Abstract
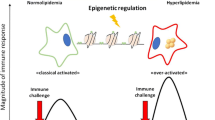
Plasma cholesterol levels have been strongly associated with atherogenesis, underscoring the role of lipid metabolism in defining cardiovascular disease risk. However, the atherosclerotic plaque is highly dynamic and contains elements of both the innate and adaptive immune system that respond to the aberrant accumulation of lipids in the subendothelial space. Previous research has focused on defining how proinflammatory cytokines synthesized by macrophages, such as interleukin-1β (IL-1β), modulate the progression of atherosclerosis, supporting the notion that chronic inflammation accelerates atherogenesis. More recently, emphasis has been placed on the elucidation of the mechanisms that contribute to pro–IL-1β production and finally its processing via multiprotein complexes termed the inflammasomes, a family of cytosolic multiprotein complexes that serve as sensors of either pathogen invasion or cellular stress (ie, cholesterol crystals) and work via triggering caspase-1–mediated processing of pro–IL-1β to IL-1β. Based on this link between cholesterol metabolism, NLRP3 inflammasome activation, and IL-1β release, it is important to re-evaluate how the atherogenic environment stimulates immune cells to produce IL-1β.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipid metabolism and atherosclerosis have been intimately linked and studied for many decades. However, the atherosclerotic plaque is by no means just a static accumulation of lipids [1]; instead, it contains elements of both the innate and adaptive immune system that respond to the aberrant accumulation of lipids in the arterial wall [2]. Of these cells, blood monocytes, which differentiate into macrophages when they enter the plaque, have been shown to play a key role in plaque progression [3]. Due to this recognition by researchers, the mechanistic understanding of atherosclerotic plaque progression has increased dramatically in the past decade. Much of this research has focused on defining how proinflammatory cytokines synthesized by macrophages, such as interleukin 1-β (IL-1β), modulate the progression of atherosclerosis, with the basic conclusion that inflammatory markers such as IL-1β accelerate atherogenesis. Despite all of this research, the mechanistic factors linking aberrant lipid metabolism to production of IL-1β and inflammation remained poorly defined. Within the past few years, there has been a flurry of publications elucidating the elements that contribute to IL-1β production due to a better understanding of the cell biology and biochemical processes that lead to its synthesis as pro–IL-1β and finally its processing via a multiprotein complex termed the inflammasome.
The inflammasomes are actually a family of different cytosolic multiprotein complexes that serve as sensors of either pathogen invasion or cellular stress and work via triggering caspase-1– mediated processing of pro–IL-1β to IL-1β [4]. The basic organization of these complexes involves two or three essential components depending on the Nod-like receptor (NLR) member in question. Because most of the work on atherosclerosis has focused on the NLRP3 inflammasome, we will limit our review to the complexes formed by these pyrin domain–containing leucine-rich–repeat sensors [5]. Caspase-1, the final enzyme responsible for processing pro–IL-1β, to IL-1β, contains a CARD domain but not a pyrin domain. Consequently, NLRP family members must use the adaptor protein ASC to nucleate and activate caspase-1. Despite the understanding of these essential components for IL-1β release, a complete mechanistic understanding for the specific triggering of NLRP3 by diverse ligands, from monosodium urate crystals to silica, is still unclear. The NLRP3 inflammasome is of particular interest in atherosclerosis due to a recent provocative article showing how cholesterol crystals, dependent on NLRP3 and other components of the inflammasome, can trigger IL-1β release [6•]. Furthermore, mice susceptible to atherosclerosis reconstituted with NLRP3- or ASC-deficient bone marrow exhibited reduced atherogenesis. Based on this novel mechanistic link between cholesterol and IL-1β release, it is important to once again re-evaluate how the atherogenic environment stimulates immune cells to produce IL-1β. One very important consideration in attempting to make this mechanistic link is that members of the NLRP3 inflammasome are not highly expressed in resting cells. Consequently, two signals are essential in order to drive IL-1β release: one mediating the expression of the components and the second their activation. Novel candidates for the first signal, traditionally delivered via lipopolysaccharide (LPS) in vitro, have recently been identified within commonly present plaque components. This review focuses on these two main signals, particularly within the context of atherogenesis and atherosclerosis.
