Abstract
Total Knee Arthroplasty (TKA) is a highly successful surgical procedure with more than 600,000 TKA’s performed annually in the US. Interest in improving surgical outcomes has led to improvements in surgical technique, instrumentation, and implant design. Computer navigation and robotic systems were introduced to further refine the mechanical alignment of joint replacement procedures. The cost to implement some of these technologies and the additional time required in the operating room to utilize these developments has limited the acceptance of them broadly. The introduction of custom instrumentation and cutting blocks based on computed tomography (CT) or magnetic resonance imaging (MRI) has allowed for better restoration of mechanical alignment. Unfortunately, little has changed in patient satisfaction in the past ten years. The recent introduction of patient specific instrumentation and patient specific implants is another step forward to restore the pre-deformity anatomy and joint geometry. This new technology can benefit the hospital by improving operating room time efficiencies through having shorter set-up times, and the elimination of cleaning, sterilization and inventory costs. The patient can potentially benefit by a shorter operative time, improved postoperative alignment and better fitting implants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traditionally, custom implants have been used to treat unusual cases when a standard ‘off the shelf’ implant would not fit. This would have been for patients with juvenile rheumatoid arthritis (JRA), unusual bone geometry, or extremely large or, extremely small patients. Customized implants have also been used for the repair of previously failed implants where additional fixation features are required to adequately secure the revision implant to the bone. They have also been used in revision surgery where bone erosion is present and the standard implant cannot adequately fill the space left behind from the failed implant. Custom implants in the past would be custom in the area of bone fixation; however, the bearing surface was always standard using standard, fixed geometries similar to those used for primary TKA.
The typical process used to manufacture the custom implants was to supply radiographs to the implant manufacturer with embedded measurement devices visible within the field of the radiograph. Measurements were then taken from the radiograph image and used to design the custom implant. Radiographic templates were then supplied back to the surgeon that is representative of the proposed design. A written approval was generally required from the surgeon before manufacture of the implant could begin.
In more recent years the need for customization of implants for revision needs has diminished due to the prevalence of modular implant systems.
The present technology for providing data to manufacture a patient matched implant or instrumentation system is with magnetic resonance imaging (MRI) or computer tomography (CT) scans. There are advantages to both methods, but each is better suited to a particular type of joint analysis.
In both cases, MRI and CT, the scan data is provided to the implant manufacturer. This data is then converted into a design engineering friendly software system through a process referred to as segmentation. The segmentation process relies on laying individual points along the outer surface of each individual scan slice. When the entire joint surface has been segmented, a point cloud has been created that is representative of the outer surface of the joint. The point cloud is then imported into a traditional computer assisted design (CAD) program. The point cloud that now resides within the CAD system is then converted into a solid model that can be used to design a product around.
The MRI data is better suited for the analysis of soft tissue. This means that the segmentation process is typically performed on the surface of the articular cartilage.
The CT scan is better suited for the imaging of hard tissue. In the case of the CT scan, the segmentation process is performed on the subchondral surface creating an image of the bone.
At present, the customized implant market in the United States encompasses unicompartmental, bicompartmental, patello-femoral, and total knee replacement implants. The customized knee instrumentation market is comprised of primary placement jigs to set the initial femoral and tibial bone cuts. All 5 of the major implant manufacturers and many of the smaller implant companies have some form of customized instrumentation available for both partial and total knee replacement.
Total knee arthroplasty is a highly successful medical procedure. Annually in the United States more than 600,000 procedures are performed. However, patient satisfaction is as not as high as the survivorship suggests it should be. Factors such as persistent pain and unsatisfactory function have been identified that influence the patient’s satisfaction. Mahoney [1] has reported that implant overhang of greater than 3 mm is associated with a twofold increased risk of a painful outcome 2 years after surgery.
