Abstract
We investigated the role of therapeutic dose monitoring (TDM) in the treatment of fungal infections with voriconazole through 49 analyses of 34 patients who received treatment for hematologic diseases. Voriconazole concentration was highly variable among patients regardless of renal, liver functions, or age, and the effect of dose enhancement was not constant. This indicates the difficulty of predicting voriconazole concentration without TDM. We evaluated the outcome with the composite assessment system where patients were assumed non-responders when they failed to show improvement in at least 2 of the following 3 criteria: clinical, radiologic, and mycologic. We showed that concentration–response relationship depended on the status of underlying hematologic diseases; this relationship was observed only in cases without refractory hematologic diseases, but not in those with refractory diseases. In the former group, cases with >2 mg/L of concentration were associated with good response to voriconazole. On the other hand, elevation of hepatic enzyme was frequently observed when voriconazole concentration was >6 mg/L. From these results, we concluded that TDM should be executed and targeted to 2–6 mg/L to improve efficacy and to avoid side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fungal infection is a life threatening complication in patients with hematological disorders [1, 2]. The analysis of autopsied cases showed that the prevalence of invasive aspergillosis in patients with hematologic malignancies was about 30% during 1999–2003 [3]. Initial therapy with voriconazole has been shown to be the most effective approach for patients with definitive invasive aspergillosis [4]. In addition, voriconazole is an effective and well-tolerated treatment option for refractory and less-common invasive fungal infections [5]. Furthermore, patients with neutropenia and persistent fever treated with voriconazole show low incidence of breakthrough aspergillus infections [6].
Voriconazole exhibits a nonlinear pharmacokinetic profile [7]. Voriconazole is mainly metabolized by the cytochrome P450 family enzyme CYP2C19 and allelic polymorphisms of CYP2C19 have been shown to be the most important determinants of the clearance efficiency of voriconazole. Patients lacking function of CYP2C19 reveal poor metabolism of voriconazole [8–10]. In Japanese, the rate of poor metabolizers is shown to be 15–20%, a higher figure than that of other races [11]. It raises the necessity for us to monitor voriconazole plasma concentrations.
A relationship between progression of fungal infection and voriconazole concentrations was demonstrated in recent studies [12, 13]. They showed that monitoring voriconazole concentration and adjusting dosage to attain appropriate concentration is necessary to ensure antifungal effect and to avoid toxicity. However, no evidence is available for Asian patients, which shows relationship between voriconazole concentrations and clinical effect. Therefore, we conducted a retrospective study to examine the impact of voriconazole concentration on the clinical effects and side effects in Japanese patients who received chemotherapy for hematologic diseases at Tokyo University Hospital.
2 Materials and methods
2.1 Patients
We conducted therapeutic drug monitoring (TDM) of voriconazole for adult patients with hematologic malignancies who were given voriconazole at Tokyo University Hospital, Japan, between February 2007 and July 2008. The underlying hematological disease was assumed refractory when blastic cells account for more than 5% in bone marrow nucleated cells after at least one course of induction therapies in leukemia cases, and when the lesions are judged stable or progressive diseases despite standard chemotherapies according to the criteria proposed by Malik et al. in lymphoma cases [14].
2.2 Antibacterial therapy
In our institute, patients who received intensive chemotherapies received prophylactic administration of fluoroquinolones (tosufloxacin or moxifloxacin) against bacterial infections and azoles (fluconazole or itraconazole oral solution) against fungal infections. Patients with febrile neutropenia were initially treated with fourth generation cephems or carbapenems. Response of initial therapies was evaluated every 3–5 days and antibiotics were altered if indicated, and indications for glycopeptides were checked up on each evaluation. Definitive diagnosis of bacterial infection is made when more than two blood cultures yielded the pathogen. Suspected bacterial infection is defined as either of the following cases: (1) when the bacteria showed abnormal growth in terms of quantity and bacterial species from sputum, stool, or urine and (2) when radiological findings of lung showed infiltrative shadow. The detailed information of the species of bacteria we detected is listed in Table 1.