Signal 1: Lipid Triggering of Toll-Like Receptors
The Toll-like receptor family is generally considered to have evolved in response to evolutionary pressure from pathogens and possesses the capacity to respond to a wide variety of pathogen-associated molecular patterns (PAMPs). Whereas endosomal TLRs that recognize double-stranded RNA (TLR3), ssRNA (TLR7), or dsDNA (TLR9) are tightly regulated via compartmentalization and processing steps to ensure that their encounters with host-derived ligands are kept to a minimum in order to avoid autoimmunity, lipid-binding TLRs such as TLR1, TLR2, TLR4, and TLR6 are present at the cell surface and ready to respond to bacterial ligands such as lipoteichoic acid (TLR2) or lipopolysaccharide (TLR4) [7]. However, the placement of this set of TLRs also allows them to interact with host-derived danger molecules that arise either from cellular damage (eg, danger-associated molecular patterns [DAMPs]) as well as modified versions of host lipids that have been shown to be present in the atherosclerotic plaque [8, 9]. Although the majority of TLR biology has been done in specialized cells of the immune system such as macrophages, conventional dendritic cells, or plasmacytoid dendritic cells, a growing body of evidence has highlighted the presence and functional significance of TLRs, particularly TLR2 and TLR4, on the endothelium and smooth muscle in atherosclerotic lesions [9, 10].
The significance of TLRs to inflammasome activation is that, at steady state, the components of the inflammasome (NLRP3, ASC, caspase-1), and in particular their target substrates (pro–IL-1β and pro–IL-18) are expressed at very low or undetectable levels [11]. As a result, it is necessary to induce the expression of the inflammasome components as well as pro–IL-1β and pro–IL-18 before triggering caspase-1 activation and target processing [11]. Some of the classical DAMPs that can interact with TLRs include Hsp60 and HMGB1, which are released from injured or necrotic cells [8]. Elevated levels of these DAMPs have been associated with increased risk of cardiovascular events [9]. Of particular interest are the more recent findings that saturated fatty acids as well as modified lipoproteins can act as endogenous ligands for TLRs [9]. As evidence for the interaction of dietary-derived ligands for TLRs mounts, perhaps it is possible to consider a new class of nutrition-associated molecular patterns or NAMPs. The importance of both DAMPs and NAMPs are underscored by evidence suggesting that apolipoprotein E knockout (ApoE KO) mice in a germ-free environment still develop atherosclerosis as ApoE KO mice do in specific pathogen-free barrier facilities [12]. With a greater understanding of the interaction between NAMPs and TLRs, some of the unknown mechanistic links between diet and inflammation may be clarified.
Some of the first studies that tested if TLR4, and its associated factors CD14 and MD-2, could respond to minimally modified low-density lipoprotein (mmLDL) provided inconclusive evidence for the role of TLR4 in the process of direct recognition of the putative ligands [13]. However, more recent work shows that a newly described heterodimer of TLR4 and TLR6, in cooperation with CD36, is actually the receptor complex for oxidized low-density lipoprotein (oxLDL) [14•]. This study provided convincing evidence that CD36/TLR4/TLR6 sufficient cells responded to oxLDL and maximally induced IL-1β mRNA whereas macrophages deficient in either TLR4 or TLR6 did not upregulate IL-1β in response to oxLDL [14•]. In response to mmLDL, macrophages also generate reactive oxygen species (ROS) in a TLR4-, Syk-, and Nox2-dependent pathway [15]. It is important to emphasize this finding because the ability of mmLDL and oxLDL to induce both IL-1β mRNA as well as ROS production in a TLR-dependent way provides two of the essential events prior to NLRP3 inflammasome activation and IL-1β release.
In terms of the effects of TLRs on atherogenesis, it has been shown that ApoE KO mice also deficient in TLR4 or MyD88 (a critical signaling adaptor for all TLR and IL-1R signaling except TLR3) show a reduced burden of atherosclerotic lesions [16]. Although the mice showed a reduced level of atherosclerosis and inflammatory mediators, they still suffered from hypercholesterolemia. This suggests that if it is possible to uncouple elevated lipid levels from their deleterious effects in triggering TLRs and providing priming signals for NLRP3 activation, perhaps it would be possible to ameliorate atherosclerosis. Subsequently, TLR4 in adipocytes and macrophages has been shown to be responsive to free fatty acids [17], and it has also been shown that that TLR4 is important in modulating the effects of a high-fat diet on vascular inflammation [18].
