF) [60–62]. Once SAHF are formed after the engagement of the p16-Rb pathway, the cells become permanently growth arrested and senescent. It has, therefore, been suggested that diverse stimuli can induce cellular senescence via various upstream signal transduction cascades (including the p53-p21 pathway) that converge on the p16-Rb pathway, whose activation provides an inescapable barrier preventing senescent cells from re-entering the cell cycle. This suggestion is supported by the finding that activation of p53 and induction of p21 in cells undergoing senescence are transient events that occur during the onset of senescence and then subside when the expression of p16 starts rising [63–65]. Inactivation of p53 prior to upregulation of p16 can prevent senescence induction. However, once p16 is highly expressed, cell cycle arrest becomes irreversible by downregulation of p53, indicating that activation of the p53-p21 pathway plays an important role in the initiation of senescence, but induction of p16 is required for the maintenance of senescence [63, 66]. In agreement with this suggestion, we found that IR induces p53 activation and p21 expression in HSCs prior to the induction of p16 [59, 67]. While p53 activation and p21 upregulation gradually declined within a few weeks after IR, p16 expression in irradiated HSCs remained elevated and the cells subsequently became senescent, exhibiting positive SA-β-gal staining. In contrast, the biological action of Arf relies on the p53 pathway. This is because Arf can directly bind to MDM2 and cause the accumulation of p53 by segregating MDM2 from p53 and by inhibiting MDM2’s E3 ubiquitin protein ligase activity for p53 [60, 61, 68]. Therefore, activation of p53 by Arf can induce not only cellular senescence, but also apoptosis, depending on which gene downstream of p53 is induced following its activation.
Although increased expression of p16 and Arf has been found in Bmi1 −/− HSCs, the expression of p16, but not that of Arf, appears to be important for the induction of Bmi1 −/− HSC senescence [27]. In addition, it has been found that knockout of both the p16 and Arf genes in mice significantly increases the clonal expansion of HSCs in vitro but modestly promotes HSC self-renewal in vivo [69, 70]. However, knockout of the Arf gene alone does not provide any advantage for HSC/HPC expansion and self-renewal [70]. In contrast, knockout of p16 increases the lifespan of HSCs by promoting HSC self-renewal [70, 71]. Furthermore, mutation of the ATM gene also results in upregulation of p16 and Arf in HSCs [5, 70]. Inactivation of the p16-Rb pathway by retroviral transfection of HPV E7 proteins restores the reproductive function of ATM −/− HSCs, while inhibition of the Arf-p53 pathway by E6 transfection has no such effect. These findings suggest that p16 plays a more significant role than that of Arf (and p53/p21) in the regulation of HSC self-renewal and induction of HSC senescence, even though both proteins are overexpressed in senescent HSCs (Fig. 1).
8 Role of p38 in induction of HSC senescence
p38 belongs to the MAPK family of signal transduction kinases [70, 72]. It is activated in a sequential cascade, e.g. mitogen-activated or extracellular signal-regulated kinase kinase [MEKK]-MAPK kinase 3/6 [MKK3/6]-p38, after exposure to a variety of stress, including oxidative stress. It regulates a variety of cellular processes such as inflammation, cell cycle arrest, and apoptosis. In addition, it also plays a critical role in the induction of senescence via upregulation of p16. For example, a high level of Ras or Raf activation induces senescence by stimulating the sustained activation of p38, which in turn upregulates the expression of p16 [70, 73–75]. Activation of the p38 pathway also contributes to the induction of p16 and cellular senescence in response to other stimuli, including DNA damage resulting from exposure to genotoxic and oxidative stress, and telomere shortening due to extensive replication [76]. Furthermore, activation of p38 by ectopic transfection of MKK3 and/or MKK6 increases p16 expression and induces senescence [77, 78]. In contrast, inhibition of p38 activity or downregulation of p38 expression attenuates the induction of p16 and cellular senescence by oncogenic stress, DNA damage, and telomere shortening [73, 76–78]. These findings suggest that p38 functions as a key molecule that mediates upregulation of p16 induced by diverse stimuli and cellular senescence.
