Abstract
In this work, an ultrasensitive fluorescence immunoassay has been proposed for the detection of chloramphenicol. The proposed assay is based on using the carboxyl-functionalized NaYF4: Yb/Tm upconversion nanoparticles with maximum emission at 482 nm under excitation at 980 nm conjugated with anti-chloramphenicol (CAP) antibody as the signal probe and the magnetic polystyrene microspheres conjugated with coating antigen as the sensing probe. The coating antigens on the sensing probes compete with CAPs to bind with the antibodies on the signal probes and the immune complexes form. Via magnetic action, these complexes can be separated to measure their fluorescence intensity. The limit of detection (LOD) of this assay for CAP in PBS is 0.01 pg mL−1, and the linear range extends from 0.05 to 100 pg mL−1 with a linear equation of y = 387.64 lgx + 1153.93 (R2 = 0.9911). The recoveries of CAP in spiked animal-derived foods range from 76.85 to 105.18%. Trace CAP levels have been measured in the real sample by the proposed fluorescence immunoassay, and the results have been verified by commercial ELISA test kit and liquid chromatography-tandem mass spectrometry. The quantitation limits of this immunoassay for CAP in the muscle tissue, milk, and honey samples are 0.25 pg g−1, 0.4 pg g−1, and 0.4 pg g−1, respectively. The fluorescence immunoassay proposed in this study can be used to highly sensitively, accurately, and rapidly detect CAP residues in animal-derived foods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics as the veterinary drug are widely used to treat animal diseases (Wang et al. 2019). However, the abuse of antibiotics could lead to their residue and accumulation in animal-derived foods, which has caused toxicological effect on human health (Wang et al. 2017a; Yan et al. 2016). Chloramphenicol (CAP) is a broad-spectrum antibiotic and reacts via binding to 50S ribosomal subunits of bacterial to inhibit protein synthesis (Pathak et al. 2017; Qin et al. 2019a). CAP has been widely used in the treatment of aquaculture and other animal infectious diseases caused by various bacteria (Samsonova et al. 2012). The USA, China, and many other countries have banned the use of CAP in the production of animal-derived food because of its side effect on humans, including the inhibition of bone marrow hematopoietic function, aplastic anemia, and fetotoxicity (Chatzitakis et al. 2008; Li et al. 2019; Sharma et al. 2019). Residues of CAP in animal-derived foods have reported in many literatures (Shakila et al. 2006; Sniegocki et al. 2015). It is necessary to develop the highly sensitive, simple, rapid test method to monitor CAP residues in order to ensure human health.
There are many analytical methods to detect CAP residues, including liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Imran et al. 2018), gas chromatography-mass spectrometry (GC-MS) (Liu et al. 2014b), real-time fluorescent quantitative PCR (Duan et al. 2017), photo-electrochemical aptasensor (Qin et al. 2019b), and microbial assays (Shakila et al. 2007). Many immunoassays have been used for detecting CAP, such as enzyme-linked immunosorbent assay (Liu et al. 2014a), photoresponsive colorimetric (Chang et al. 2017), chemiluminescence (Wang et al. 2017b), electrochemical (Liu et al. 2014c), and fluorescence immunoassay (Wang et al. 2018a; Gasilova and Eremin 2010; Zhang et al. 2008). Among these test techniques, the immunoassay based on the specific recognition of antigen-antibody has the advantages of good specificity, high sensitivity, easy to operate, and saving time. In particular, the fluorescence immunoassay using various fluorescence nanoparticles and test strategy has attracted great attention with researchers.
The upconversion nanoparticles (UCNPs) have unique luminescent mechanism and superior spectroscopic properties compared with the traditional organic dyes as follows: high stability for photochemical properties, especially suitable for the fluorescent tags in complex biological samples, excellent penetrability, low damage to biological samples, and non-bleaching emission (Liu et al. 2015; Liu et al. 2009; Vijayan et al. 2019). And the upconversion phenomenon follows the anti-Stokes luminescent process, which can greatly reduce the interference of background and then improve the detection sensitivity (Shao et al. 2017; Tu et al. 2015). Recently, the upconversion nanoparticles have been widely used in bioimaging (Shen et al. 2013), fluorescent marker (Zhang et al. 2017), medical diagnostics (Cai et al. 2018; Wang et al. 2018b), and other fields. Magnetic polystyrene microspheres (MPMs) have been widely used as the rapid separation material due to their unique advantages: better monodispersity, lower toxicity, chemical stability, and superparamagnetic properties (Lu et al. 2007; Tran et al. 2019). It has been widely applied in wastewater treatment (Wang et al. 2015), drug delivery (Blazkova et al. 2013), biomedicine (Ma and Liu 2007), and other fields.
In this study, a highly sensitive fluorescence immunoassay has proposed to detect CAP; it is making use of UCNPs as the fluorescent marker to prepare the signal probe and MPMs as separable component to prepare the sensing probe.