It has also been found that combinations of signals from scavenger receptors and TLRs result in differential signals in terms of cellular survival than do TLR signals alone, with a combined signal leading to proapoptotic signals that may contribute to enhanced plaque necrosis [19]. In this situation, SR-A and TLR4 signaling in endoplasmic reticulum (ER)-stressed macrophages led to an uncharacteristically proapoptotic signal rather than the normal survival signal that is typically mediated due to TLR4 stimulation [19]. Using a different combination of scavenger receptor and TLR, another study also showed that CD36 and TLR2 signaling concurrently leads to apoptosis in macrophages and subsequent plaque necrosis [20]. This study found that oxLDL, oxidized phospholipids, saturated fatty acids, and lipoproteins triggered apoptosis in ER-stressed macrophages and that LDL receptor KO mice transplanted with TLR2/4 double-KO bone marrow were protected from plaque necrosis in a model of advanced atherosclerosis [20].
Much attention has been given to the balance between saturated and polyunsaturated fatty acids in diet and their effects on cardiovascular disease risk. Several groups have shown that, with respect to TLRs, saturated fatty acids provide activating signals whereas omega-3 polyunsaturated fatty acids provide negative regulatory signals [21–23]. Integrating these findings with the recent identification of the omega-3 fatty acid sensor, GPR120, it is possible to hypothesize a model by which saturated fatty acids directly trigger TLR2, TLR4, TLR6 or certain heterodimers to provide a priming signal as LPS would for TLR4. Simultaneously, omega-3 polyunsaturated fatty acids signaling through GPR120 help to attenuate the proinflammatory effects of the fatty acids and thus may potentially limit the “Signal 1” necessary for NLRP3 inflammasome assembly [24•]. Within the context of the inflammasome and atherosclerosis, it will be of great interest to determine how the saturated fatty acid to omega-3 fatty acid ratio present in serum and atherosclerotic plaques integrates at the level of “Signal 1” to correlate with the expression of components of the inflammasome.
Human genetic evidence has shown that the TLR4 polymorphism Asp299Gly shows a reduced level of acute phase and proinflammatory cytokines with a reduced risk for carotid atherosclerosis [25]. However, after the publication of this article, other groups raised questions about the validity of the statistical analysis and TLR4 has not been highly replicated in the human genetics of atherosclerosis. In terms of other genes involved, CD36 (a member of the CD36/TLR4/TLR6 complex that is responsive to oxLDL) has been associated with a higher likelihood for metabolic syndrome [26]. Another scavenger receptor, SR-B1, has also been shown to determine levels of plasma lipoprotein concentrations and particle size [27]. With our current updated knowledge of the molecular wiring of Signal 1, it will be interesting to look at genes that are known to directly interact at the protein level to see how SNPs in one or several of the components interact at the level of risk factors for atherosclerosis as well as atherogenesis itself.
Signal 2: NLRP3 Activation in Atherosclerosis
Considering the atherosclerotic plaque as an unresolved inflammatory lesion, it is not surprising that one of the critical pathways mediating pro–IL-1β release has been strongly linked with the progression of atherosclerotic lesions. The first study to suggest that one of the inflammasome family members played a critical role in atherogenesis used ASC KO mice to show that mice deficient in this critical adaptor for inflammasome formation had significantly reduced neointimal formation after injury [28]. Furthermore, this work also characterized that IL-1β and IL-18 production from bone-marrow derived cells play a larger role than endothelial-derived cytokines in neointima formation [28]. This first study still left the unanswered question of which NLR family member is responsible for triggering the formation of the mature IL-1β processing machinery. The seminal paper in the inflammasome field that provided a critical hint for other investigators to follow demonstrated that the NLRP3 inflammasome is responsible for sensing monosodium urate crystals (MSU), the etiologic agent of gout [29]. Subsequently, it has been shown that other crystalline substances, such as asbestos, alum, and silica, are capable of triggering the NLRP3 inflammasome [30, 31]. This observation, along with others that followed, has sparked much debate as to the mechanism of how the NLRP3 inflammasome is activated, as MSU, asbestos, alum, and silica are structurally and chemically distinct substances. Two different yet complementary explanations to NLRP3 inflammasome activation have emerged, including ROS generation from NADPH oxidase as well as lysosomal damage releasing proteolytic enzymes such as cathepsin B into the cytosol [30, 31]. It is particularly interesting to consider the strong role that ROS play in NLRP3 inflammasome activation due to the link between oxidative stress and progression of atherosclerotic plaques [32].