2.3 Examinations for fungal infection
The hospitalized patients who fulfill one of the “host factors” of revised version of the Invasive Fungal Infection Group of the European Organization for Research and Treatment of Cancer and Mycosis study Group (EORTC/MSG) criteria [15] are assumed to be at high risk for invasive fungal diseases and are checked for vital signs daily and screened with β-d-glucan (Wako Pure Chemical Industries Ltd., Tokyo, Japan) and Aspergillus galactomannan (Platelia Aspergillus; Sanofi Diagnostics Pasteur, Marnes-La-Coquette, France) weekly. β-d-glucan test was judged positive if the measured value was >6.0 pg/mL. The threshold optical density index (ODI) of Aspergillus galactomannan was set 0.5 according to our investigations and that of others [16, 17]. CT scan of the lung was performed when patients remained febrile for 3–5 days despite broad-spectrum antibiotics, or when abnormal shadow emerged in routine chest X-ray examinations, or when fungal marker elevated to the significant level. The halo sign is defined as a macronodule with diameter >1 cm and is surrounded by a perimeter of ground-grass opacity [18].
2.4 Voriconazole administration
At first, voriconazole was administered orally unless patients were incapable of oral intake. Intravenous route was selected at the physician’s discretion that oral preparations lead to insufficient concentration. Voriconazole dose was initially set in accordance with manufacturer’s instructions, and was adjusted by taking prior TDM results into consideration.
2.5 Measurements of voriconazole plasma concentrations
Voriconazole trough plasma concentrations were studied in citrated plasma by high-performance liquid chromatography (TSL Inc., Tokyo, Japan) [19].
2.6 Evaluation
Overall efficacy was determined according to the criteria proposed by Nikolaos et al. [20]. Briefly, we applied composite outcome assessment in which non-responders are defined as having infection associated with worsening of at least 2 of the following 3 types of criteria: clinical, radiologic, and mycologic. Clinical failure was defined as either development of new fever or persistent fever for at least 1 week after voriconazole initiation or dose adjustment. More than 25% expansion of abnormal shadow area in CT image was assumed radiologic failure. Mycological failure included a rise within the abnormal range or positive conversion of serum markers (either β-d-glucan or galactomannan).
Adverse events, including hepatic damage and their causal relationship with voriconazole therapy were defined according to the criteria of the National Cancer Institute (NCI) [21]. Liver damage was judged when serum glutamic oxaloacetic transaminase (AST), serum glutamic pyruvic transaminase (ALT), gamma glutamyl transpeptidase (GGT), or serum bilirubin elevation was in grade 2–4 according to the NCI criteria.
2.7 Statistical methods
The relationship between the administered dose per patient body weight (mg/Kg) and the trough concentration (mg/L) of voriconazole was illustrated with a scattergraph method and determined with a coefficient of determination. Furthermore, each assessment was distinguished according to basal renal function (glomerular filtration rate (GFR) <75 mL/min or not), liver excretion (total bilirubin (T.Bil.) >0.65 mg/dL or not), and age (>64 years old or not) of the patients before voriconazole administration. These thresholds were the median of all the subjects except for ages.
The relationship between trough concentration of voriconazole and treatment efficacy or liver damage was analyzed with logistic regression model. p values are one-sided, with a type I error rate fixed at 0.05. Statistical analyses were performed with R 2.6.1 software (The R Foundation for Statistical Computing, 2007). This study was approved by the Institutional Review Board of Tokyo University.
3 Results
3.1 Reasons for voriconazole administration
The reasons for administering voriconazole were proven fungal infection in one (Candida parapsilosis in blood cultures), probable in five, possible in 29 according to the 2008 EORTC/MSG criteria. The remaining 14 cases did not meet these criteria, but voriconazole was applied because of persistent fever despite broad-spectrum antibiotics in 11 patients (10 with neutropenia, and one without), and positive aspergillus galactomannan tests in three cases.
3.2 Measurements of voriconazole plasma concentrations
A total of 49 trough voriconazole concentrations were assessed from 34 immunocompromised patients with hematologic diseases. The detailed information of the patients and underlying conditions are shown in Table 1. In sputum from patients suspected to bacterial combined infection, we detected enterococcus faecalis (resistant to third generation cephems and calbapenems) in sputum of one case and pseudomonas aeruginosa (resistant to fluoroquinolons and third generation cephems in two cases, resistant to carbapenems in one case).
TDM was checked once for 22 patients, twice for nine patients, and three times for three patients. The median concentration was 3.01 mg/L (range 0.22–12.77). TDM was assessed at a median of 9 days (range 3–40) after voriconazole was started or continued with dose adjustment. Information of TDM that was assessed after several weeks of voriconazole administration or dose adjustment was included because TDM in a steady state was considered constant.
