Abstract
Inherited antithrombin deficiency, an established risk factor for venous thromboembolism (VTE), can be classified into type I (quantitative deficiency) or type II (qualitative deficiency). In the present study, we assessed the VTE risk associated with the phenotypes of antithrombin deficiency in patients admitted to our hospital. We found that patients with type I deficiency (n = 21) had more VTE events and earlier onset of VTE than those with type II deficiency (n = 10). The VTE-free survival analysis showed that the risk for VTE in patients with type I deficiency was sevenfold greater than that in patients with type II deficiency (hazard ratio: 7.3; 95% confidence interval: 1.9–12.2; P = 0.0009). The prevalence of type I deficiency in the VTE group (5.6%, 6/108) was higher than that in the general population (0.04%, 2/4,517) (odds ratio: 132.8; 95% confidence interval: 26.5–666.1; P < 0.0001). However, the prevalence of type II deficiency was not different between the VTE group and the general population. Our study indicated that the risk for VTE in patients with type I deficiency was much higher than that in patients with type II deficiency. Thus, simple phenotypic classification of antithrombin deficiency is useful for assessment of VTE risk in Japanese.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Antithrombin is a serine protease inhibitor and functions as a potent natural anticoagulant by inactivating proteases in the coagulation cascade [1–3]. Since Egeberg’s [4] first report of antithrombin deficiency in a Scandinavian family in 1965, numerous additional families with antithrombin deficiency have been reported [5, 6]. The majority of individuals with antithrombin deficiency are heterozygotes, and homozygotes are extremely rare.
Antithrombin deficiency is phenotypically classified into two types. In type I deficiency, both antithrombin activity and antigen levels are low in the plasma, indicating that the protein is not produced by the mutant allele. In type II deficiency, low antithrombin activity within normal antigen limits is observed, indicating a functional impairment of the molecule. Type II deficiency can be further divided into three subtypes: a heparin-binding site subtype, in which heparin binding is abnormal; a reactive site subtype, in which the reactive center loop is abnormal; and a pleiotropic effect subtype, in which the influence is pleiotropic [3, 5].
Antithrombin deficiency or low antithrombin levels are associated with increased incidence of VTE and are a risk for first and recurrent VTE [1–3, 7–12]. Asymptomatic antithrombin deficiency is present as frequently as 1:600 [9, 13, 14], and type II deficiency is more prevalent than type I deficiency in the general population [13]. In a cumulative analysis of the literature, more than half of the patients with antithrombin type I deficiency or the reactive site or pleiotropic effect subtypes of type II deficiency manifested VTE events [15–17]. However, the incidence of VTE events in patients with the heparin-binding site subtype of type II deficiency was very low (only 6%), although patients who were homozygous or compound heterozygous for the heparin-binding site subtype mostly manifested VTE events [15–18]. These observations were obtained from cumulative evidence collected worldwide from patients with antithrombin deficiency. However, the methods used for identifying antithrombin deficiency in each institution were different and not standardized. To date, there has been no direct comparison of the differential thrombotic risk associated with type I and type II antithrombin deficiency at a single institution.
We have measured hemostatic factors including antithrombin, protein C, and plasminogen in patients admitted to the National Cerebral and Cardiovascular Center Hospital [9, 19, 20]. We have thus far identified 31 patients with antithrombin deficiency based on their heparin-dependent activity [9]. We also measured antithrombin activity in a Japanese general population consisting of more than 4,500 individuals, who were selected randomly from the municipal population registry stratified by sex and 10-year age group. Comparing the prevalence of antithrombin deficiency in the VTE patient group with that in the general population, we concluded that antithrombin deficiency is a strong risk factor for VTE with an odds ratio of 38 [9].
Here, we extend our previous study to clarify the VTE risk associated with the antithrombin deficiency phenotypes (type I and type II) using two studies. In the first study, 31 patients with antithrombin deficiency admitted to our hospital were classified as type I or type II and their VTE events were retrospectively collected and compared between the two types. In the second study, we compared the prevalence of type I and type II antithrombin deficiency in the VTE patient group and that in the general population and assessed whether the phenotypes of antithrombin deficiency affected VTE events. Both studies showed that type I antithrombin deficiency was a stronger risk factor for VTE than type II deficiency. Thus, the phenotypic classification of antithrombin deficiency is important for assessing VTE risk, and a simple phenotypic classification for type I and type II is useful for assessment of VTE risk in Japanese.