Based on the evidence linking crystalline substances to NLRP3 inflammasome activation and the observation that minute cholesterol crystals are present in diet-induced atherosclerotic lesions, Duewell et al. [6•] published the provocative study linking crystalline cholesterol to activation of the NLRP3 inflammasome and to atherogenesis. Using reflection microscopy, cholesterol crystals were visualized both in the aortic sinus during high-fat diet–induced atherosclerosis in LDL-R KO mice as well as in macrophages that phagocytosed crystalline cholesterol [6•]. The most compelling data showed that LDL-R KO mice reconstituted with NLRP3-KO bone marrow showed aortic lesion sizes 2.5-fold smaller than mice reconstituted with wild-type bone marrow [6•]. The lesions were also smaller in size in mice transplanted with ASC-KO or IL-1α/β KO bone marrow [6•]. In most studies involving the NLRP3 inflammasome, it is common to use LPS as a “Signal 1” to prime cells to stimulate greater expression of inflammasome subunits as well as synthesis of pro–IL-1B. This study also provided an alternative mechanism via which mmLDL is capable of providing the priming signal, thus showing that NLRP3 inflammasome formation and IL-1β release can occur in macrophages due to endogenously present material within a nascent atherosclerotic lesion [6•]. Shortly after the publication of the role of NLRP3 in the murine model, another group recapitulated the findings in vitro by showing that human macrophages sense cholesterol crystals via the NLRP3 inflammasome as well, which should provide a strong impetus for therapeutics targeting NLRP3 inflammasome activation [33].
However, the role of the NLRP3 inflammasome in atherogenesis is still an unresolved matter and shows inter-model variability. A very recent study investigating the role of the NLRP3 inflammasome in atherosclerosis using the ApoE KO mouse model crossed to either NLRP3, ASC, or Caspase 1 KO mice followed by high-fat diet showed no differences in atherogenesis or plaque stability across genotypes [34•]. Although this study, for now, stands alone in finding no role for the NLRP3 inflammasome in atherogenesis, it will be important to reconcile some of the differences in the mouse models used as well as the use of double-KO mice compared to bone marrow chimeras. One potential explanation is that the deletion of ApoE from all cell types in this model, an essential protein involved in reverse-cholesterol transport from macrophages, is necessary for macrophages to remain in an inducible rather than constitutively active “M1” state with respect to proinflammatory cytokine secretion beyond IL-1β. If these macrophages accumulate so much cholesterol that they are constitutively secreting IL-6, IL-12, and other proinflammatory cytokines, then perhaps the lack of the NLRP3 inflammasome does not affect the disease course in this model. In agreement with this hypothesis, ApoE has been shown to be important in maintaining an “M2” phenotype in macrophages associated with tissue repair and depressing production of proinflammatory and thus proatherogenic mediators [35, 36]. HDL has also been shown to be directly anti-inflammatory to macrophages bearing functional ApoE by not only mediating M2 polarization but also migration out of the plaque [37]. Other data from independent groups continue to support both oxidative stress and NLRP3 inflammasome activation in the context of ApoE KO mice in atherogenesis [38]. It would be of great interest to see the results of an experiment where ApoE KO mice are transplanted with NLRP3-deficient but ApoE-sufficient bone marrow to isolate the defects in lipid metabolism from that of inflammasome activation. Future work may reveal the importance of lipid metabolism in monocyte to macrophage maturation or macrophage activity within an atherosclerotic plaque or even within the context of infection.