3.3 Dose–concentration relationship
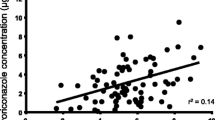
No correlation was found between administered dosage (per patient weight) and trough concentration of voriconazole as shown in Fig. 1a (coefficient of determination: r 2 = 0.058). Although >90% of voriconazole is eliminated into urine, we found that renal function (determined by glomerular filtration rate) has no effect on TDM (Fig. 1b). Likewise, bilirubin level or age did not influence voriconazole concentration (Fig. 1c, d).
Relationship between voriconazole dosages per patient weight and voriconazole trough plasma concentration (a). Each point represents a measurement. The linear regression curve is presented with coefficient of determination (r 2). Each measurement is discriminated according to whether glomerular filtration ratio (GFR) (b), total bilirubin level (T.Bil) (c), and patient age (d) of each subject is over the median value or not
Intra-patient dose–concentration relationships are shown in Fig. 2. Eight out of 10 patients who had voriconazole dosage increased according to the previous TDM demonstrated higher concentrations (Fig. 2, black lines), but two patients did not show elevation of voriconazole concentration despite dose escalation (Fig. 2, gray lines).
Intra-patient relationship between voriconazole dosages per patient weight and voriconazole trough plasma concentrations. Each point represents a measurement and repetitive measurements in a patient are linked by lines. Voriconazole concentration was positively correlated with administered dose in eight patients (black lines), however, not in two patients (gray lines)
3.4 Clinical efficacy
Fever resolution after voriconazole therapy was observed in eleven out of 32 cases (34%). Improvement in CT findings was shown in 16 out of 46 cases (35%, no radiological finding was observed in three cases). More specifically, cases which showed nodular shadow in chest CT revealed 41% (13 out of 32 cases) improvement in CT findings and cases which showed halo sign revealed 44% (four out of nine cases).
3.5 Relationship between efficacy and TDM of voriconazole
Using the composite outcome assessment [20], there was no definite correlation between voriconazole TDM and efficacy (Fig. 3a). However, when we conducted a sub-population analysis where subjects with refractory underlying hematological diseases were omitted, voriconazole concentration was significantly correlated with the efficacy (Fig. 3b). Concretely, none of the cases with TDM >2 mg/L failed to respond to voriconazole while two out of six cases with TDM below this threshold were non-responders. This indicates that 2 mg/L is the lower limit of voriconazole TDM to ensure adequate efficacy.
The influence of voriconazole trough concentrations on the efficacy for fungal infections. All the subjects are included in analysis (a), or those with refractory underlying hematological diseases are excluded (b). The curve in b represents the logistic regression model predicting the probability of treatment failure for each concentration
3.6 Side effects
There were no severe adverse events except temporal visual disturbance and elevation of hepatic enzymes. We observed a trend for increased hepatic toxicity in cases with high voriconazole TDM as shown in Fig. 4; this adverse event was observed in eight out of eleven cases with 6 mg/L or higher.
4 Discussion
We report our experience with voriconazole TDM in 39 Japanese adult patients with hematological disorders. According to this study, no significant correlation was observed between voriconazole dosage and trough concentrations. This relationship was not affected by renal function, liver function, or patients’ age. These results indicate that predicting voriconazole concentration is difficult, and that we have to monitor trough concentration and adjust voriconazole dosage according to the results. Additionally, our results indicate that dose–concentration relationship is inconstant among different patients and this highlights the necessity of TDM after each dose-adjustment. Presumably, this volatility of concentration is derived from the fact that a wide variety of drugs interact with voriconazole through inhibiting its metabolizing enzymes or transporters [22]. From this viewpoint, it is seen that repetitive TDM is desirable in each occasion of changing medications that are known to interact with voriconazole. It has not been elucidated whether administration route influences voriconazole TDM, and only a provisional investigation with a limited number of healthy volunteers indicated that the oral bioavailability of this drug is almost 100% [23]. Although systemic approach for this effect is impossible in our series because only a few patients received voriconazole intravenously, we experienced a case in which altering administration route from oral (10.53 mg/kg/day) to intravenous (8.42 mg/kg/day) effectively enhanced the voriconazole concentration from 0.22 to 0.64 mg/L and improved clinical efficacy. Further analysis of oral bioavailability in patients who are under treatment is required to clarify this point.
