Abstract
Infection is a major obstacle in cancer chemotherapy. Neutropenia has been considered to be the most important risk factor for severe infection; however, other factors, such as impaired neutrophil function, may be involved in susceptibility to infection in patients undergoing chemotherapy. In this study, we analyzed neutrophil function in children with acute lymphoblastic leukemia (ALL). Whole blood samples were obtained from 16 children with ALL at diagnosis, after induction chemotherapy, and after consolidation chemotherapy. Oxidative burst and phagocytic activity of neutrophils were analyzed by flow cytometry. Oxidative burst of neutrophils was impaired in ALL patients. The percentage of neutrophils with normal oxidative burst after PMA stimulation was 59.0 ± 13.2 or 70.0 ± 21.0% at diagnosis or after induction chemotherapy, respectively, which was significantly lower compared with 93.8 ± 6.1% in healthy control subjects (P = 0.00004, or 0.002, respectively); however, this value was normal after consolidation chemotherapy. No significant differences were noted in phagocytic activity in children with ALL compared with healthy control subjects. Impaired oxidative burst of neutrophils may be one risk factor for infections in children with ALL, especially in the initial periods of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Infection is a major obstacle in cancer chemotherapy. Recent improvements in leukemia treatment have been brought about by progress in chemotherapy as well as by progress in supportive care, including prophylaxis and treatment of infection. In patients with acute lymphoblastic leukemia (ALL), the mortality rate from infection during induction chemotherapy has been decreasing, but is still 1–3% among children [1–3] and 3–11% among the adult population [4, 5]. Neutropenia has been considered to be the most important risk factor for severe infection during chemotherapy [6]. However, other factors, such as impaired neutrophil function, may be involved in susceptibility to infection in patients undergoing chemotherapy [7]. Several studies have shown the presence of impaired neutrophil function in patients with acute leukemia [8–10]. However, most such studies were performed in the adult population, and few have been done in pediatric patients [10], who usually undergo more intensive chemotherapy compared with adults. In the present study, we analyzed neutrophil function, phagocytic activity, and the ability of oxidative burst in children with ALL before or after administration of cytotoxic agents.
2 Patients and methods
2.1 Patients
Overall, 16 patients aged 0–18 years with ALL were involved in this study. Three patients had relapsed disease, and the others were newly diagnosed. The diagnosis of ALL was done by standard procedures. All patients were treated with the Tokyo Children’s Cancer Study Group (TCCSG) ALL protocols [11]. These protocols consisted of 6 weeks of remission induction therapy using prednisolone, vincristine, and L-asparaginase with or without cyclophosphamide or anthracycline depending on the risk group, followed by phases of consolidation therapy, reinduction therapy, and maintenance therapy. The first consolidation therapy consisted of 6-mercaptopurine, cyclophosphamide and cytarabine. Patients’ characteristics are summarized in Table 1.
Residual blood samples after ordinary clinical use were utilized in this study with informed consent from the parents or guardians. This study has been approved by the Ethics Board of Yokohama City University Hospital. Analyses of neutrophil function were done at diagnosis, after remission induction therapy, or after consolidation chemotherapy. Among 16 patients, 8 were analyzed at diagnosis before the start of chemotherapy, 12 after remission induction therapy, and 8 after consolidation chemotherapy. Neutrophil counts at diagnosis were 160–13,311/μl (median 778/μl). Analyses after chemotherapy were performed when the absolute neutrophil count reached more than 500/μl, as a rule. Samples from healthy adult volunteers were used as normal controls.
2.2 Measurement of oxidative burst
Oxidative burst of neutrophils upon phorbol myristate acetate (PMA)-stimulation was determined by flow cytometry, using the methods of Bass et al. [12], with modifications. In this study, we used dihydrorhodamine 123 (DHR) instead of 2′,7′-dichlorofluorescein diacetate (DCFH) because DHR is more specifically responsive to H2O2 accumulation than DCFH [13]. Heparinized whole blood (100 μl) was incubated in 2 ml of phosphate buffered saline (PBS) containing dihydrorhodamine 123 (DHR, Sigma–Aldrich, St. Louis, MO, USA) at 1 μM for 15 min at 37°C. 500 μl of 25 mM EDTA, and for PMA-stimulation, 10 μl of 25 μg/ml PMA (Sigma–Aldrich) were added. After further incubation at 37°C for 20 min, the reaction was terminated by cooling samples on ice, and erythrocytes were removed by hemolysis using FACS Lysing Solution (Becton–Dickinson, Franklin Lakes, NJ, USA). The remaining white blood cells were washed in PBS, and resuspended in 0.5 ml of PBS. Oxidative burst was indicated by the production of reactive oxygen species (ROS) in neutrophils, which oxidize DHR 123 to fluorescent rhodamine 123. For the negative control, samples without PMA-stimulation were also prepared.