Activation of p38 has been implicated in BM suppression in various pathological conditions, including aplastic anemia and myelodysplastic syndromes [79, 80]. Furthermore, oxidative stress, mutation of the ATM gene and serial BM transplantation can activate p38 selectively in HSCs through ROS, which leads to increased expression of p16 and premature exhaustion of HSCs [4]. These defects can be corrected by inhibition of ROS production or p38 inactivation, suggesting that p38 acts downstream of ROS but upstream of p16 in the induction of HSC senescence (Fig. 1).
The mechanisms by which p38 upregulates p16 and induces senescence have yet to be elucidated. The positive and negative transcriptional factors that are involved in regulation of p16 transcription include the Jun, Ets, Id and Bmi1 families [60, 61]. p38 may regulate the transcription of p16 mRNA through these transcriptional factors [81]. However, there is a growing body of evidence indicating that the expression of p16 can also be regulated at the post-transcriptional level [82, 83]. It is well established that p38 primarily regulates the expression of various genes by stabilizing mRNA [84]. Activated p38 can phosphorylate the downstream substrate-MAPK-activated protein kinase 2 (MK2) which in turn phosphorylates various targets including adenylate/uridylate-rich element (ARE)-binding proteins (ARE-BPs) to regulate the stability of mRNA via their interaction with ARE [84, 85]. Several ARE-BPs have been implicated in regulation of mRNA stability through the p38-MK2 pathway [84]. Genes regulated by p38 through MK2-mediated stabilization of mRNA include various inflammatory cytokines, transcription factors, cyclins, and CDK inhibitors [84]. It has been found that p16 mRNA is highly stable in senescent cells [82]. The p16 mRNA 3′ untranslated region (3′UTR) contains an ARE which acts as an mRNA destabilizing determinant [83]. One of the ARE-binding proteins (ARE-BPs), AUF-1, can interact with the ARE presented in the 3′-UTR of p16 mRNA, resulting in destabilization of p16 mRNA [83]. The increase in p16 expression in senescent cells is in part attributable to downregulation of AUF-1 [83]. It will be interesting to determine whether a major role of p38 in regulation of p16 expression is to stabilize p16 mRNA via its ARE, which leads to increased expression of p16 and induction of senescence.
9 Conclusion
In this review, we have summarized a number of recent findings regarding the role of ROS in regulation of HSCs, with an emphasis on the induction of HSC senescence. These findings provide new insights into HSC defects. First, they show that HSCs are susceptible to the induction of imbalance of reduction/oxidation (redox) reactions under various pathological conditions, leading to a persistent and prolonged increase in ROS production. Second, HSCs are more sensitive to ROS-induced oxidative damage than are their progeny. Moreover, it appears that ROS injure HSCs not by a nonspecific cytotoxic effect as previously hypothesized. Instead, the damage is at least partially mediated by induction of cellular senescence through redox-dependent activation of the p38-p16 pathway (Fig. 1). These findings also provide new opportunities for intervention of BM suppression resulting from an increased production of ROS in HSCs in patients with ataxia-telangiectasia or after exposure to ionizing radiation by targeting ROS with a potent antioxidant or the p38 pathway with a specific p38 inhibitor.
References
Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300.
Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–14.
Salmon AB, Richardson A, Perez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48:642–55.
Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–51.
Ito K, Takubo K, Arai F, Satoh H, Matsuoka S, Ohmura M, Naka K, Azuma M, Miyamoto K, Hosokawa K, Ikeda Y, Mak TW, Suda T, Hirao A. Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J Immunol. 2007;178:103–10.
Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39.
Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–56.
Cho J, Shen H, Yu H, Li H, Cheng T, Lee SB, Lee BC. Ewing sarcoma gene Ews regulates hematopoietic stem cell senescence. Blood. 2011;117:1156–66.
Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY, Ahn BH, Kumar NG, Rovira II, Xu XL, van Lohuizen M, Motoyama N, Deng CX, Finkel T. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–92.
Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–12.
Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C, Sarkar A, Grisotto M, Taneja R, Ghaffari S. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem. 2008;283:25692–705.
Reya T. Regulation of hematopoietic stem cell self-renewal. Recent Prog Horm Res. 2003;58:283–95.
Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403.
Wilson A, Laurenti E, Trumpp A. Balancing dormant and self-renewing hematopoietic stem cells. Curr Opin Genet Dev. 2009;19:461–8.
Wang Y, Schulte BA, Zhou D. Hematopoietic stem cell senescence and long-term bone marrow injury. Cell Cycle. 2006;5:35–8.
Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 2007;1106:41–53.
Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–31.
Wilson A, Oser GM, Jaworski M, Blanco-Bose WE, Laurenti E, Adolphe C, Essers MA, Macdonald HR, Trumpp A. Dormant and self-renewing hematopoietic stem cells and their niches. Ann N Y Acad Sci. 2007;1106:64–75.
Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–96.
Eliasson P, Jonsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222:17–22.
Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104:5431–6.
Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Levesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116:375–85.
Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–90.
Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, Suda T. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402.
Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–63.
Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112:126–35.
Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–5.
Schuringa JJ, Vellenga E. Role of the polycomb group gene BMI1 in normal and leukemic hematopoietic stem and progenitor cells. Curr Opin Hematol. 2010;17:294–9.
Abbas HA, Maccio DR, Coskun S, Jackson JG, Hazen AL, Sills TM, You MJ, Hirschi KK, Lozano G. Mdm2 is required for survival of hematopoietic stem cells/progenitors via dampening of ROS-induced p53 activity. Cell Stem Cell. 2010;7:606–17.
Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–408.
Du W, Adam Z, Rani R, Zhang X, Pang Q. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid Redox Signal. 2008;10:1909–21.
Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95.
Piccoli C, Ria R, Scrima R, Cela O, D’Aprile A, Boffoli D, Falzetti F, Tabilio A, Capitanio N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J Biol Chem. 2005;280:26467–76.
Piccoli C, D’Aprile A, Ripoli M, Scrima R, Lecce L, Boffoli D, Tabilio A, Capitanio N. Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem Biophys Res Commun. 2007;353:965–72.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–47.
Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–14.
Kinder M, Wei C, Shelat SG, Kundu M, Zhao L, Blair IA, Pure E. Hematopoietic stem cell function requires 12/15-lipoxygenase-dependent fatty acid metabolism. Blood. 2010;115:5012–22.
Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–8.
Lewandowski D, Barroca V, Duconge F, Bayer J, Van Nhieu JT, Pestourie C, Fouchet P, Tavitian B, Romeo PH. In vivo cellular imaging pinpoints the role of reactive oxygen species in the early steps of adult hematopoietic reconstitution. Blood. 2010;115:443–52.
Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–41.
Pazhanisamy SK, Li H, Wang Y, Batinic-Haberle I, Zhou D. NADPH oxidase inhibition attenuates total body irradiation-induced haematopoietic genomic instability. Mutagenesis. 2011. doi:10.1093/mutage/ger001 [First published online: March 17, 2011].
Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85.
von Zglinicki T, Saretzki G, Ladhoff J, d’Adda di Fagagna F, Jackson SP. Human cell senescence as a DNA damage response. Mech Ageing Dev. 2005;126:111–7.
Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–36.
Campisi J, Kim SH, Lim CS, Rubio M. Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol. 2001;36:1619–37.
Marcotte R, Wang E. Replicative senescence revisited. J Gerontol A Biol Sci Med Sci. 2002;57:B257–69.
Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102:517–20.
Goytisolo FA, Samper E, Martin-Caballero J, Finnon P, Herrera E, Flores JM, Bouffler SD, Blasco MA. Short telomeres result in organismal hypersensitivity to ionizing radiation in mammals. J Exp Med. 2000;192:1625–36.
Greenwood MJ, Lansdorp PM. Telomeres, telomerase, and hematopoietic stem cell biology. Arch Med Res. 2003;34:489–95.
Samper E, Fernandez P, Eguia R, Martin-Rivera L, Bernad A, Blasco MA, Aracil M. Long-term repopulating ability of telomerase-deficient murine hematopoietic stem cells. Blood. 2002;99:2767–75.
Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–24.
Allsopp RC, Morin GB, Horner JW, DePinho R, Harley CB, Weissman IL. Effect of TERT over-expression on the long-term transplantation capacity of hematopoietic stem cells. Nat Med. 2003;9:369–71.