2 Materials and methods
2.1 Study population: retrospectively followed patients with antithrombin deficiency classified by phenotypes
We measured antithrombin and protein C activities, in response to a doctor’s request, in addition to routine hemostatic parameters including prothrombin time and activated partial thromboplastin time in patients admitted to the National Cerebral and Cardiovascular Center Hospital from January 1986 to May 2006. We ultimately identified 31 patients with antithrombin deficiency from 30 families on the basis of antithrombin and protein C activities and the family study. We classified them into type I or type II antithrombin deficiency groups based on the antithrombin activity/antigen ratio. These patients were admitted to our hospital due to cardiovascular problems and did not always have VTE. We, therefore, retrospectively followed them for VTE events and VTE onset age by their medical records for assessment of thrombotic risk. Information on hypertension, hyperlipidemia, diabetes mellitus, and current smoking status was also collected. The diagnosis of deep vein thrombosis was based on radioisotope venography, contrast venography, magnetic resonance imaging, and/or continuous-wave Doppler ultrasonography. The diagnosis of pulmonary embolism was confirmed by pulmonary angiography, computed tomography, and/or magnetic resonance imaging.
2.2 Study populations: the VTE group and the general population
We previously enrolled 108 outpatients with VTE (54 men and 54 women), who were admitted to the National Cerebral and Cardiovascular Center Hospital between 1994 and 1998, and identified 6 patients with antithrombin deficiency [9]. These 6 patients were included in the 31 patients with antithrombin deficiency described above. We also previously enrolled 4,517 individuals (2,090 men and 2,427 women), who were randomly selected from the residents of Suita city in Japan and stratified by sex and 10-year age group as a general population, and identified 7 individuals with antithrombin deficiency [9, 21, 22]. Here, we measured the antigen levels of antithrombin in these 13 patients (6 with antithrombin deficiency described above and 7 from Suita city), classified them as having type I or type II deficiency, and compared the prevalence of the phenotypes of antithrombin deficiency in the VTE patient group with that in the general population.
2.3 Assay methods
Blood samples were collected in siliconized plastic vacuum tubes containing a 1/10 volume of 3.13% trisodium citrate. The tubes were centrifuged at 4,000 rpm for 10 min at room temperature and the plasma samples were obtained. Antithrombin activity was determined as heparin-cofactor activity based on the antithrombin assay by the use of chromogenic substrate S-2238 (CHROMOGENIX AB, Stockholm, Sweden) [9, 19]. Protein C activity was measured using the chromogenic substrate S-2236 (CHROMOGENIX AB) after activation by Protac (Pentapharm, Basel, Switzerland) [9, 19]. Antithrombin antigen levels were determined using a latex photometric immunoassay kit (Mitsubishi Chemical Medience Co., Tokyo, Japan) and LPIA-A700, a fully automated quantitative latex photometric immunoassay instrument (Mitsubishi Chemical Medience Co.). The activity and antigen levels were expressed as percentages of the levels obtained from commercially available standard human plasma (Siemens AG, Bayern, Germany). As measured in our laboratory, the inter-assay coefficients of variation were 2.2% for the antithrombin activity assay, 1.9% for the antithrombin antigen assay, and 2.4% for the protein C activity assay. Antithrombin deficiency was defined by the antithrombin and protein C activities and the family study. In this study, patients having an antithrombin activity <3 standard deviations below the mean (70%) and a protein C/antithrombin activity ratio >3 standard deviations above the mean (1.65) [9] and at least one relative with antithrombin deficiency were considered to have antithrombin deficiency.
2.4 Statistical analysis
Statistical analysis was performed using Prism (GraphPad Software, La Jolla, CA, USA). Quantitative data were assessed using the Mann–Whitney test. Associations between qualitative variables were analyzed with the Chi-square test. Odds ratios with 95% confidence intervals were used to assess the differences between prevalence rates in the different groups. VTE-free curves in patients with type I or type II antithrombin deficiency were created using the method of Kaplan–Meier and compared using the log-rank test.