Materials and Methods
Materials and Apparatus
Chloramphenicol (99.2%) was purchased from Dr. Ehrenstorfer GmbH (Augsburg, German). C6H9O6Yb·4H2O (99.9%, MW: 422.23 g mol−1), C6H9O6Tm·xH2O (99.9%, MW: 346.07 g mol−1), C6H9O6Y·xH2O (99.9%, MW: 266.04 g mol−1), polyacrylic acid (PAA), ethyl-3-[3-(dimethylamino)propyl] carbodiimide (EDC), N-hydroxysuccinimide (NHS), N, N-dimethylformamide (DMF), dicyclohexylcarbodiimide (DCC), bovine serum albumin (BSA), ovalbumin (OVA), and diethylene glycol (DEG) were acquired from Sigma-Aldrich (St. Louis, MO, USA). Oleic acid (OA, 90%) and 1-octadecene (ODE, 90%) were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Magnetic polystyrene microspheres (MPMs) were obtained from Tianjin BaseLine ChromTech Research Centre (Tianjin, China). Chloramphenicol succinate sodium was purchased from Toronto Research Chemicals (Toronto, Canada). The phosphate-buffered saline (PBS) was prepared by mixing the solution of 0.1 mol L−1 NaH2PO4, 0.1 mol L−1 Na2HPO4, and 0.25 mol L−1 NaCl. The 2-(N-morpholino) ethanesulfonic acid (MES) buffer was prepared by dissolving 10 mmol MES in deionized water at a constant volume of 1000 mL and adjusting the pH value to 5.5. The 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES) buffer was prepared by dissolving 10 mmol HEPES in deionized water at a constant volume of 1000 mL and adjusting the pH value to 7.2. The anti-CAP monoclonal antibody (heavy chain: IgG1, light chain: Kappa) and CAP-hapten were acquired in our laboratory. Sodium hydroxide, ammonium fluoride, and other reagents used in this work were analytical grade and purchased form Sinopharm Chemical Reagent Co., Ltd. (Tianjin, China). The commercial ELISA test kit was purchased from R-Biopharm AG (Darmstadt, Germany). The quality control samples were purchased from Beijing Meizheng Testing Co., Ltd. (Beijing, China). F-2500 fluorescence spectrophotometer (Hitachi, Japan) equipped with an external 980 nm laser source (Hi-Tech Optoelectronics Co., Ltd., Beijing, China) was applied to measure upconversion fluorescence intensity.
Synthesis and Surface Modification of Upconversion Nanoparticles
UCNPs (NaYF4: Yb, Tm) were synthesized by thermolysis method according to previous study (Chen et al. 2010) with slight modification. Here, 239.44 mg C6H9O6Y·xH2O, 38 mg C6H9O6Yb·4H2O, and 3.46 mg C6H9O6Tm·xH2O were added into a 100-mL three-necked flask containing 17 mL of 1-octadecene and 6 mL of oleic acid, and the above solution was mixed under agitation. Under vacuum, the mixture was heated to 100 °C in 10 min. Subsequently, the mixture was continually heated to 160 °C and maintained at this temperature for half an hour under argon protection, and then cooled naturally to the room temperature. The solution of sodium hydroxide (2.5 mmol) and ammonium fluoride (4 mmol) dissolved in 6 mL of methanol was added dropwise to the above mixture within 10 min and reacted for 30 min. Then, the mixed solution was heated to 80 °C to eliminate the methanol and quickly heated to 300 °C for 1 h under argon shielding. The reactants were allowed to cool to room temperature, collected in a plastic centrifuge tube, and washed with ethanol four times by centrifugation. Finally, the resulting products dried hydrophobic OA-capped UCNPs (OA-UCNPs) in an oven under 60 °C were collected for subsequent use.
The hydrophilic PAA enwrapped UCNPs (PAA-UCNPs) were synthesized according a study reported previously by using PAA as a new ligand to replace the OA ligand on the surface of OA-UCNPs (Liu et al. 2011). In a 100-mL three-necked flask, 30 mL of DEG and 1.5 g PAA were mixed together and heated to 110 °C with rabbling vigorously, and reacted 1 h under the protection of argon. At the same temperature conditions, 90 mg of OA-UCNPs were dispersed in 6 mL of methylbenzene by sonication and dropped on the above flask instantly under agitating for 1 h in argon. After treating by above steps, the above mixed solution was directly heated to 240 °C for 1 h. Finally, the reaction solution was allowed to cool to room temperature; the excessive dilute hydrochloric acid solution was added. The resulting solution was centrifuged and the white precipitates were obtained and washed three times with deionized water. The synthesized hydrophilic PAA-UCNPs were dried in the drying oven under 60 °C.
Preparation of Chloramphenicol Coating Antigen
The chloramphenicol coating antigen (CAP-OVA) was produced via the active eater method according to our previous study (Wang et al. 2010) with slight modification. Briefly, 13.78 mg of CAP-hapten, 3.75 mg of NHS, and 7.38 mg of DCC were dissolved in 2 mL of anhydrous DMF and reacted with rabbling for 5 h at room temperature. The above reacted solution was centrifuged to obtain supernatant. Under the condition of stirring constantly, the supernatant was dropwise added into 10 mL of OVA protein solution (20 mg of OVA was dissolved in 10 mL of 0.05 mol L−1, pH 8.0 phosphate buffer) followed by incubation for 24 h at 4 °C. The resulting solution was dialyzed with PBS for 3 days and used for further experiment.