With respect to the mechanisms that contribute to NLRP3 inflammasome activation either preceding or in parallel with the sensing of cholesterol crystals, ROS have been shown to be a large contributor [30, 39]. One intriguing study demonstrates that damaged mitochondria can provide the source of ROS for inflammasome activation and furthermore postulates a connection between mitochondrial damage and chronic inflammatory diseases [39]. Very recent data suggest that ROS may actually be critical not for activating the NLRP3 inflammasome directly but instead for providing “Signal 1” leading to expression of NLRP3 and the other components of the inflammasome [40]. Because atherosclerosis has also been linked with mitochondrial dysfunction, it would be predicted that damage induced by nutritional stresses, such as hypercholesterolemia, or aging contribute significantly to ROS production and consequently lower the threshold required to trigger NLRP3 inflammasome activation [32]. Another pathway that has very recently been described for NLRP3 inflammasome activation shows that saturated fatty acids such as palmitate can induce its activation upstream of mitochondrial ROS and a signaling cascade controlling autophagy [41]. This study also showed that oleate, a polyunsaturated fatty acid, did not show the same activating effects that provide a link between dietary lipids and the mechanistic production of IL-1β and its effects on the progression of type 2 diabetes [41].
Although the pathogenesis of type 2 diabetes is distinct from that of atherosclerosis, they share common etiologic agents such as high-fat diet, and many individuals are affected by both diseases under the umbrella of metabolic syndrome. One recent study links obesity-induced inflammation in both humans and mice to insulin resistance via an NLRP3 inflammasome–mediated mechanism [42•]. Because very few studies have been published to date focusing on humans, this study is invaluable in demonstrating that exercise and calorie restriction lead to less NLRP3 expression in adipose tissue of humans and that this correlates with a decrease in IL-1β expression as well [42•]. NLRP3 KO mice were also protected from obesity-induced inflammasome activation in adipose tissue. The inflammasome has also been shown to play a role in the progression of type 2 diabetes via a thioredoxin-interacting protein (TXNIP)-initiated mechanism, an ROS sensitive trigger of NLRP3 that also has the ability to sense islet amyloid polypeptide (IAPP) [43, 44]. Individuals with type 2 diabetes may consequently be at even higher-risk for uncontrolled NLRP3 inflammasome activation at distal sites such as atherosclerotic lesions due to the elevated levels of circulating IL-1β produced in the pancreas and within adipose tissue, which can further accelerate the progression of atherosclerosis and vascular complications. In addition to these co-morbidities, risk factors and direct effectors generated during atherogenesis such as hyaluronan and serum amyloid A (SAA) have been shown to trigger the assembly of the NLRP3 inflammasome. This may provide a positive feedback loop to the system as both hyaluronan and SAA are acute phase proteins generated in response to IL-1β [45, 46].
Besides NLRP3, ASC, and caspase-1, pyrin (MEFV) has been reported to interact with inflammasome components in such a way that it helps to downregulate pro–IL-1β processing [47]. Patients with homozygous mutations in pyrin suffer from periodic fevers known as familial Mediterranean fever, and knock-in mice recently generated with human disease alleles demonstrate that mutant pyrin can constitutively activate IL-1β processing in an ASC-dependent yet NLRP3-independent fashion [48]. This study is important because it demonstrates that mutant pyrin is a constitutively active variant of the protein and can lead to uncontrolled IL-1β secretion with only “Signal 1” and without a second insult such as a cholesterol crystal [48]. Although it has not been well studied, there is some suggestion that patients with familial Mediterranean fever have more vascular complications such as atherosclerosis [49]. Based on this evidence, it would also seem likely that other components of the inflammasome, in particular NLRP3, may be associated independently with cardiovascular risk factors. Three recent studies looking at large and racially diverse cohorts have identified NLRP3 as a predictor of fibrinogen and C-reactive protein levels [39, 50, 51].
It will be important to further pursue these findings to identify if NLRP3 or MEFV mutations are predictive of atherogenesis and vascular disease and if modulating NLRP3 inflammasome activity results in not only decreasing levels of bona-fide risk factors but can also perhaps contribute to plaque stabilization or even regression. Before transitioning these findings to the clinic, it will be necessary to determine the role of the NLRP3 inflammasome in normal tissue homeostasis as well as in host defense in order to appropriately tailor and modulate therapies. However, due to the fact that other inflammasomes such as the IPAF inflammasome (bacterial infections) or AIM2 inflammasome (double-stranded DNA) would still remain functional and capable of producing IL-1β, therapeutics targeting the NLRP3 inflammasome axis are particularly appealing because they would not completely block IL-1β, as is the main option now with monoclonal antibodies directed towards IL-1β. Another possibility would be to titrate the levels of the US Food and Drug Administration–approved drug Anakinra (IL-1Ra) in order to offset the elevated production of IL-1β in patients at high risk for cardiovascular disease.