3 Results
3.1 Classification of type I and type II antithrombin deficiency
To classify antithrombin deficiency into type I and type II, the antithrombin activity/antigen ratio (AT-act/AT-ag ratio) was calculated. Figure 1 shows the distribution and box plots of the AT-act/AT-ag ratio in the 31 patients with antithrombin deficiency. We arbitrarily divided the patients into two groups based on this ratio; the median (range) of the ratio in the two groups was 0.98 (0.84–1.29) and 0.52 (0.42–0.62), respectively. We considered 0.75 as the cut-off value for discrimination between the two groups. This cut-off value was calculated as the mean of the median in the two groups. There was no overlap of the AT-act/AT-ag ratio between the two groups. Based on these criteria, 21 patients with type I (circles) and 10 patients with type II (triangles) were identified.
Discrimination of antithrombin deficiency phenotypes using the antithrombin activity/antigen ratio. Thirty-one patients with antithrombin deficiency admitted to our hospital were classified into type I (circles, n = 21) or type II (triangles, n = 10) deficiency groups using the antithrombin activity/antigen ratio (AT-act/AT-ag) of 0.75. In the box plots, the error bars represent minimum and maximum values, the horizontal rules indicate median values, and the ends of the boxes indicate interquartile ranges
3.2 Retrospective follow-up study to compare VTE-free curves in patients with type I and type II antithrombin deficiency
We retrospectively collected the VTE events of the 21 patients with type I antithrombin deficiency and the 10 patients with type II antithrombin deficiency (Table 1). In the type I group, 33 VTE events occurred in 18 of the 21 patients (85.7%), and 16 patients (76.2%) experienced a VTE event before 50 years of age. Recurrent VTE events were observed in 6 of the 18 patients with VTE. The median age for VTE events was 37 years with a range of 15–89 years of age. In contrast, in the type II group, only 3 VTE events occurred in 2 of the 10 deficient patients (20.0%), and the first VTE events occurred at the ages of 57 and 70. There were no significant differences in the risk factors for coronary artery disease, including hypertension, hyperlipidemia, diabetes mellitus, and smoking, between the type I and type II groups.
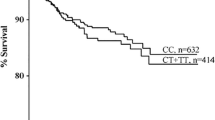
To evaluate the VTE risk for patients with type I and type II antithrombin deficiency, we compared the VTE-free curves between the groups (Fig. 2). The VTE risk was increased by approximately sevenfold in patients with type I deficiency relative to those with type II deficiency (hazard ratio: 7.3; 95% confidence intervals: 1.9–12.2; P = 0.0009).
VTE-free curves of patients with type I or type II antithrombin deficiency. In type I antithrombin deficiency, 18 out of 21 patients developed VTE. In type II antithrombin deficiency, only 2 out of 10 patients developed VTE. For the Kaplan–Meier analysis, the remaining three or eight patients were treated as the censored patients
3.3 Comparison of the prevalence of type I and type II antithrombin deficiency in the VTE patient group and the general population
We previously identified 6 patients with antithrombin deficiency among 108 consecutive outpatients with VTE [9]. We also identified 7 individuals with antithrombin deficiency in a general population consisting of 4,517 individuals. Comparing these frequencies, we reported that antithrombin deficiency was a strong risk factor for VTE with an odds ratio of 38 [9]. To extend this previous work, we measured the antithrombin antigen levels in these patients in the present study. We found that all 6 VTE patients with antithrombin deficiency were type I (Table 2). In the general population, two individuals were type I and the remaining 5 were type II (Table 2). The prevalences of type I antithrombin deficiency in the VTE group (6/108, 5.56%) and the general population (2/4,517, 0.04%) were significantly different, and the odds ratio between the two groups was 132.8 (95% confidence intervals: 26.5–666.1; P < 0.0001). In contrast, the prevalences of type II antithrombin deficiency in the VTE group (0/108, 0.00%) and the general population (5/4,517, 0.11%) were not significantly different (odds ratio: 3.8; 95% confidence interval: 0.2–68.9; P = 0.7294).
4 Discussion
In the present study, we identified 21 patients with type I antithrombin deficiency and 10 patients with type II antithrombin deficiency. Among these patients, more than 75% with type I deficiency had VTE events before the age of 50; in contrast, only 2 out of 10 patients with type II deficiency had VTE events, and these occurred at the ages of 57 and 70. The VTE-free curves showed that type I antithrombin deficiency was a strong risk factor for VTE, with a hazard ratio of 7.3 compared to type II deficiency. These findings were reinforced by a comparison of the prevalence of type I and type II deficiency in the VTE group and the general population, which showed very high odds ratio for VTE for type I, but not for type II.