Preparation of Fluorescence Signal Probe and Sensing Probe
The fluorescence signal probe and sensing probe were synthesized by the classical active ester method. In a round-bottom flask, 5 mg of PAA-UCNPs was completely dispersed in 2 mL of MES buffer by sonication. Next, 5-mg NHS and 10-mg EDC were added to excite carboxyl groups on PAA-UCNPs and stirred for 3 h at 30 °C in water bath. Then, the mixed solution was purified by centrifuging 10 min (4000 rpm) and the precipitate was collected and washed with deionized water three times. Subsequently, the residual precipitation was redispersed in 1 mL of HEPES buffer followed by adding anti-CAP monoclonal antibody (McAb) to react for 4 h at 30 °C with constant stirring. And then, the uncombined sites on the surface of PAA-UCNPs were blocked by adding 15 mg BSA into the above reaction mixture and incubating for 1 h at 30 °C. Afterwards, the resulting mixture was purified by centrifuging to obtain the precipitate followed by washing with HEPES buffer. After that, the white precipitate was redispersed in 1 mL of HEPES buffer as fluorescence signal probe.
CAP-OVA was conjugated with carboxyl-functional MPMs by the classic EDC/NHS coupling reaction between the amine groups on CAP-OVA and carboxyl groups on MPMs. Firstly, 5 mg of MPMs were washed twice by PBS and redissolved in 1 mL of PBS. Then, 5 mg of NHS and 10 mg of EDC were added into the above solution and incubated for 80 min on the oscillator (250 rpm) at room temperature. Then, the precipitate (activated carboxyl-functional MPMs) was collected by magnetic separation and washed twice with PBS, and dispersed in 1 mL of PBS. Subsequently, the CAP-OVA was added into the aforesaid mixed solution and incubated at room temperature for 4 h followed by adding 15-mg BSA into the above mixture in order to cover protein unconjugated sites on the surface of MPMs. After magnetic separation, the obtained precipitate was washed five times with PBS, and redispersed in 5 mL of PBS as sensing probe.
Immunoassay Principle and Procedure
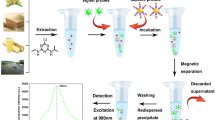
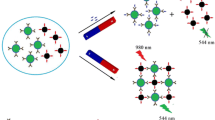
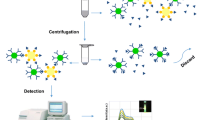
The test principle of the competitive upconversion fluorescence immunoassay is exhibited in Fig. 1. In the test system, the sensing probe and CAP combined competitively with signal probe based on antibody–antigen-specific recognition. Via magnetic force, the immune complex of signal probe and sensing probe was obtained and the fluorescence intensity of immune complex was measured by using fluorescence spectrophotometer (F-2500) equipped with a 980-nm laser as excitation light source. The fluorescence intensity of immune complex achieved maximum in the absence of CAP in the test system, because all the sensing probes combined with signal probe. When CAP was present in the test system, the amount of the immune complex decreased with the increase of concentration of CAP, leading to the decrease of fluorescence intensity. There is a negative correlation relationship between the change of fluorescence intensity and the concentration of CAP; this relationship can be used to achieve the quantitative detection of CAP.
Test procedures of the fluorescence immunoassay are as follows: 50 μL of CAP standard solution (or sample solution) and 50 μL of the sensing probe were added to each centrifuge tube, and then 50 μL of the signal probe was subsequently dropped. Then, the mixed solution was incubated on a reciprocating oscillator (280 rpm) for 20 min at room temperature. The immune complex was separated from the test system using an external magnet followed by washing with PBS three times. Finally, the immune complex was redispersed in 400 μL of PBS, and the fluorescence intensity of immune complex (at 482 nm) was measured by F-2500 fluorescence spectrophotometer (scan speed: 1500 nm min−1) equipped with a 980-nm laser (working current: 1.0 A).
Preparation of Samples
For the muscle tissue sample, 2 g of the homogenized muscle tissue sample was mixed with 6-mL ethyl acetate with vortex extraction for 5 min. After the resulting mixture was centrifuged (10 min, 5000 rpm), the obtained supernatant was moved to a clean glass tube. The supernatant was dried at 60 °C under a nitrogen stream and the dried residue was redissolved in 2-mL PBS. And then 2 mL of n-hexane was added to eliminate fat. After centrifuging, the supernatant organic phase was discarded and the remaining extract solution was diluted with PBS in the appropriate multiple for analysis.
For the honey sample, 2-g honey sample was mixed with 4-mL ethyl acetate and 2-mL deionized water and then the sample was extracted by vortexing vigorously for 5 min. The remaining steps were repeated as the treatment method for the muscle tissue sample.
For the milk sample, 2-g milk sample was mixed with 2-mL trichloroacetic acid aqueous solution (3%) with vortex extraction for 5 min. Afterwards, the resulting mixture was centrifuged at 10,000 rpm for 10 min, and then 1 mol L−1 NaOH solution was added into the obtained supernatant to adjust the pH to neutral. Finally, the supernatant was diluted by PBS in the appropriate multiple for analysis.