Inflammasome-Related Genetics and Cardiovascular Disease Risk Factors
Although most of this work has been carried out in murine models of atherosclerosis or using human cells in vitro, it is important to turn to human genetic studies to see if the inflammasome is associated with human cardiovascular disease risk. Genetic studies involving inflammasome-related genes (ie, NLRP3, ACS, CARD8, and P2RX7) have focused, for the most part, on immune traits and only a handful of reports investigated associations with cardiovascular traits. Some of the findings came from agnostic approaches using genome-wide association studies (GWAS). This was the case for the reported association between the NLRP3 locus and fibrinogen levels based on a GWAS analysis of six population-based studies, including 22,096 participants of European ancestry [51]. The NLR family pyrin domain containing three isoforms (NLRP3) of the gene (rs1539019) was one of the four loci that demonstrated genome-wide significance (P < 5.0 × 10−8) in this study. Moreover, the significant association of this locus with fibrinogen was independently replicated by Wassel et al. [52] using a vascular gene-centric array (47,539 common and lower-frequency variants) in 23,634 European American and 6657 African American participants from six studies comprising the Candidate Gene Association Resource project.
C-reactive protein (CRP) was the focus of another GWAS that included 66,185 participants from 15 population-based studies in the discovery phase and 16,540 individuals from 10 independent studies in the replication phase [50]. This analysis identified NLRP3 as one of the 18 loci associated with CRP levels. Both studies highlight the importance of immune response in the regulation of chronic inflammation and cardiovascular disease risk.
Roberts et al. [53] took a hypothesis-driven approach based on the fact that cholesterol crystals have been shown to cause inflammation, and ultimately atherosclerotic lesions, through the activation of the NLRP3 inflammasome. As cholesterol crystals have also been found in the walls of patients with abdominal aortic aneurysms (AAA), the authors proposed that the NLRP3 inflammasome may be involved in AAA and that genetic variability within this protein complex could alter disease risk. Therefore, they tested for associations of AAA with functional single nucleotide polymorphisms (SNPs) in the CARD8 (rs2043211) and NLRP3 (rs35829419) genes in AAA patients (n = 1151) and controls (n = 727). These authors found that homozygosity for the common C allele of NLRP3 rs35829419 was associated with significantly higher mean concentration of plasma IL1-β (P = 0.010). These finding suggests that genetic variability within the NLRP3 inflammasome may be important in the pathophysiology of AAA.
None of the other inflammasome-related genes reached genome-wide statistical significance in other GWAS examining these or other cardiovascular disease traits. However, some investigators have taken a more hypothesis-driven approach to investigate potential associations between these genes and cardiovascular disease risk factors. Thus, Stokes et al. [54] investigated the relation between genetic variation at the P2RX7 locus and IL-1β secretion. The P2X(7) receptor is an ATP-gated cation channel expressed in immune cells that mediates the activation of the NLRP3 inflammasome and the expression of pro–IL-1β via interaction with a number of ligands such as cholesterol crystals, particulate materials, and serum amyloid protein (SAA). In this study, Stokes et al. [54] investigated 12 functionally relevant single SNPs in the human P2RX7 gene in 3430 white subjects. Haplotype analysis revealed some gain of function combinations associated with increased receptor activity and increased IL-1β secretion from LPS-primed monocytes; therefore, genetic variability at the P2X7 locus may predispose to increased proinflammatory cytokine secretion and consequently to increased cardiovascular disease risk.
The P2X receptor channels are also involved in transducing aldosterone-mediated signaling in the distal renal tubule and are potential candidate genes for blood pressure regulation. Therefore, Palomino-Doza et al. [55] performed a family-based genetic association study in 248 families ascertained through a proband with hypertension to investigate the relationship between genetic variation in the P2X4, P2X6, and P2X7 genes and ambulatory blood pressure. They genotyped 28 SNPs, which, according to the authors, captured most of the common variation at these loci. They found significant evidence of association between the SNP rs591874 in the first intron of the P2X7 gene and blood pressure, with the strongest association being for night-time diastolic blood pressure (P = 0.0032). These data suggest that genetic variability at the P2X7 gene may be a predictor of hypertension and that therapies targeted to the P2X receptor signaling may be promising antihypertensive agents.