The orthopedic community has been searching for surgical techniques that can provide a more reliable method for installing implants into proper alignment and implant position. Studies have shown that alignment of the lower limb can have a significant effect on survivorship [2, 3]. Mason [4] has suggested that a mechanical alignment of neutral with a varus to valgus tolerance of ±3° is preferred. In the 1980s, highly instrumented knee systems became the standard of care for total knee replacement. These instrument systems allowed a cook book approach to knee replacement, making the installation more reproducible and provided reliable restoration of mechanical alignment. Between 1995 and 2005 the application of computer technology became more applicable to orthopedic implant procedures, spawning the first surgical robots and then surgical planning and navigation. Most orthopedic robots have not gained widespread acceptance. This is primarily due to the cost to acquire the devices, the set up time required preoperatively, and the limited application they presented. Computer navigation on the other hand enjoyed several years of active market acceptance, with many major and minor orthopedic suppliers having them available for commercial use. The navigation systems were successful in closing the gap for postoperative mechanical alignment by eliminating the outliers on the distribution curve; however, they still required a significant capital outlay for the medical institution, additional preoperative set up, and are cumbersome in the operating room, taking up substantial floor space. Thus, the use of computer navigation in the operating room has declined sharply in the last 5 years.
With the desire to improve patient satisfaction, new technologies have been investigated. Gender specific implants were introduced in an attempt to better accommodate the infinite variation in patient geometry. At the dawn of joint replacement surgery, implant sizes were limited to a few sizes. The Total Condylar (Howmedica, Rutherford, NJ), when first introduced in 1974, was available in just 3 sizes. By the time the PFC (Johnson & Johnson, Raynham, MA) came along in 1986, it was available in 6 sizes, and had left and right components. Today, total knee replacement systems can have as many as 8 to 10 femoral sizes in left and right components and a similar number of tibial base plate sizes. These size variations do not take into account for the variations in the level of constraint that may be available within a knee system. These variations can easily triple the femoral components available within a knee system. All of the size and constraint variation is accompanied by mountains of reusable instrumentation and instrument trays. The financial investments required for the companies to purchase and maintain this inventory is substantial.
Total hip arthroplasty procedures in the US number about 300,000. Patient satisfaction in hip replacement patients is much higher than for total knee patients. This may be due to the near anatomic restoration of the joint total hip that arthroplasty achieves compared with total knee arthroplasty. The natural hip geometry is a ball in socket joint, and the hip replacement product is also a ball in socket. When natural soft tissue balance is achieved in the hip surgery, the replaced joint moves in the identical path that the native joint did prior to the disease process. The same cannot be said for knee replacement patients. Bourne [5•] has reported a dissatisfaction rate for their total knee replacement patients to be 19 %, while Noble [6] has reported a dissatisfaction rate of 14 % for their patients. Scott [7] has reported that his patients that have both a unicondylar replacement in one knee and a total knee in the opposite leg prefer the unicondylar replacement over the TKR. This is due to several factors. In the case of the unicondylar replacement, the anterior cruciate ligament is preserved, thus retaining all of the natural structures of the knee. The unicondylar replacement also is more of a resurfacing of the distal and posterior condyle, thus retaining 60 % of the natural articular surfaces of the patient’s knee. These 2 factors work in harmony to provide a repair that not only moves in a very similar fashion to the patient’s pre-disease state, but also feels more like the patient’s natural knee.
In the case of the total knee replacement, a significant alteration to the pre-diseased anatomy occurs. The anterior cruciate ligament is always excised and in 65 % of cases the posterior cruciate ligament is removed. The articular surface of the tibial plateau is placed perpendicular to the mechanical axis, which is about 3° of valgus from the natural alignment. The femoral component is placed in external rotation, primarily to account for the larger lateral gap created by the valgus cut on the tibial plateau. And the size and shape of the femoral component is an engineered interpretation of what an average distal femur is geometrically. When viewed in the sagittal plane, the natural human knee has a medial condyle that is typically more distal and has a larger radius than the lateral condyle. The lateral condyle is typically shorter in the anterior to posterior direction. The medial condyle is also wider in the coronal plane than the lateral condyle. Most knee femoral components do not respect these anatomic differences in favor of a simplified near symmetrical design. All of these factors conspire to produce a motion in the total knee replacement patient that is different from what the patient had in the pre-disease state. Several publications have shown this in in-vivo kinematic studies [8–10].