Results and Discussion
Characterization of UCNPs
The morphology of the prepared OA-UCNPs and PAA-UCNPs has been characterized by transmission electron microscope (TEM), as indicated in Fig. 2a and Fig. 2b, which shows that the nanoparticles are spheroidal and their surface is smooth. The average particle diameters of the OA-UCNPs and PAA-UCNPs are approximately 28 nm and 30 nm (the particle size distribution is given in Fig. 2c and Fig. 2d), respectively. Because of the hydrophilic PAA layer that has been capped on the surface of OA-UCNPs, the average particle size of PAA-UCNPs is slightly larger than that of OA-UCNPs. Figure 2e indicates the fluorescence emission spectra of UCNPs by excitation at 980 nm, and the narrow emission peak at 482 nm with strong fluorescence intensity is observed. In order to ulteriorly confirm the successful modification of hydrophobic UCNPs, the OA-UCNPs and PAA-UCNPs have been characterized by Fourier transform infrared spectrometer (FTIR spectrometer). As illustrated in Fig. 2f, the OA-UCNPs have two stretching vibration peaks at 2928 cm−1 and 2856 cm−1, which correspond to the characteristic peaks of asymmetric and symmetric stretching vibration of methylene in OA molecules on the surface of OA-UCNPs. However, these two peaks at the corresponding wavenumber in the PAA-UCNPs significantly weakened, indicating that most of the OA molecules have been replaced. In addition, the stretching vibration peaks of hydroxyl at 3436 cm−1 are observed in the spectra of the OA-UCNPs and PAA-UCNPs. And the PAA-UCNPs have a new characteristic peak at 1731 cm−1, which represents the stretching vibration of carbonyl, further proving that the carboxyl groups have introduced into PAA-UCNPs, and the OA-UCNPs have been successfully modified into the PAA-UCNPs.
Characterization of the OA-UCNPs and PAA-UCNPs. (a): TEM image of OA-UCNPs, (b): TEM image of PAA-UCNPs, (c): Particle size distribution of OA-UCNPs, (d): Particle size distribution of PAA-UCNPs, (e): Fluorescence emission spectra (under excitation at 980 nm), (f): Fourier transform infrared spectra
Preparation of Probes and Optimization of Test Conditions
For achieving a better fluorescent signal response and high sensitive detection, several trial parameters such as the addition quantity of anti-CAP McAb in preparation of signal probe, the addition quantity of coating antigen (CAP-OVA) in preparation of sensing probe, the addition volume of sensing probe, and incubation time were all investigated. The content of McAb in the signal probe will directly affect the combining capacity of the signal probe with the sensing probe, thus can affect the fluorescence intensity of test system. Therefore, 100-μL aliquot of the activated PAA-UCNPs solution was respectively distributed in each centrifuge tube, and then various amounts of McAb (10, 15, 20, 25, 30, 35, and 40 μg) were seriatim added to the above activated solution to make the signal probe. The signal probe can be collected by centrifugation for 10 min (4 °C, 4000 rpm), and the amount of unconjugated McAb in the obtained supernatant can be measured by the BCA protein quantitation kit. The conjugated amount of McAb with PAA-UCNPs is calculated by subtracting the unconnected amount of McAb from the initial addition amount of McAb. Furthermore, the conjugation rate of McAb with PAA-UCNPs is calculated as the percentage of the amount of conjugated McAb and the initial addition amount of McAb. As shown in Fig. 3a, when the addition amount of McAb is 10 μg, the conjugation rate is close to 100%, indicating that the addition amount of McAb is less than the maximum conjugated amount of McAb and PAA-UCNPs. The conjugated amount of McAb gradually increases with increase in the addition quantity of McAb. When the addition quantity of McAb is greater than or equal to 25 μg, the amount of conjugation is tended to be changeless, indicating that the amount of McAb conjugated with the activated PAA-UCNPs has reached the maximum. Moreover, the conjugation rate gradually decreases with the increase of addition quantity of McAb, which indicates that the surface of activated PAA-UCNPs has no more binding site to conjugate the McAb; the excess addition causes the waste of McAb. Therefore, 25 μg of addition quantity of McAb with 80.80% of conjugation rate was used for the preparation of signal probe.
Optimization of the working parameters. (a): Optimization of the added amount of anti-chloramphenicol antibody in preparation of signal probe, (b): Optimization of the added amount of coating antigen in preparation of sensing probe, (c): Optimization of the added volume of sensing probe with 50 μL of signal probe in detection process, (d): Optimization of the incubation time. Each data point is the mean of triplicate analyses
Furthermore, the addition quantity of CAP-OVA was optimized by adding different amounts of CAP-OVA (10, 15, 20, 25, 30, 35, and 40 μg) into 100 μL of the activated MPMs solution in each centrifuge tube to prepare sensing probe. The supernatant was obtained by magnetic separation and detected by the BCA protein quantitation kit to calculate the amount of unconjugated CAP-OVA. The conjugated amount of CAP-OVA with MPMs is calculated by subtracting the unconnected amount of CAP-OVA from the initial addition amount of CAP-OVA. And the conjugation rate of CAP-OVA with MPMs is expressed as the percentage of the amount of conjugated CAP-OVA and the initial addition amount of CAP-OVA. The optimization results (Fig. 3b) are similar with those results of the added amount of McAb. When the addition quantity of CAP-OVA is greater than or equal to 25 μg, the amount of conjugation is tended to be changeless, indicating that the amount of CAP-OVA conjugated with the activated MPMs has reached the maximum; the excess addition causes the waste of CAP-OVA.