In addition to traditional genetic association studies, at least one of the loci has been examined for potential epigenetic mechanisms regulating inflammation. In this regard, Nakajima et al. [56] reported an interesting relation between physical activity and methylation of the ASC gene that could help us to understand the mechanism by which chronic moderate exercise reduces proinflammatory cytokines. These investigators analyzed DNA methylation from peripheral blood DNA extracts from young control (n = 34), older control (n = 153), and older exercise (n = 230) groups. Methylation of ASC decreased significantly with age, consistent with an age-dependent increase in ASC expression. Moreover, compared to the older control group, the degree of ASC methylation was higher in the older exercise group, which should be associated with lower ASC expression. Therefore, these results indicate that exercise may offset the age-dependent decrease in ASC methylation, inferring suppression of excess proinflammatory cytokines through reduction of ASC expression.
Overall, and despite the general paucity of studies, the current data suggest that genetic variation at inflammasome-related loci has a significant influence over a number of cardiovascular risk factors, including inflammatory markers, fibrinogen, and blood pressure. Future studies should aim to replicate these initial findings and to investigate potential gene-environment interactions that could unravel new preventive approaches to decreased CVD risk.
Conclusions
As the prevalence of cardiovascular disease continues to increase dramatically, any mechanistic understanding that can educate the search for novel therapies is paramount to enhancing the quality of life for many individuals. Now, the challenge for both basic researchers as well as clinicians is to attempt to extend and integrate this new mechanistic insight into atherogenesis in order to identify preventive approaches or druggable targets in the pathway. The most important work still to be done is to clarify the role of the NLRP3 inflammasome in atherosclerosis and atherogenesis, particularly due to conflictive findings that may be model dependent. A suggested experiment for future work would be to generate bone marrow chimeras within an ApoE−/− mouse with either NLRP3-sufficient and ApoE-sufficient bone marrow or NLRP3-deficient and ApoE-deficient bone marrow. As a result, the role of the inflammasome in the hematopoietic cells, particularly macrophages, could be isolated in this scenario from defects in reverse cholesterol transport. Nevertheless, the bulk of the evidence still suggests that the NLRP3 inflammasome plays a role in atherogenesis and the progression of diseases classified under the umbrella of metabolic syndrome. Consequently, the one drug target that stands out is the NLRP3 inflammasome itself. The hypothetical ideal drug would be a specific blocker or stabilizer of NLRP3 inflammasome activation that allows those responsible for sensing bacterial infection, such as NLRC4, to continue functioning. As with any therapy targeting a branch of the immune system, it will be important to consider on a case-by-case basis the potential benefits and drawbacks to a specific patient in terms of reduced atherosclerotic burden while balancing a state of potential immunosuppression and risk of severe infection. For some patients, the best intervention may remain diet and exercise, whereas others may benefit from novel drugs targeting components of “Signal 1” or “Signal 2.”
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74.
Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12.
Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–55.
Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33.
Schroder K, Tschopp J. The Inflammasomes. Cell. 2010;140:821–32.
• Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010; 464:1357–61. This the seminal article linking NLRP3 to sensing damage induced by cholesterol crystals and playing a role in the progression of atherosclerosis.
Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–42.
Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–37.
Curtiss LK, Tobias PS. Emerging role of Toll-like receptors in atherosclerosis. The Journal of Lipid Research. 2008;50:S340–5.
Lundberg AM, Hansson GK. Innate immune signals in atherosclerosis. Clin Immunol. 2010;134:5–24.
Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–11.
Wright SD et al. Infectious agents are not necessary for murine atherogenesis. J Exp Med. 2000;191:1437–41.
Miller YI. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2002;278:1561–8.
• Stewart CR, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2009;11:155–61. This is a description of a novel trimeric receptor for oxidized LDL, which has great relevance for understanding how endogenous ligands can provide proinflammatory signals.
Bae YS et al. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104:210–8.
Michelsen KS et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. PNAS. 2004;101:10679–84.
Shi H et al. TLR4 links innate immunity and fatty acid–induced insulin resistance. J Clin Invest. 2006;116:3015–25.