2.3 Measurement of phagocytic activity
Phagocytic activity of neutrophils was determined by flow cytometory, using the methods of Dunn et al. [14], with modification. Fluorescent microspheres (fluospheres carboxylate-modified 2.0 μm, red fluorescent, 2% solid, Molecular Probe, Eugene, OR, USA) were used as target particles. Five μl of microspheres (1 × 109/ml) was added to 100 μl of heparinized whole blood, and then incubated at 37°C in a water bath for 25 min. The reaction was terminated by chilling on ice. Erythrocytes were removed by hemolysis using FACS lysing solution. The resultant pellet was collected, washed in PBS, and resuspended in 0.5 ml of 3 mM EDTA in PBS. The percentage of cells phagocytizing fluorescent microspheres was measured by flow cytometry.
2.4 Flow cytometric assay
A FACScan (Becton–Dickinson) was used to detect both rhodamine and microsphere fluorescence. Fluorescence parameters from single cells were collected using a logarithmic amplifier after gating on the neutrophils by a combination of forward and side light scatter. In all samples, counts of eosinophils or basophils were minimal, and the effect of contamination by those cells was considered to be insignificant. Green fluorescence from rhodamine was collected through an FL1 channel, and red fluorescence from microspheres through an FL2 channel. Ten thousand cells were analyzed per tube and data were acquired. For the oxidative burst, the fluorescence threshold of normal ROS production was determined from values from healthy control subjects. Fluorescence distribution was displayed as a single histogram, and the fluorescent cells over the threshold were determined as having normal function.
2.5 Reagents
DHR 123 dissolved in dimethyl sulfoxide (DMSO) to give a final concentration of 1 mM was stored as the stock solution at −20°C, and diluted to 1 μM with PBS for use. PMA, which was initially dissolved in absolute ethanol to a concentration of 2 mg/ml, was stored at −20°C and diluted to 25 μg/ml with PBS for use. Microspheres were diluted with PBS to give a final concentration of 1 × 106 beads/μl for use.
2.6 Statistical analyses
The results were presented as mean ± standard deviations. Statistical analyses were performed using the unpaired Student t test. The significance level was determined as p < 0.05.
3 Results
3.1 Oxidative burst
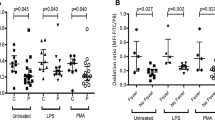
The percentage of fluorescent neutrophils with normal oxidative burst was 59.0 ± 13.2% in children with ALL at diagnosis, which was significantly lower than the 93.8 ± 6.1% in control subjects (P = 0.00004). After induction chemotherapy, the percentage was 70.0 ± 21.0%, which was higher than the value at diagnosis but was still lower than control values (P = 0.002). After consolidation chemotherapy, values were at a normal level (83.1 ± 16.9%, P = 0.12; vs. controls) (Table 2; Fig. 1).
Oxidative burst of neutrophils in children with ALL. Green fluorescence of oxidized dihydrorhodamine 123 in neutrophils before or after phorbol myristate acetate (PMA)-stimulation was detected, and % of neutrophils with normal oxidative burst was measured by flow cytometry. Representative results are shown in (a), and data on all patients and control subjects are summarized in (b). Horizontal lines indicate the mean in each therapeutic phase. Percentage of fluorescent neutrophils with normal oxidative burst was 59.0 ± 13.2, 70.0 ± 21.0, or 83.1 ± 16.9% in children with ALL at diagnosis, after induction chemotherapy, or after consolidation chemotherapy, respectively. Compared to 93.8 ± 6.1% for normal control subjects, values were significantly lower at diagnosis (P = 0.00004) or after induction chemotherapy (P = 0.002)
As for the values at diagnosis, there were no differences in results, when analyzing by WBC or neutrophil counts, the risk groups of ALL, or CRP values. In the patients with high fever (>38°C, n = 4) at diagnosis, the percentage of neutrophils with normal oxidative burst was 51.2 ± 13.8%, which tended to be lower compared with 66.8 ± 7.3% in the patients without high fever (n = 4), although the difference was not significant (P = 0.10).