For the immunoassay with competitive format, the less contents of coating antigen on the particles is more beneficial to achieve a higher sensitivity. With 50 μL of the signal probe and 30 min of incubation time, varying added volumes of the sensing probe (10, 20, 30, 40, 50, 60, 70, and 80 μL) prepared using different added amounts of the CAP-OVA (10, 15, 20, and 25 μg) were applied to carry out test process, and the fluorescence intensity of test system under varying conditions is shown in Fig. 3c. The fluorescence intensity of test system is relatively low with the sensing probe prepared using 10 and 15 μg of added amounts of CAP-OVA. When 20 and 25 μg of added amounts of CAP-OVA have been applied to prepare the sensing probe, the fluorescence intensity of test system is increased with increase of added volumes of the sensing probe. When added volume of the sensing probe is greater than or equal to 50 μL, the fluorescence intensity of test system achieved the maximum and tended to be changeless, which indicates that there are no more signal probes that can be conjugated with the sensing probes; the excess addition causes the waste of the sensing probe. Finally, 20 μg of added amount of CAP-OVA with 93.39% of conjugation rate was used to prepare the sensing probe, and 50 μL of added volume of the sensing probe was applied in test process of the fluorescence immunoassay. Under optimal conditions, the incubation time has been optimized. As shown as Fig. 3d, the fluorescence intensity of immune complex increases gradually and become level off at 20 min. Therefore, 20 min of incubation time was applied in test process of the fluorescence immunoassay in order to save test time.
Evaluation of Assay Sensitivity
Under optimum conditions, the various concentrations of CAP standard solutions were measured by the developed fluorescence immunoassay. The emission wavelength at 482 nm was selected as the test wavelength, and a fluorescence spectrophotometer with a 980 nm laser was used to obtain the fluorescence intensity for the test system in the presence of different concentrations of CAP standard solution. As depicted in Fig. 4a, the fluorescence intensity of the immune complex in test system at 482 nm has achieved maximum when the concentration of CAP is 0 pg mL−1. And the fluorescence intensity decreases gradually with the increased concentration of CAP. The amount of the immune complex decreased gradually with the increased concentration of CAP based on competitive format, resulting in the decrease of fluorescence intensity. Figure 4b shows the decrease of fluorescence intensity (∆I, ∆I = I0 − I) corresponding to different concentrations of CAP, wherein I is the fluorescence intensity in the presence of CAP and I0 is the fluorescence intensity in the absence of CAP. When the concentration of CAP is more than 100 pg mL−1 (or less than 0.05 pg mL−1), the ∆I value tends to be flat. There is a linear correlation between the decrease in fluorescence intensity (∆I) and the concentration of CAP in a range that extends from 0.05 to 100 pg mL−1 (Fig. 4c) with a linear equation of y = 387.64 lg (x) + 1153.93 (R2 = 0.9911). According to the 3σ rule, the LOD of the proposed assay for the CAP in PBS is 0.01 pg mL−1, which means that the proposed assay have a high sensitivity for CAP detection.
Detection of CAP with the fluorescence immunoassay. (a): Fluorescence intensity of the immune complexes in the different concentrations of CAP, (b): The decreased fluorescence intensity (ΔI) corresponding to varying concentrations of CAP, (c): Standard curve of the fluorescence immunoassay for the detection of CAP in PBS
Evaluation of Assay Specificity
CAP, the analogues (florfenicol, thiamphenicol), and the other common antibiotics (malachite green, leucomalachite green, basic violet, semicarbazide, 1-aminohydantoin, 3-amino-2-oxazolidinone, 3-amino-morpholinomethyl-2-oxazolidinone, enrofloxacin, norfloxacin, sulfamethoxazole, sulfadimidine, tetracycline, salbutamol, gentamycin, metronidazole) at the same concentration of 100 pg mL−1 were analyzed to assess the specificity of the proposed assay. As shown in Fig. 5, the analogues and other common antibiotics have caused negligible decrease of fluorescence intensity (∆I) compared with CAP. The proposed assay has high specificity for CAP, which proved that this fluorescence immunoassay has good selectivity for discriminating of CAP from other interfering antibiotics.
Sample Analysis
The matrix components from sample can be extracted along with the analyte. These matrix components can block the combining of antibody with the analyte in immunoassay, thereby affect the accuracy of analysis results. In order to achieve rapid test, the dilution method generally is used to eliminate the matrix effect. In this work, the extraction solutions of honey, milk, and muscle tissue samples (crucian, carp, turbot, chicken, weever, shrimp) are diluted at running multiple with PBS followed by analysis using the proposed assay. It is considered that the matrix effect has been eliminated when the fluorescence intensity in test system for sample solution with applicable multiple is approximately equal with the fluorescence intensity in test system for PBS. Figure 6a shows that the matrix effect can be completely eliminated with fivefold dilution of the extraction solutions of muscle tissue samples. And an eightfold dilution of the extraction solutions using PBS can eliminate the matrix effect from milk and honey samples (Fig. 6b). The limits of quantitation of CAP in muscle tissue samples, milk, and honey were 0.25 pg g−1, 0.4 pg g−1, and 0.4 pg g−1, respectively.
To investigate the effectiveness of the method proposed in this study for detecting CAP, the animal-derived foods (honey, milk, and muscle tissue samples) spiked with CAP at four low levels were detected by this method. As shown as Table 1, the detected concentrations of CAP by the proposed assay are in good agreement with the corresponding spiked level. The recoveries of CAP ranged from 76.85 to 105.18%, and the coefficients of variation (CVs) varied from 0.38 to 15.38%.