Our results imply that the clinical state of the underlying hematological disease had the tendency to affect the outcome of anti-fungal therapy. Ten out of the 31 cases with refractory hematologic diseases had not responded to voriconazole, while only two out of the other 18 cases had no response (refractory rate : 32 vs. 11%, p = 0.168). The concentration–efficacy relationship was observed only when cases with non-refractory underlying diseases were subjected to analysis. Under this condition, 2 mg/L was considered the lower limit of voriconazole concentration to secure its efficacy. In contrast, concentration seemed to have no effect on antifungal effect for those with refractory hematological diseases. In fact, voriconazole concentration was high (>8 mg/L) in eight out of 10 refractory cases in which voriconazole was not effective. Although provisional as yet, the advanced status of the underlying disease would affect the basal immunological function through prolonged neutropenia or accumulated administration of chemotherapeutic agents.
Defining non-responsive fungal infection is a challenging issue because of several reasons as appropriately pointed out by Almyroudis et al. [20], and we applied composite outcome assessment according to their recommendation as described in Sect. 2. One of the major points under discussion is the paradoxical change of radiological findings; sequential CT findings showed that the fungal lesions expand in the first week of treatment and remain stable during the second week in spite of effective antifungal treatment [24]. This is referred to as Pulmonary Immune Reconstitution Inflammatory Syndrome (PIRIS) [25] and poses a challenge to the proper evaluation of the effectiveness of anti-fungal treatment with radiological basis only. Indeed, we experienced a case in which temporal expansion of fungal lesion was observed simultaneously with neutrophil recovery and marked shrinkage of the lesion followed subsequently (Fig. 5). In this case, monotonic decrease of Aspergillus galactomannan ODI and CRP showed successful treatment of voriconazole. Another issue to be discussed is the limited usability of mycological markers. For example, β-d-glucan tests can be false-positive in patients who received plasma components [26] or amoxicillin clavulanic acid [27]. Similarly, administration of piperacillin/tazobactum can lead to false-positive marks of aspergillus galactomannan [28]. These facts highlight the risk of judging clinical responses with one barometer and show the relevance of introducing composite outcome assessment.
Pasucual [13] reported that there is an increase in neurological toxicity when voriconazole trough concentration is over 5.5 mg/L. Imhof [29] reported that the trough concentration under 4.0 mg/L is appropriate to avoid neurological toxicity. However, we observed no severe neurological toxicities except temporal disturbances of vision. In contrast, our present study indicates that there is a clear increase in hepatic damage when voriconazole trough concentration is over 6 mg/L, although Pascual’s report did not reveal a clear relationship between voriconazole trough concentration and frequency of hepatic damage. It is possible that concentration–toxicity relationship shows different profile among different races.
Our study has several limitations. (1) We had a limited number of cases, especially in the sub-population analyses. (2) Reasons for voriconazole administration were wide-ranging from target therapy for a proven case of EORTC/MSG criteria to empirical medications, (3) This is a retrospective study, and terms of voriconazole administration and strategy for dose-adjustment was under the discretion of attendant physicians. So, further study with prospective design and larger subjects is warranted to clarify the role of voriconazole TDM.
In conclusion, the present study shows the significance of monitoring voriconazole trough concentrations in patients with hematological disorders who receive voriconazole treatment. Because neither inter- nor intra-patient relationship is allowed between voriconazole dosage and TDM, monitoring should be executed not only in the initial evaluation but also after each change of dose and/or route of voriconazole administration. Moreover, voriconazole trough concentration should be kept 2–6 mg/L to improve the efficacy and safety.
References
Patterson TF, Kirkpatrick WR, White M, et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus study group. Medicine (Baltimore). 2000;79:250–60. doi:10.1097/00005792-200007000-00006.
Hachem R, Hanna H, Kontoyiannis D, Jiang Y, Raad I. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer. 2008;112:2493–9. doi:10.1002/cncr.23466.
Chamilos G, Luna M, Lewis RE, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003). Haematologica. 2006;91:986–9.
Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–15. doi:10.1056/NEJMoa020191.
Perfect JR, Marr KA, Walsh TJ, et al. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003;36:1122–31. doi:10.1086/374557.
Powers JH, Dixon CA, Goldberger MJ. Voriconazole versus liposomal amphotericin B in patients with neutropenia and persistent fever. N Engl J Med. 2002;346:289–90. doi:10.1056/NEJM200201243460414.
Purkins L, Wood N, Ghahramani P, Greenhalgh K, Allen MJ, Kleinermans D. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob Agents Chemother. 2002;46:2546–53. doi:10.1128/AAC.46.8.2546-2553.2002.
Dodds Ashley ESLR, Lewis JS, Martin C, Andes D. Pharmacology of systemic antifungal agents. Clin Infect Dis. 2006;43:28–39. doi:10.1086/504492.