Allsopp RC, Weissman IL. Replicative senescence of hematopoietic stem cells during serial transplantation: does telomere shortening play a role? Oncogene. 2002;21:3270–3.
Effros RB, Globerson A. Hematopoietic cells and replicative senescence. Exp Gerontol. 2002;37:191–6.
Serrano M, Blasco MA. Putting the stress on senescence. Curr Opin Cell Biol. 2001;13:748–53.
Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–60.
Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–7.
Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–66.
Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev. 2003;13:77–83.
Sharpless NE, DePinho RA. The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev. 1999;9:22–30.
Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–16.
Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22.
Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–23.
te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–83.
Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–22.
Meng A, Wang Y, Van ZG, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63:5414–9.
Sherr CJ, Weber JD. The ARF/p53 pathway. Curr Opin Genet Dev. 2000;10:94–9.
Lewis JL, Chinswangwatanakul W, Zheng B, Marley SB, Nguyen DX, Cross NC, Banerji L, Glassford J, Thomas NS, Goldman JM, Lam EW, Gordon MY. The influence of INK4 proteins on growth and self-renewal kinetics of hematopoietic progenitor cells. Blood. 2001;97:2604–10.
Stepanova L, Sorrentino BP. A limited role for p16Ink4a and p19Arf in the loss of hematopoietic stem cells during proliferative stress. Blood. 2005;106:827–32.
Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–6.
Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69.
Deng Q, Liao R, Wu BL, Sun P. High intensity ras signaling induces premature senescence by activating p38 pathway in primary human fibroblasts. J Biol Chem. 2004;279:1050–9.
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602.
Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007.
Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–44.
Haq R, Brenton JD, Takahashi M, Finan D, Finkielsztein A, Damaraju S, Rottapel R, Zanke B. Constitutive p38HOG mitogen-activated protein kinase activation induces permanent cell cycle arrest and senescence. Cancer Res. 2002;62:5076–82.
Wang W, Chen JX, Liao R, Deng Q, Zhou JJ, Huang S, Sun P. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22:3389–403.
Katsoulidis E, Li Y, Yoon P, Sassano A, Altman J, Kannan-Thulasiraman P, Balasubramanian L, Parmar S, Varga J, Tallman MS, Verma A, Platanias LC. Role of the p38 mitogen-activated protein kinase pathway in cytokine-mediated hematopoietic suppression in myelodysplastic syndromes. Cancer Res. 2005;65:9029–37.
Verma A, Deb DK, Sassano A, Kambhampati S, Wickrema A, Uddin S, Mohindru M, Van BK, Platanias LC. Cutting edge: activation of the p38 mitogen-activated protein kinase signaling pathway mediates cytokine-induced hemopoietic suppression in aplastic anemia. J Immunol. 2002;168:5984–8.
Voncken JW, Niessen H, Neufeld B, Rennefahrt U, Dahlmans V, Kubben N, Holzer B, Ludwig S, Rapp UR. MAPKAP kinase 3pK phosphorylates and regulates chromatin association of the polycomb group protein Bmi1. J Biol Chem. 2005;280:5178–87.
Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–67.
Wang W, Martindale JL, Yang X, Chrest FJ, Gorospe M. Increased stability of the p16 mRNA with replicative senescence. EMBO Rep. 2005;6:158–64.
Dean JL, Sully G, Clark AR, Saklatvala J. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell Signal. 2004;16:1113–21.
Gaestel M. MAPKAP kinases—MKs—two’s company, three’s a crowd. Nat Rev Mol Cell Biol. 2006;7:120–30.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (R01-CA086688, CA102558, and AI080421), and a grant from the National Natural Science Foundation of China (NSFC 30828011), the Winthrop Rockefeller Endowment for Leukemia Research, and the Arkansas Research Alliance Scholarship from the Arkansas Science & Technology Authority.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shao, L., Li, H., Pazhanisamy, S.K. et al. Reactive oxygen species and hematopoietic stem cell senescence. Int J Hematol 94, 24–32 (2011). https://doi.org/10.1007/s12185-011-0872-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-011-0872-1