Kim F et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–96.
Seimon TA et al. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. PNAS. 2006;103:19794–9.
Seimon TA et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metabol. 2010;12:467–82.
Lee JY. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2002;44:479–86.
Weatherill AR et al. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–7.
Joosten LAB et al. Engagement of fatty acids with toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum. 2010;62:3237–48.
• Da Young Oh, et al. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell. 2010;142:687–98. This article identifies a receptor that is expressed on macrophages that can mediate the inflammatory effects of omega-3 fatty acids.
Kiechl S et al. Toll-like receptor 4 polymorphisms and atherogenesis. NEJM. 2002;347:185–92.
Noel SE et al. Variants of the CD36 gene and metabolic syndrome in Boston Puerto Rican adults. Atherosclerosis. 2010;211:210–5.
Osgood D et al. Genetic variation at the scavenger receptor class B type I gene locus determines plasma lipoprotein concentrations and particle size and interacts with type 2 diabetes: the Framingham study. J Clin Endocrinol Metab. 2003;88:2869–79.
Yajima N et al. Critical role of bone marrow apoptosis-associated speck-like protein, an inflammasome adaptor molecule, in neointimal formation after vascular injury in mice. Circulation. 2008;117:3079–87.
Martinon F et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41.
Dostert C et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–7.
Hornung V et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–56.
Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–73.
Rajamäki K et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765.
• Menu P, et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. 2011;2:e137–4. This article suggests that the NLRP3 inflammasome may not play a role in atherogenesis in an ApoE-deficient mouse model.
Khallou-Laschet J et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852.
Baitsch D et al. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1160–8.
Feig JE et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. PNAS. 2011;108:6166–7171.
Freigang S et al. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–51.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2010;469:221–5.
Bauernfeind F et al. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–7.
Wen H, et al. Fatty acid–induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature Publishing Group 1–9 (2011).doi:10.1038/ni.2022
• Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88. This article links the NLRP3 inflammasome both in humans and mice to diseases falling under the umbrella of metabolic syndrome.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature Publishing Group. 2009;11:136–40.
Masters SL et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nature Publishing Group. 2010;11:897–904.
NLRP3 is necessary for IL1 release in response to hyaluronan endogenous trigger. 1–10 (2009).
Niemi K et al. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol. 2011;186:6119–28.
Papin S et al. The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1β processing. Cell Death Differ. 2007;14:1457–66.
Chae JJ et al. Gain-of-function pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice. Immunity. 2011;34:755–68.
Özçakar ZB, Yalçınkaya F. Vascular comorbidities in familial Mediterranean fever. Rheumatol Int. 2011;31:1275–81.
Dehghan A et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple Loci for C-reactive protein levels. Circulation. 2011;123:731–8.
Dehghan A et al. Association of novel genetic loci with circulating fibrinogen levels: a genome-wide association study in 6 population-based cohorts. Circulation: Cardiovascular Genetics. 2009;2:125–33.
Wassel CL et al. Association of genomic loci from a cardiovascular gene SNP array with fibrinogen levels in European Americans and African-Americans from six cohort studies: the Candidate Gene Association Resource (CARe). Blood. 2011;117:268–75.
Roberts RL et al. Interaction of the inflammasome genes CARD8 and NLRP3 in abdominal aortic aneurysms. Atherosclerosis. 2011;218:123–6.
Stokes L et al. Two haplotypes of the P2X7 receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1 secretion. FASEB J. 2010;24:2916–27.
Palomino-Doza J et al. Ambulatory blood pressure is associated with polymorphic variation in P2X receptor genes. Hypertension. 2008;52:980–5.
Nakajima K et al. Exercise effects on methylation of ASC gene. Int J Sports Med. 2010;31(9):671–5.
Acknowledgments
Supported by National Heart, Lung, and Blood Institute grants HL-54776, and U01 HL72524; National Institute of Diabetes and Digestive and Kidney Diseases, Grant Number DK075030 and by contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture Research.
Disclosure
No conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ordovas-Montanes, J.M., Ordovas, J.M. Cholesterol, Inflammasomes, and Atherogenesis. Curr Cardiovasc Risk Rep 6, 45–52 (2012). https://doi.org/10.1007/s12170-011-0212-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12170-011-0212-2