Based on a cumulative analysis of the literature, type I antithrombin deficiency as well as both the reactive site and the pleiotropic subtypes of type II deficiency have an increased risk for VTE [15–17]. However, the heparin-binding site subtype of type II deficiency showed a relatively low risk for VTE [15–17]. Thus, the VTE risk conferred by type I antithrombin deficiency was consistent between our study and the cumulative analysis. However, the risk assessment of type II antithrombin deficiency for VTE is in part debatable. In our analysis, we could not classify our patients with type II deficiency into three subtypes, a heparin-binding site subtype, a reactive site subtype, and a pleiotropic effect subtype, due to the sample limitations. Therefore, we could not evaluate the risk assessment for VTE by subtypes. Despite this limitation, we demonstrated that type I antithrombin deficiency was a greater risk for VTE than type II.
The plausible explanation for type II deficiency as a low VTE risk would be that mutations responsible for a low VTE risk, the heparin-binding defects, would be more common than those for a high VTE risk, the reactive site defects and pleiotropic effect defects, in a Japanese population. This could explain the clearly differentiated risk between type I and type II deficiency in our study. In the public database, the cases of at least three Japanese VTE patients homozygous for the R47C mutation, that causes a heparin-binding defect, have been deposited (Japanese Thrombophilia Mutation Database, http://hes.met.nagoya-u.ac.jp/KENSAWEB/labo/blood/bunshi_hp/mutation.html, Antithrombin Mutation Database, http://www1.imperial.ac.uk/medicine/divisions/olddivisions/is/haematology/coag/antithrombin/) [18, 23, 24], suggesting the prevalence of this mutation in the Japanese population. As for the reactive site defects in the Japanese population, two heterozygous patients, one with the R425C mutation [25] and another with the R425H mutation [26] have been deposited in the public database. Thus, the frequency of patients with the reactive site defects seemed lower than those with the heparin-binding defects.
We identified 2 individuals with type I antithrombin deficiency and 5 individuals with type II deficiency out of 4,517 individuals, who were selected randomly from the municipal population registry stratified by sex and 10-year age group. Thus, the prevalences of type I and type II deficiency in the Japanese general population were 0.04% (2/4,517) and 0.11% (5/4,517), respectively, which were similar to those obtained in western Scotland (type I: 0.02%, type II: 0.15%) [13], although the selection criteria for antithrombin deficiency were different. The Japanese population is now about 128 million. Extrapolating from the prevalence, we estimate that as many as 57,000 Japanese have type I antithrombin deficiency and are at risk of developing VTE.
In conclusion, considerable differences in VTE risk were observed between Japanese individuals with type I and type II antithrombin deficiency and a simple phenotypic classification for type I and type II antithrombin deficiency was shown to be useful for assessment of VTE risk in Japanese.
References
De Stefano V, Finazzi G, Mannucci PM. Inherited thrombophilia: pathogenesis, clinical syndromes, and management. Blood. 1996;87:3531–44.
Perry DJ, Carrell RW. Molecular genetics of human antithrombin deficiency. Hum Mutat. 1996;7:7–22.
Lane DA, Mannucci PM, Bauer KA, Bertina RM, Bochkov NP, Boulyjenkov V, et al. Inherited thrombophilia: Part 1. Thromb Haemost. 1996;76:651–62.
Egeberg O. Inherited antithrombin deficiency causing thrombophilia. Thromb Diath Haemorrh. 1965;13:516–30.
Lane DA, Bayston T, Olds RJ, Fitches AC, Cooper DN, Millar DS, et al. Antithrombin mutation database: 2nd (1997) update For the Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 1997;77:197–211.
Miyata T, Sato Y, Ishikawa J, Okada H, Takeshita S, Sakata T, et al. Prevalence of genetic mutations in protein S, protein C and antithrombin genes in Japanese patients with deep vein thrombosis. Thromb Res. 2009;124:14–8.
Koster T, Rosendaal FR, Briet E, van der Meer FJ, Colly LP, Trienekens PH, et al. Protein C deficiency in a controlled series of unselected outpatients: an infrequent but clear risk factor for venous thrombosis (Leiden Thrombophilia Study). Blood. 1995;85:2756–61.
Sanson BJ, Simioni P, Tormene D, Moia M, Friederich PW, Huisman MV, et al. The incidence of venous thromboembolism in asymptomatic carriers of a deficiency of antithrombin, protein C, or protein S: a prospective cohort study. Blood. 1999;94:3702–6.