The goal of customized knee surgery should be to restore the patient’s knee to as close as possible to their pre-disease state, corrected for any underlying deformity. Presently customized total knee replacement advertised by the large implant manufacturers are actually customized placement jigs for a specific patient using standard off-the-shelf knee implants. Either a CT or MRI is taken of the patient’s knee. The data is processed in a computer assisted design (CAD) system, and the primary femoral and tibial placement jigs are designed and manufactured for that patient. Once the primary distal femoral cut and proximal tibial cut are completed, the remaining bone preparation is accomplished with standardized off the shelf instrumentation. The case is then completed by implanting standardized off the shelf implants.
Review of recent publications
Radermacher [11] published the use of an individual template for the placement of an implant in 1998. He showed that computerized tomography (CT) data can be used to produce an orthopedic instrument for use with pedicle screws, triple pelvic osteotomies, and knee replacements. Howell [12] used MRI images to create customized tibial and femoral cutting guides using standard implants aligned to the articular surfaces. As of today, all of the major orthopedic manufacturers have introduced a customized instrumentation option for their standard knee replacement products. ConforMIS Inc., (Burlington, MA), has taken the patient specific concept further by offering a true patient matched implant and instrumentation system.
Hafez [13] demonstrated experimentally in 2006 that a simple pair of patient shape matched instruments can be used to install a total knee replacement. In their study they scanned 16 cadaveric knees and 29 plastic knee models. A set of femoral aligning and cutting guides as well as a tibial cutting guide were fabricated by selective laser sintering. Surgery was performed on all of the cadavers and plastic bone models. In 6 randomly selected postoperatively CT scanned cadaveric legs, the maximum deviation from the planned alignment was 2.3° and the maximum deviation from the planned resection thickness was 1.2 mm. They also used the preoperative planning process to determine which size implant would be used. They determined that their method was accurate in predicting both the femoral component and tibial component size, but determining tibial liner thickness was not accurate. This outcome is not unexpected as the CT method can accurately determine bone condition and spatial alignment, yet it cannot assess soft tissue condition which would affect the selection of the tibial insert required to achieve the desired balance and laxity.
Sisto [14] described a CT based customized patella-femoral implant system in a 2006 paper. In this publication, they reported on 25 implants in 22 patients with a mean follow-up of 23 months. Sisto describes a process where a CT of the knee is taken that is used to create a model of the subject distal femur. The model is sent to the surgeon, who can use it to recommend which osteophytes are to be removed. The bone model is returned to the implant manufacturer who then produces the customized patella femoral implant. At the time of publication, no revisions had been performed and the mean Knee Society functional score was 89 points and the mean Knee Society objective score was 91 points.
Lombardi [15] described a set of MRI based instruments which place Steinman pins in the distal femur and proximal tibial plateau at the Current Concepts in Joint Reconstruction meeting in December of 2007. In his presentation, he describes a process where a MRI is taken of the patient’s hip, knee and ankle and a map of the knee joint is constructed within a CAD software program. The implant size is derived from the size of the virtual knee model. A virtual surgery of the knee is conducted where the distal femoral cut and proximal tibial cut are placed perpendicular to the mechanical axis, which has been derived from the hip and ankle data. This basic plan is then provided to the surgeon where they can make adjustments to the operative plan. Upon approval of the plan, custom tibial and femoral pin placement guides are fabricated using a rapid prototype method. The customized pin placement guides are used to establish the placement of the distal femoral cut in the coronal plane and the proximal tibial cut in the same plane. Standard ‘off the shelf’ implants are used and the remaining bone preparation is performed using standard reusable instrumentation. Lombardi [16] later published his preliminary results of their first 273 cases. They compared 91 patients that underwent total knee replacement with the custom pin guides to 91 patients that underwent a standard total knee replacement surgery. In a postoperative radiographic study of the 182 patients, they concluded that the custom jigs produced a more accurate result that is statistically significant compared with standard instrumentation. Furthermore the custom jigs produced less outliers (1.1 %, 1/91 %) compared with the standard instrumentation group (4.4 %, 4/91 %).