3.2 Phagocytic activity
Phagocytic parameters showed wide deviations both in ALL children and control subjects. In children with ALL, neutrophil phagocytic activity at diagnosis, after induction chemotherapy, or after consolidation chemotherapy was 94.1 ± 3.5, 89.7 ± 11.8, or 79.7 ± 21.7%, respectively, which was not significantly different from 76.7 ± 20.5% in control subjects (P = 0.24, 0.81, or 0.51, respectively) (Table 3; Fig. 2).
Neutrophil phagocytic activity in children with ALL. Fluorescent signals are increased when neutrophils phagocyte microspheres. Representative results are shown in Fig. 2. Phagocytic activity of neutrophils varied from 52 to 95% in control subjects. In children with ALL, neutrophil phagocytic activity was 94.1 ± 3.5% at diagnosis, 89.7 ± 11.8% after induction chemotherapy, and 79.7 ± 21.7% after consolidation chemotherapy. These values were not significantly different from those of control subjects
3.3 Oxidative burst of neutrophils in patients with various diseases
Additionally, we measured neutrophil function in nine patients with diseases other than ALL. Underlying diseases were neuroblastoma, osteosarcoma, Wilms’ tumor, acute myelogenous leukemia (AML), chronic myelogenous leukemia (CML), non-Hodgkin lymphoma (NHL, T lymphoblastic lymphoma), aplastic anemia (AA), or acute idiopathic thrombocytopenic purpura (aITP) (Table 4). The analysis was performed at diagnosis before therapeutic intervention in all patients. As summarized in Table 4, oxidative burst of neutrophils was suppressed to 54.8 ± 7.9% in all patients with malignant diseases (P = 0.003; vs. control subjects), but values were normal (95.2–98.2%) in patients with AA or aITP. In this connection, of the patients with malignant diseases, three (neuroblastoma, osteosarcoma, Wilms’ tumor) did not have bone marrow disease at diagnosis.
4 Discussion
Infection is the most frequent complication during chemotherapy for ALL [15]. Especially, remission induction therapy is often complicated by severe myelosuppression, and infection in this phase is sometimes causative of therapy-related death. The period of induction therapy is usually 5–8 weeks, which is almost one tenth of the 2- or 3-year total chemotherapy course for ALL; however, the rate of fatal infection has been reported to be highest during this phase compared with subsequent chemotherapy phases [1, 2].
During chemotherapy, the risk of infection has been reported to increase according to decreased number and suppressed function of neutrophils. Several studies have evaluated neutrophil function in patients with hematological malignancies by various methods, although the underlying disease and disease status at the time of examination were heterogenous. In patients with AML, it has been reported that impairment of neutrophil phagocytic activity before chemotherapy was associated with the occurrence of severe infection [8]. Hofmann et al. [9] reported that neutrophil function was impaired at disease onset but recovered to normal levels after achievement of complete remission in patients with ALL or AML. These studies were mostly conducted among adult populations, and few studies referred to pediatric patients, who usually undergo more intensive chemotherapy than older patients.
Lejeune et al. [10] reported chemiluminescence and microbicidal activity were significantly depressed in ALL children. Chemiluminescence, a reflection of the H2O2 and O2-production, returned to normal values in maintenance therapy, but bactericidal activity remained depressed throughout the maintenance therapy, although few patients were analyzed at disease diagnosis before chemotherapy.