Sakata T, Okamoto A, Mannami T, Matsuo H, Miyata T. Protein C and antithrombin deficiency are important risk factors for deep vein thrombosis in Japanese. J Thromb Haemost. 2004;2:528–30.
Vossen CY, Walker ID, Svensson P, Souto JC, Scharrer I, Preston FE, et al. Recurrence rate after a first venous thrombosis in patients with familial thrombophilia. Arterioscler Thromb Vasc Biol. 2005;25:1992–7.
Vossen CY, Conard J, Fontcuberta J, Makris M, VDM FJ, Pabinger I, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459–64.
Lijfering WM, Brouwer JL, Veeger NJ, Bank I, Coppens M, Middeldorp S, et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood. 2009;113:5314–22.
Tait RC, Walker ID, Perry DJ, Islam SI, Daly ME, McCall F, et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol. 1994;87:106–12.
Wells PS, Blajchman MA, Henderson P, Wells MJ, Demers C, Bourque R, et al. Prevalence of antithrombin deficiency in healthy blood donors: a cross-sectional study. Am J Hematol. 1994;45:321–4.
Finazzi G, Caccia R, Barbui T. Different prevalence of thromboembolism in the subtypes of congenital antithrombin III deficiency: review of 404 cases. Thromb Haemost. 1987;58:1094.
Hirsh J, Piovella F, Pini M. Congenital antithrombin III deficiency. Incidence and clinical features. Am J Med. 1989;87:34S–8S.
Demers C, Ginsberg JS, Hirsh J, Henderson P, Blajchman MA. Thrombosis in antithrombin-III-deficient persons. Report of a large kindred and literature review. Ann Intern Med. 1992;116:754–61.
Koide T, Odani S, Takahashi K, Ono T, Sakuragawa N. Antithrombin III Toyama: replacement of arginine-47 by cysteine in hereditary abnormal antithrombin III that lacks heparin-binding ability. Proc Natl Acad Sci USA. 1984;81:289–93.
Okamoto A, Sakata T, Mannami T, Baba S, Katayama Y, Matsuo H, et al. Population-based distribution of plasminogen activity and estimated prevalence and relevance to thrombotic diseases of plasminogen deficiency in the Japanese: the Suita study. J Thromb Haemost. 2003;1:2397–403.
Miyata T, Kimura R, Kokubo Y, Sakata T. Genetic risk factors for deep vein thrombosis among Japanese: importance of protein S K196E mutation. Int J Hematol. 2006;83:217–23.
Kokubo Y, Nakamura S, Okamura T, Yoshimasa Y, Makino H, Watanabe M, et al. Relationship between blood pressure category and incidence of stroke and myocardial infarction in an urban Japanese population with and without chronic kidney disease: the Suita study. Stroke. 2009;40:2674–9.
Kokubo Y, Kamide K, Okamura T, Watanabe M, Higashiyama A, Kawanishi K, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease in a Japanese urban cohort: the Suita study. Hypertension. 2008;52:652–9.
Ueyama H, Murakami T, Nishiguchi S, Maeda S, Hashimoto Y, Okajima K, et al. Antithrombin III Kumamoto: identification of a point mutation and genotype analysis of the family. Thromb Haemost. 1990;63:231–4.
Shimizu K, Toriyama F, Ogawa F, Katayama I, Okajima K. Recurrent leg ulcers and arterial thrombosis in a 33-year-old homozygous variant of antithrombin. Am J Hematol. 2001;66:285–91.
Okajima K, Abe H, Wagatsuma M, Okabe H, Takatsuki K. Antithrombin III Kumamoto II; a single mutation at Arg393-His increased the affinity of antithrombin III for heparin. Am J Hematol. 1995;48:12–8.
Nagaizumi K, Inaba H, Amano K, Suzuki M, Arai M, Fukutake K. Five novel and four recurrent point mutations in the antithrombin gene causing venous thrombosis. Int J Hematol. 2003;78:79–83.
Acknowledgments
This study was supported by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO), a Grant-in-Aid from the Ministry of Health, Labor and Welfare of Japan, and the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mitsuguro, M., Sakata, T., Okamoto, A. et al. Usefulness of antithrombin deficiency phenotypes for risk assessment of venous thromboembolism: type I deficiency as a strong risk factor for venous thromboembolism. Int J Hematol 92, 468–473 (2010). https://doi.org/10.1007/s12185-010-0687-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-010-0687-5