To further verify the accuracy of assay for sensitive detection of CAP, the proposed fluorescence immunoassay and commercial ELISA test kit were used simultaneously to detect CAP at three relatively large spiked levels in eight samples (carp, crucian, weever, turbot, chicken, shrimp, honey, milk). The results are provided in Table 2; the detection results of this two methods show good correlation with R2 = 0.9972, indicating that the fluorescence immunoassay has good accuracy for the detection of CAP.
The real samples have been also analyzed to validate the reliability and practicability of this immunoassay, and the test results are listed in Table 3. The trace amount of CAP residue with a concentration of 0.0022 ng g−1 has been measured in a crucian sample purchased in a small local market, and CAP residues have not been detected in the other samples purchased by us. The trace amount of CAP in the crucian sample has not been detected by the commercial ELISA test kit and LC-MS/MS, because this trace concentration is lower than the LOD of the ELISA kit and LC-MS/MS. In addition, two chicken positive quality control samples for CAP were purchased and analyzed simultaneously by the proposed fluorescence immunoassay, commercial ELISA test kit, and LC-MS/MS. In the chicken sample no.1, the concentrations of CAP detected by this immunoassay, ELISA test kit, and LC-MS/MS are 0.19, 0.19, and 0.21 ng g−1, respectively. And the concentrations of CAP detected by these three methods are 1.06, 1.01, and 1.12 ng g−1, respectively, in the chicken sample no.2. The results of three kinds of analytical methods were consistent, further proving the reliability of the developed method to test CAP residue in animal-derived foods. The announcement (no. 235) enacted by the Ministry of Agriculture of the People’s Republic of China has stipulated that CAP is forbidden to be used as a veterinary drug, and it has not been allowed to be detected in the animal-derived foods. Trace of CAP has been detected in the randomly purchased crucian sample, which may be the CAP antibiotics were illegally used in breeding process, resulting in residue of CAP in the crucian.
Analytical Performance Evaluation of the Fluorescence Immunoassay
The traditional commercial ELISA test kit for CAP needs at least 50 min to achieve the whole test process containing incubation (30 min), washing (5 min), color development (15 min), and measurement. The developed method is relatively easy to operate and rapid for detection process, including incubation (20 min), washing (5 min), and measurement. Especially, the washing process becomes more rapid and convenient due to the application of magnetic separation operation. The quantitation limits of CAP for commercial ELISA kit in muscle tissue, milk, and honey samples were 8 pg g−1, 24 pg g−1, and 25 pg g−1, respectively. The proposed fluorescence immunoassay has high sensitivity with the quantitation limits of CAP in muscle tissue, milk, and honey samples at 0.25 pg g−1, 0.4 pg g−1, and 0.4 pg g−1, respectively. This fluorescence immunoassay and an indirect competitive ELISA were simultaneously established by using the same CAP-OVA and anti-CAP monoclonal antibody. The half-maximum inhibition concentration (IC50) and LOD (concentration calculated as IC15) of this ELISA for CAP are 3.70 μg L−1 and 0.38 μg L−1. Compared with this ELISA, the proposed fluorescence immunoassay with a LOD of 0.01 pg mL−1 has shown higher sensitivity. The proposed fluorescence immunoassay has the characteristics of high sensitivity, easy to operate, and saving time; it can be applied as an alternative approach to detect the trace amount of CAP residue in animal-derived foods.
Conclusions
A fluorescence immunoassay in combination with magnetic separation using upconversion nanoparticles NaYF4: Yb/Tm with emission peak at 482 nm as labels has been established to test CAP. The proposed assay has high specificity for CAP and can be used for the specific detection of CAP. This immunoassay is ultrasensitive with the LODs of 0.01 pg mL−1 for CAP in PBS, of 0.05 pg g−1 in muscle tissue, of 0.08 pg g−1 in milk, and of 0.08 pg g−1 in honey, respectively. It is more sensitive than the LC-MS/MS, the commercial ELISA test kit, and the indirect competitive ELISA established using same coating antigen and antibody. The test results for the spiked- and real animal-derived food samples by this assay are in good agreement with the testing results of LC-MS/MS and commercial ELISA kit, indicating good reliability and practicability of this assay. The trace amount of CAP has been found by this assay in a crucian sample and it has not been detected by the commercial ELISA test kit and LC-MS/MS due to their insufficient sensitivity. This method is relatively easy to operate and rapid, and only needs 25 min to finish the entire test process. Although CAP has been forbidden to use as the veterinary drug in animal-derived foods, the CAP residues have been detected sporadically. In order to ensure human health, it is necessary to develop the high sensitive, simple, rapid test method to monitor CAP residues. The fluorescence immunoassay proposed in this work can be used as an ultrasensitive rapid test tool to accurately detect CAP residues in animal-derived foods.