In this study, we evaluated neutrophil function at different phases of the chemotherapy course in pediatric patients with ALL. The oxidative burst of neutrophils was significantly suppressed in ALL patients at diagnosis and after induction chemotherapy. Among the patients with ALL in this study, two patients developed severe infection during induction chemotherapy, and their oxidative burst of neutrophils were relatively low (67.8, 48.1%, respectively). The number of patients included in this study was small, and we could not conclude if suppressed neutrophil function was associated with infection. However, it is important to note that neutrophils of ALL patients are functionally suppressed at initial phases, even after achievement of complete remission.
The mechanisms underlying suppressed neutrophil function in leukemic patients are not clear. Considering results of a study showing that neutrophil function was impaired in patients with hypoplastic myelodysplastic syndrome, but not with aplastic anemia, the lower neutrophil function could be due to derivation of abnormal neutrophils from a leukemic stem cells in patients with hematological malignancies [16]. In our examination of oxidative burst of neutrophils in patients with various diseases, values were within the normal level in patients with non-malignant diseases. However, the oxidative burst of neutrophils was impaired in patients with solid tumors without bone marrow disease, suggesting an indirect effect of malignant cells to suppress neutrophil function.
Activated CD4+CD25+ T regulatory cells were reported to have a negative regulatory effect on neutrophil function, and this role of T regulatory cells was noted to be partially mediated by IL-10 [17]. It has been shown that the number of T regulatory cells increases in patients with cancer, and that most ALL blasts produce IL-10 [18]. An in vitro study showed that IL-10 suppresses neutrophil function [19]. In some patients included in this study, we measured serum levels of IL-10 by ELISA at diagnosis. In the patient with high IL-10 level (>20 pg/ml, n = 5), the percentage of neutrophils with normal oxidative burst was 54.1 ± 13.6%, which tended to be lower compared with 67.1 ± 8.9% in the patients with normal IL-10 level (<10 pg/ml, n = 3), although the difference was not significant (P = 0.15). The serum levels of IL-10 in two patients who developed severe infection during induction chemotherapy were higher than the normal level (34.0, 31.6 pg/ml, respectively), accompanied by low oxidative burst of neutrophils (67.8, 48.1%, respectively). We could not declare that high level of IL-10 were responsible for the suppressed neutrophil function, because of the number of patients included in this study was small. However it was suggested that direct or indirect production of IL-10 by ALL blasts might be responsible for the impaired neutrophil function at diagnosis.
In the present study, the function of neutrophils in the oxidative burst was still low after induction chemotherapy, even though all tested patients achieved complete remission after induction chemotherapy. Tsurumi et al. [16] studied the influence of steroids and other chemotherapeutic drugs on hydroperoxide (H2O2) production of neutrophils, and presumed that the reduction of H2O2 production was caused primarily by the action of steroids, since the H2O2 production of neutrophils was reduced in patients with malignant neoplasms who underwent chemotherapy with steroids but not reduced in those not administered steroids. The prolonged use of high-dose glucocorticoid in induction chemotherapy may be responsible for the impaired function of neutrophils in ALL patients.
The values for phagocytic activity varied widely among patients, and we could not find a difference in values between patients and control subjects in any phases. In this study, whole blood was employed instead of using purified neutrophil fractions because of concern about non-specific neutrophil stimulation by the isolation procedure. There were differences in neutrophil concentration among samples; however, in our preliminary study, we confirmed that the neutrophil concentration in the samples did not affect the results of neutrophil function assays (data not shown).
In conclusion, our study revealed that neutrophil function in relation to oxidative burst was suppressed in pediatric patients with ALL at diagnosis and after remission induction chemotherapy. Because of the small number of study subjects, we could not conclude if suppressed neutrophil function was actually associated with the frequent occurrence of severe infection during the period around induction chemotherapy. However, in order to improve management of children with ALL, it is clinically significant to note that neutrophils are functionally abnormal in such phases.
References
Christensen MS, Heyman M, Mottonen M, Zeller B, Jonmundsson G, Hasle H. Nordic Society of Paediatric Haematology and Oncology (NOPHO): treatment-related death in childhood acute lymphoblastic leukaemia in the Nordic countries: 1992–2001. Br J Haematol. 2005;131:50–8.
Rubnitz JE, Lensing S, Zhou Y, Sandlund JT, Razzouk BI, Ribeiro RC, et al. Death during induction therapy and first remission of acute leukemia in childhood: the St. Jude experience. Cancer. 2004;101:1677–84.