Howell [12] published his initial experience with a custom-fit positioning jig set on 48 patients in September of 2008. In his procedure a MRI is obtained of the diseased knee. The MRI image is converted to a virtual model of the knee that includes osteophytes, cartilage, and bone. The virtual knee model is then converted into a ‘naturally aligned’ model by removing the osteophytes, filling articular defects, and approximating the pre-arthritic joint surface. A secondary software routine is then employed to shape match the bone models to a library of standard off the shelf knee components. Of particular interest here is the implants are aligned to the patient’s natural geometry, and are not necessarily placed perpendicular to a reconstructed mechanical axis. The primary bone resection planes from the femoral component and tibial plateau are transferred to the bone model. The bone model, with the distal femoral cut and the proximal tibial plateau cut, is then used to model custom cut guides for each of the primary bone cuts. The pin holes are also placed in the femoral and tibial cut guide to provide transverse rotation of the implants according to the shape matched position of the selected femoral implant. Howell later demonstrated this technique via a live surgery feed at the Current Concept in Joint Replacement meeting in May of 2009.
Klatt [17] reported on 4 patients that underwent the OtisMed surgical technique utilizing the MRI based custom cutting guides to implant Triathlon (Stryker, Mahwah, NJ) knee implants. All 4 patients underwent preoperative MRI that was provided to the manufacturer. Custom guides were fabricated according to the standard protocol of the manufacturer. Intraoperative navigation was employed to measure the position of the bone cuts relative to the mechanical axis. The recommended femoral valgus angle ranged from 5.5° of valgus to 0.5° of varus and the recommended flexion cut ranged from 4° to 9° of flexion. The femoral rotation was within 1° of the epicondylar axis. The recommended tibial positioning of the guides ranged from 3° of varus to 7.5° of valgus. The recommended posterior slope ranged from 5.5° of anterior slope to 0.5° of posterior slope. Postoperative radiographic evaluation of the tibial component was assessed with the posterior slope ranging from 1° of posterior slope to 8° of anterior slope. Postoperative evaluation of the femoral component was not reported. From these results they concluded that this system does not follow accepted standards for postoperative alignment to the mechanical axis and therefore these patients are at risk for premature failure.
Noble [18] has shown that postoperative alignment can be improved with a customized instrumentation set as compared with a standard instrumentation set. In this study, 15 patients were operated on with a customized instrument set and 14 randomly selected patients were operated on with a standard instrumentation set for their primary total knee replacements. The customized instrument group obtained closer to neutral mechanical alignment than the standard instrumentation group. The customized group also benefited from a shorter hospital stay, shorter operative time, shorter incision length, and the use of fewer instrument trays compared with the standard instrumentation group. Although this study is small in scope, it certainly demonstrates what is possible with a patient customized system.
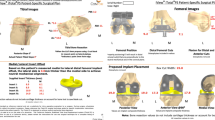
Fitz [19] described a patient specific unicompartmental implant and instrumentation system. In this paper, he described a CT based method to design a patient specific resurfacing unicompartmental implant. A CT is acquired of the patient’s hip knee and ankle according to the recommended protocol. The CT data is segmented using proprietary software and converted into a solid CAD model. A secondary proprietary software system is utilized to design the patient specific femoral and tibial components. The patient’s own biomechanical and anatomic axes are utilized to place the cut planes that allow restoration of a neutral mechanical axis. The femoral implant geometry in the sagittal plane follows the patient’s natural geometry while it has an engineered coronal radius to reduce contact stress. The tibial component is designed to cover the cortical rim of the cut tibial plateau, achieving cortical bone support close to100 %. The articular geometry of the tibial bearing is derived from the femoral component to provide a truly patient specific implant. The single use instrumentation is also designed from the CT data and is supplied sterile with the implant.