References
Blazkova I, Nguyen HV, Dostalova S, Kopel P, Stanisavljevic M, Vaculovicova M, Stiborova M, Exkschlager T, Kizek R, Adam V (2013) Apoferritin modified magnetic particles as doxorubicin carriers for anticancer drug delivery. Int J Mol Sci 14:13391–13402. https://doi.org/10.3390/ijms140713391
Cai Q, Xu JT, Yang D, Dai YL, Yang GX, Zhong CN, Gai S, He F, Yang PP (2018) Polypyrrole-coated UCNPs@mSiO2@ZnO nanocomposite for combined photodynamic and photothermal therapy. J Mater Chem B 6:8148–8162. https://doi.org/10.1039/c8tb02407c
Chang HH, Lv J, Zhang HC, Zhang B, Wei WL, Qiao Y (2017) Photoresponsive colorimetric immunoassay based on chitosan modified AgI/TiO2 heterojunction for highly sensitive chloramphenicol detection. Biosens Bioelectron 87:579–586. https://doi.org/10.1016/j.bios.2016.09.002
Chatzitakis A, Berberidou C, Paspaltsis I, Kyriakou G, Sklaviadis T, Poulios I (2008) Photocatalytic degradation and drug activity reduction of chloramphenicol. Water Res 42:386–394. https://doi.org/10.1016/j.watres.2007.07.030
Chen GY, Ohulchanskyy TY, Kumar R, Agren H, Prasad PN (2010) Ultrasmall monodisperse NaYF4:Yb3+/Tm3+ nanocrystals with enhanced near-infrared to near-infrared upconversion photoluminescence. ACS Nano 4:3163–3168. https://doi.org/10.1021/nn100457j
Duan Y, Wang LH, Gao ZQ, Wang HS, Zhang HX, Li H (2017) An aptamer-based effective method for highly sensitive detection of chloramphenicol residues in animal-sourced food using real-time fluorescent quantitative PCR. Talanta 165:671–676. https://doi.org/10.1016/j.talanta.2016.12.090
Gasilova NV, Eremin SA (2010) Determination of chloramphenicol in milk by a fluorescence polarization immunoassay. J Anal Chem 65:255–259. https://doi.org/10.1134/S1061934810030081
Imran M, Fazal-e-Habib MS, Tawab A, Rauf W, Rahman M, Umer M, Iqbal M (2018) LC-MS/MS-based determination of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in poultry meat from the Punjab-Pakistan. Food Addit Contam A 35:1530–1542. https://doi.org/10.1080/19440049.2018.1463569
Li XQ, Li HM, Xu S, Gao Y, Zhang QH, Zhang Y, Feng MY (2019) Rapid quantification of trace chloramphenicol in honey under ambient conditions using direct analysis via real-time QTRAP mass spectrometry. Food Chem 276:50–56. https://doi.org/10.1016/j.foodchem.2018.09.130
Liu XM, Zhao JW, Sun YJ, Song K, Yu Y, Du C, Kong XG, Zhang H (2009) Ionothermal synthesis of hexagonal-phase NaYF4:Yb3+, Er3+/Tm3+ upconversion nanophosphors. Chem Commun 43:6628–6630. https://doi.org/10.1039/b915517a
Liu CH, Wang Z, Wang XK, Li ZP (2011) Surface modification of hydrophobic NaYF4:Yb, Er upconversion nanophosphors and their applications for immunoassay. SCIENCE CHINA Chem 54:1292–1297. https://doi.org/10.1007/s11426-011-4319-6
Liu N, Song SQ, Lu L, Nie DX, Han Z, Yang XL, Zhao ZH, Wu A, Zheng XD (2014a) A rabbit monoclonal antibody-based sensitive competitive indirect enzyme-linked immunoassay for rapid detection of chloramphenicol residue. Food Agric Immunol 25:523–534. https://doi.org/10.1080/09540105.2013.847065
Liu TS, Xie J, Zhao JF, Song GX, Hu YM (2014b) Magnetic chitosan nanocomposite used as cleanup material to detect chloramphenicol in milk by GC-MS. Food Anal Methods 7:814–819. https://doi.org/10.1007/s12161-013-9686-5
Liu BQ, Zhang B, Chen GN, Tang DP (2014c) Biotin-avidin-conjugated metal sulfide nanoclusters for simultaneous electrochemical immunoassay of tetracycline and chloramphenicol. Microchim Acta 181:257–262. https://doi.org/10.1007/s00604-013-1096-2
Liu XG, Yan CH, Capobianco JA (2015) Photon upconversion nanomaterials. Chem Soc Rev 44:1299–1301. https://doi.org/10.1039/c5cs90009c
Lu AH, Salabas EL, Schuth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46:1222–1244. https://doi.org/10.1002/anie.200602866
Ma ZY, Liu HZ (2007) Synthesis and surface modification of magnetic particles for application in biotechnology and biomedicine. China Particuol 5:1–10. https://doi.org/10.1016/j.cpart.2006.11.001
Pathak BK, Mondal S, Barat C (2017) Inhibition of Escherichia coli ribosome subunit dissociation by chloramphenicol and blasticidin: a new mode of action of the antibiotics. Lett Appl Microbiol 64:79–85. https://doi.org/10.1111/lam.12686
Qin D, Wang JT, Ge CZ, Lian ZR (2019a) Fast extraction of chloramphenicol from marine sediments by using magnetic molecularly imprinted nanoparticles. Microchim Acta 186:428. https://doi.org/10.1007/s00604-019-3548-9
Qin XF, Wang QQ, Geng LP, Shu XL, Wang Y (2019b) A "signal-on" photoelectrochemical aptasensor based on graphene quantum dots-sensitized TiO2 nanotube arrays for sensitive detection of chloramphenicol. Talanta 197:28–35. https://doi.org/10.1016/j.talanta.2018.12.103