Geist MJ, Egerer G, Burhenne J, Mikus G. Safety of voriconazole in a patient with CYP2C9*2/CYP2C9*2 genotype. Antimicrob Agents Chemother. 2006;50:3227–8. doi:10.1128/AAC.00551-06.
Murayama N, Imai N, Nakane T, Shimizu M, Yamazaki H. Roles of CYP3A4 and CYP2C19 in methyl hydroxylated and N-oxidized metabolite formation from voriconazole, a new anti-fungal agent, in human liver microsomes. Biochem Pharmacol. 2007;73:2020–6. doi:10.1016/j.bcp.2007.03.012.
Shimizu T, Ochiai H, Asell F, et al. Bioinformatics research on inter-racial difference in drug metabolism I. Analysis on frequencies of mutant alleles and poor metabolizers on CYP2D6 and CYP2C19. Drug Metab Pharmacokinet. 2003;18:48–70. doi:10.2133/dmpk.18.48.
Smith J, Safdar N, Knasinski V, et al. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother. 2006;50:1570–2. doi:10.1128/AAC.50.4.1570-1572.2006.
Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201–11. doi:10.1086/524669.
Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8. doi:10.1200/JCO.2006.08.2305.
De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi:10.1086/588660.
Kawazu M, Kanda Y, Nannya Y, et al. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1→3)-beta-d-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J Clin Microbiol. 2004;42:2733–41. doi:10.1128/JCM.42.6.2733-2741.2004.
Marr KA, Balajee SA, McLaughlin L, Tabouret M, Bentsen C, Walsh TJ. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J Infect Dis. 2004;190:641–9. doi:10.1086/422009.
Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–9. doi:10.1086/509917.
Pascual A, Nieth V, Calandra T, et al. Variability of voriconazole plasma levels measured by new high-performance liquid chromatography and bioassay methods. Antimicrob Agents Chemother. 2007;51:137–43. doi:10.1128/AAC.00957-06.
Almyroudis NG, Kontoyiannis DP, Sepkowitz KA, DePauw BE, Walsh TJ, Segal BH. Issues related to the design and interpretation of clinical trials of salvage therapy for invasive mold infection. Clin Infect Dis. 2006;43:1449–55. doi:10.1086/508455.
National Cancer institute. Serious adverse events definitions. In Edition National Cancer Institute 2006.
Nivoix Y, Leveque D, Herbrecht R, Koffel JC, Beretz L, Ubeaud-Sequier G. The enzymatic basis of drug-drug interactions with systemic triazole antifungals. Clin Pharmacokinet. 2008;47:779–92. doi:10.2165/0003088-200847120-00003.
Perea JR, Diaz De Rada BS, Quetglas EG, Juarez MJ. Oral versus intravenous therapy in the treatment of systemic mycosis. Clin Microbiol Infect. 2004;10(Suppl 1):96–106. doi:10.1111/j.1470-9465.2004.00846.x.
Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001;19:253–9.
Miceli MH, Maertens J, Buve K, et al. Immune reconstitution inflammatory syndrome in cancer patients with pulmonary aspergillosis recovering from neutropenia: Proof of principle, description, and clinical and research implications. Cancer. 2007;110:112–20. doi:10.1002/cncr.22738.
Obayashi T, Yoshida M, Tamura H, Aketagawa J, Tanaka S, Kawai T. Determination of plasma (1→3)-beta-d-glucan: a new diagnostic aid to deep mycosis. J Med Vet Mycol. 1992;30:275–80. doi:10.1080/02681219280000361.
Mennink-Kersten MA, Warris A, Verweij PE. 1,3-beta-d-glucan in patients receiving intravenous amoxicillin-clavulanic acid. N Engl J Med. 2006;354:2834–5. doi:10.1056/NEJMc053340.
Sulahian A, Touratier S, Ribaud P. False positive test for aspergillus antigenemia related to concomitant administration of piperacillin and tazobactam. N Engl J Med. 2003;349:2366–7. doi:10.1056/NEJM200312113492424.
Imhof A, Schaer DJ, Schanz U, Schwarz U. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med Wkly. 2006;136:739–42.
Acknowledgments
We appreciate agency of Pfizer Japan Inc. and TSL Inc. for measuring voriconazole concentrations. We declare that we have no competing interests.
Conflict of interest statement
The authors do not have any conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ueda, K., Nannya, Y., Kumano, K. et al. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int J Hematol 89, 592–599 (2009). https://doi.org/10.1007/s12185-009-0296-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-009-0296-3