In the surgical technique described by Fitz, the cartilage is removed from the femur and thus the femoral component sits directly on the subchondral bone. Perforations to the femoral bone are made to facilitate cement fixation. A small posterior femoral cut is made and conventional fixation lugs are provided on the component. This technique of placing the femoral component on subchondral bone preserves the joint line within 1 millimeter. Fitz reports the advantages of this system includes; (1) easy positioning of the implant in the exact anatomic position it was designed for; (2) precise alignment with both the anatomic and mechanical axes by referencing the hip and ankle joints; (3) replacement of the usual multi-tray instrumentation set with a small number of disposable and self-positioning guides; (4) facilitating the precise fitting of the femoral and tibial component in either the medial or lateral compartment, and (5) improved fit with use of patient specific implant components.
Discussion
The field has progressed to the point where all 5 major orthopedic manufacturers offer some variation of a customized instrumentation approach to standard joint replacement surgery. The rationale for pursuing the customized jig approach is supported from both economical as well as potential clinical benefits based on Noble’s published work.
The theoretical clinical advantages to using a patient matched instrumentation system include the elimination of the use of intramedullary rods to determine alignment. This in turn eliminates the pressure head generated when inserting an intramedullary rod as well as the associated blood loss. The elimination of reusable instruments also theoretically reduces the potential for transmission of infection on an instrument that may have been used hundreds of times.
In the case for a partial customized instrument set, which are now widely offered by the large orthopedic manufacturers, the cost advantages are not great. The typical partial customized instrumentation includes a single femoral positioning jig and a single tibial position jig. All remaining instrumentation is the reusable type requiring multiple trays in the operating room. Unless the manufacturers reconfigure the instrumentation sets to be patient specific with only the instruments and trials that will be required to install the predetermined implant into the patient, little cost or operational efficiency will be achieved in the operating room or ancillary support services such as sterile processing, with this approach.
Much greater economic benefits can be gained from the use of a complete patient specific instrument set as described by Fitz. In the case of the complete single use kit, the elimination of several reusable instrument trays can be achieved. Using a conservative estimate for a typical unicompartmental knee surgery, the standard set of instruments typically includes 6 reusable instrument trays. Unpublished cost data for the sterilization of each tray is believed to be at least $100, and in some cases in New York City the cost can be as high as $300. This adds between $600 and $1800 to the cost of a procedure, but is not presently considered in the actual cost of the procedure. In the approach described by Fitz, there is no additional charge for the customized instrumentation as it is included as part of the implant cost. The use of the complete patient matched system also eliminates the storage space needed in the hospital for both the implants as well as the instrumentation. Although these costs are difficult to calculate, they are assuredly substantial.
The theoretical advantages with a complete patient specific implant and instrumentation extend beyond what has been described here. A complete patient specific system can potentially save operative time, set up time, operating room space, and hospital space. The set up time is saved in the elimination of bringing multiple instrumentation sets to the operating room and opening them. In the event that a barrier breach is seen in a reusable tray, a backup tray is requested. This additional request for a replacement tray adds set up time and a delay in the start time for that procedure. This is eliminated with a complete single use patient specific instrument set.
Procedural time can be reduced in the complete patient specific system that includes all instrumentation and a patient specific implant by eliminating several time consuming steps. When the instrumentation and implants are completely patient specific, the implant sizing, rotation, and positional decisions are pre-determined. These implant attributes can be either based on a standard set of design rules, or could be surgeon customized as desired.
Space needs in the operating room are decreased substantially with the limited instrumentation and the elimination of the need for multiple implant trials and implant sizes. The complete patient specific set including custom instrumentation and implants utilizes approximately 1.5 square feet of the back table space. The typical 6 reusable instrument trays can take up 7 square feet of table space, and requires the stacking of trays upon each other. The multiple trays results in greater delays in pre-operative set up time, post-operative cleanup and turnover time in addition to the intra-operative search and find time.