Hargrave DR, Hann II, Richards SM, Hill FG, Lilleyman JS, Kinsey S, et al. Medical Research Council Working Party for Childhood Leukaemia: progressive reduction in treatment-related deaths in Medical Research Council childhood lymphoblastic leukaemia trials from 1980 to 1997 (UKALL VIII, X and XI). Br J Haematol. 2001;112:293–9.
Offidani M, Corvatta L, Malerba L, Marconi M, Leoni P. Infectious complications in adult acute lymphoblastic leukemia (ALL): experience at one single center. Leuk Lymphoma. 2004;45:1617–21.
Annino L, Vegna ML, Camera A, Specchia G, Visani G, Fioritoni G, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99:863–71.
Bodey GP. Infection in cancer patients: a continuing association. Am J Med. 1986;81:11–26. Review.
Yamamoto K, Sasada M, Nishiyama H, Uchida M, Sawada H, Nakamura T, et al. Ingestion and killing of bacteria by neutrophils from patients with leukemia: a new method for evaluating phagocyte function at short incubation. Acta Haematol Jpn. 1982;45:56–65.
Hubel K, Hegener K, Schnell R, Mansmann G, Oberhauser F, Staib P, et al. Suppressed neutrophil function as a risk factor for severe infection after cytotoxic chemotherapy in patients with acute nonlymphocytic leukemia. Ann Hematol. 1999;78:73–7.
Hofmann WK, Stauch M, Hoffken K. Impaired granulocytic function in patients with acute leukaemia: only partial normalisation after successful remission-inducing treatment. J Cancer Res Clin Oncol. 1998;124:113–6.
Lejeune M, Ferster A, Cantinieaux B, Sariban E. Prolonged but reversible neutrophil dysfunctions differentially sensitive to G-CSF in children with ALL. Br J Haematol. 1998;102:1284–91.
Manabe A, Ohara A, Hasegawa D, Koh K, Saito T, Kiyokawa N. Significance of the complete clearance of peripheral blasts after 7 days of prednisolone treatment in children with acute lymphoblastic leukemia: the Tokyo Children’s Cancer Study Group Study L99-15. Haematologica. 2008;93:1155–60.
Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130:1910–7.
Walrand S, Valeix S, Rodriguez C, Ligot P, Chassagne J, Vasson MP. Flow cytometry study of polymorphonuclear neutrophil oxidative burst: a comparison of three fluorescent probes. Clin Chim Acta. 2003;331:103–10.
Dunn PA, Tyrer HW. Quantitation of neutrophil phagocytosis, using fluorescent latex beads: correlation of microscopy and flow cytometry. J Lab Clin Med. 1981;98:374–81.
Katsimpardi K, Papadakis V, Pangalis A, Parcharidou A, Panagiotou JP, Soutis M, et al. Infections in a pediatric patient cohort with acute lymphoblastic leukemia during the entire course of treatment. Support Care Cancer. 2006;14:277–84.
Tsurumi H, Shimazaki M, Takahashi T, Moriwaki H, Muto Y. Flow cytometric determination of active oxygen (hydroperoxide) produced by peripheral blood neutrophils in patients with hematological disorders. Int J Hematol. 1993;57:213–9.
Lewkowicz P, Lewkowicz N, Sasiak A, Tchorzewski H. Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J Immunol. 2006;177:7155–63.
Wu S, Gessner R, von Stackelberg A, Kirchner R, Henze G, Seeger K. Cytokine/cytokine receptor gene expression in childhood acute lymphoblastic leukemia: correlation of expression and clinical outcome at first disease recurrence. Cancer. 2005;103:1054–63.
Kuga S, Otsuka T, Niiro H, Nunoi H, Nemoto Y, Nakano T, et al. Suppression of superoxide anion production by interleukin-10 is accompanied by a downregulation of the genes for subunit proteins of NADPH oxidase. Exp Hematol. 1996;24:151–7.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tanaka, F., Goto, H., Yokosuka, T. et al. Suppressed neutrophil function in children with acute lymphoblastic leukemia. Int J Hematol 90, 311–317 (2009). https://doi.org/10.1007/s12185-009-0412-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-009-0412-4