Conclusion
We are at the dawn of the patient specific era in orthopedics. There are clear operative efficiencies that can be gained for all stake holders in the joint replacement hierarchy. The implant manufacturers gain cost efficiencies by eliminating enormous inventory investments. The hospitals benefit by improving time efficiencies with the shorter set-up times, and the elimination of cleaning, sterilization and inventory costs. With the use of patient specific rather than standard implants, the patient can potentially benefit by a shorter operative time, improved postoperative alignment and a better fitting implant that has the potential to restore near normal kinematics.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Mahoney MM, Kinsey T. Overhang of the femoral component in total knee arthroplasty: risk factors and clinical consequences. J Bone Joint Surg Am. 2010;92:1115–21.
Wasielewski RC, Galante JO, Leighty R, et al. Wear patterns on retrieved polyethylene inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31.
Ritter MA, Faris PM, Keating EM, et al. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153.
Mason JB, Fehring TK, Estok R, et al. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22:1097–106.
• Bourne RB, Chesworth BM, Davis AM, et al. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468:57–63. This study demonstrates that despite all of the technological advances made in total knee replacement surgery in recent years that patient satisfaction for total knee patients is far below what total hip replacement surgery delivers.
Noble PC, Conditt MA, Cook KF, Mathis KB. The John Insall Award: patient expectations affect satisfaction with total knee arthroplasty. Clin Orthop Relat Res. 2006;452:35–43.
Laurencin CT, Zelicoff SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. Clin Orthop. 1990;260:135.
Dennis DA, Komistek RD, Hoff WA, Gabriel SM. In vivo knee kinematics derived using an inverse perspective technique. Clin Orthop Relat Res. 1996;331:107–17.
Dennis DA, Komistek RD, Colwell CW, et al. In vivo anteroposterior femorotibial translation: a multi-center analysis. Clin Orthop. 1998;356:47–57.
Dennis DA, Komistek RD, Mahfouz MR, et al. Multi-center determination of in vivo kinematics after total knee arthroplasty. Clin Orthop. 2003;416:37–57.
Radermacher K, Portheine F, Anton M, et al. Computer assisted orthopedic surgery with image based individual templates. Clin Orthop Relat Res. 1998;354:28–38.
Howell SM, Kuznik K, Hull ML, Siston RA. Results of an initial experience with custom-fit positioning total knee arthroplasty in a series of 48 patients. Orthopedics. 2008;31:857–63.
Hafez MA, Chelule KL, Seedhom BB, Sherman KP. Computer-assisted total knee arthroplasty using patient-specific templating. Clin Orthop Relat Res. 2006;444:184–92.
Sisto DJ, Sarin VK. Custon patellofemoral arthroplasty of the knee. JBJS. 2006;88:1475–80.
Lombardi AV. Patient specific knee design: an evolution of computer navigation; current concepts in joint replacement, published abstract, Winter 2007 meeting.
Lombardi AV, Berend KR, Adams JB. Patient-specific approach in total knee arthroplasty; Orthopedics 2008;31:927–30.
Klatt BA, Goyal N, Austin MA, Hozack WJ. Custom-fit total knee arthroplasty (OtisKnee) results in malalignment. J Arthroplasty. 2008;23:26–9.
Noble JW, Moore CA, Lui N. The value of patient-matched instrumentation in total knee arthroplasty. J Arthroplasty. 2012;27:153–5.
Fitz W. Unicompartmental knee arthroplasty with use of novel patient-specific resurfacing implants and personalized jigs. J Bone Joint Surg Am. 2009;91:69–76.
Disclosure
J Slamin: employed and has patents and stock options with ConforMIS, Inc.; BS Parsley: consultant to Nimbic, Inc., receives royalties and travel expenses from ConforMIS, Inc., has stock/stock options with Nimbic and ConforMIS, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slamin, J., Parsley, B. Evolution of customization design for total knee arthroplasty. Curr Rev Musculoskelet Med 5, 290–295 (2012). https://doi.org/10.1007/s12178-012-9141-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-012-9141-z