Samsonova JV, Cannavan A, Elliott CT (2012) A critical review of screening methods for the detection of chloramphenicol, thiamphenicol, and florfenicol residues in foodstuffs. Crit Rev Anal Chem 42:50–78. https://doi.org/10.1080/10408347.2012.629951
Shakila RJ, Vyla SAP, Kumar RS, Jeyasekaran G, Jasmine GI (2006) Stability of chloramphenicol residues in shrimp subjected to heat processing treatments. Food Microbiol 23:47–51. https://doi.org/10.1016/j.fm.2005.01.012
Shakila RJ, Saravanakumar R, Vyla SAP, Jeyasekaran G (2007) An improved microbial assay for the detection of chloramphenicol residues in shrimp tissues. Innov Food Sci Emerg Technol 8:515–518. https://doi.org/10.1016/j.ifset.2007.03.002
Shao QY, Zhang GT, Ouyang LL, Hu YQ, Dong Y, Jiang JQ (2017) Emission color tuning of core/shell upconversion nanoparticles through modulation of laser power or temperature. Nanoscale 9:12132–12141. https://doi.org/10.1039/c7nr03682e
Sharma R, Akshath US, Bhatt P, Raghavarao KSMS (2019) Fluorescent aptaswitch for chloramphenicol detection-quantification enabled by immobilization of aptamer. Sensors Actuators B Chem 290:110–117. https://doi.org/10.1016/j.snb.2019.03.093
Shen JW, Yang CX, Dong LX, Sun HR, Gao K, Yan XP (2013) Incorporation of computed tomography and magnetic resonance imaging function into NaYF4: Yb/Tm upconversion nanoparticles for in vivo trimodal bioimaging. Anal Chem 85:12166–12172. https://doi.org/10.1021/ac403486r
Sniegocki T, Gbylik-Sikorska M, Posyniak A (2015) Transfer of chloramphenicol from milk to commercial dairy products-experimental proof. Food Control 57:411–418. https://doi.org/10.1016/j.foodcont.2015.04.028
Tran CTH, Craggs M, Smith LM, Stanley K, Bilek MM, McKenzie DR (2019) A plasma ion bombardment process enabling reagent-free covalent binding of multiple functional molecules onto magnetic particles. Mater Sci Eng C 98:118–124. https://doi.org/10.1016/j.msec.2018.12.106
Tu LP, Liu XM, Wu F, Zhang H (2015) Excitation energy migration dynamics in upconversion nanomaterials. Chem Soc Rev 44:1331–1345. https://doi.org/10.1039/c4cs00168k
Vijayan AN, Liu ZM, Zhao HH, Zhang P (2019) Nicking enzyme-assisted signal-amplifiable Hg2+ detection using upconversion nanoparticles. Anal Chim Acta 1072:75–80. https://doi.org/10.1016/j.aca.2019.05.001
Wang L, Zhang Y, Gao X, Duan ZJ, Wang S (2010) Determination of chloramphenicol residues in milk by enzyme-linked immunosorbent assay: improvement by biotin-streptavidin-amplified system. J Agric Food Chem 58:3265–3270. https://doi.org/10.1021/jf903940h
Wang FH, Jiang W, Fang Y, Cheng CW (2015) Preparation of Fe3O4 magnetic porous microspheres (MPMs) and their application in treating mercury-containing wastewater from the polyvinyl chloride industry by calcium carbide method. Chem Eng J 259:827–836. https://doi.org/10.1016/j.cej.2014.08.009
Wang HX, Ren LS, Yu X, Hu J, Chen Y, He GS, Jiang QW (2017a) Antibiotic residues in meat, milk and aquatic products in Shanghai and human exposure assessment. Food Control 80:217–225. https://doi.org/10.1016/j.foodcont.2017.04.034
Wang LY, Yao MW, Fang CZ, Yao X (2017b) A highly sensitive detection of chloramphenicol based on chemiluminescence immunoassays with the cheap functionalized Fe3O4@SiO2 magnetic nanoparticles. Luminescence 32:1039–1044. https://doi.org/10.1002/bio.3288
Wang JY, Wang Q, Zheng YJ, Peng T, Yao K, Xie SL, Zhang XY, Xia X, Li JC, Jiang HY (2018a) Development of a quantitative fluorescence-based lateral flow immunoassay for determination of chloramphenicol, thiamphenicol and florfenicol in milk. Food Agric Immunol 29:56–66. https://doi.org/10.1080/09540105.2017.1359498
Wang XX, Xu JT, Yang D, Sun CQ, Sun QQ, He F, Gai SL, Zhong CN, Li CX, Yang PP (2018b) Fe3O4@MIL-100(Fe)-UCNPs heterojunction photosensitizer: rational design and application in near infrared light mediated hypoxic tumor therapy. Chem Eng J 354:1141–1152. https://doi.org/10.1016/j.cej.2018.08.070
Wang YF, Xie TS, Yang J, Lei M, Fan J, Meng ZH, Xue M, Qiu LL, Qi FL, Wang Z (2019) Fast screening of antibiotics in milk using a molecularly imprinted two-dimensional photonic crystal hydrogel sensor. Anal Chim Acta 1070:97–103. https://doi.org/10.1016/j.aca.2019.04.031
Yan ZD, Gan N, Li TH, Cao YT, Chen YJ (2016) A sensitive electrochemical aptasensor for multiplex antibiotics detection based on high-capacity magnetic hollow porous nanotracers coupling exonuclease-assisted cascade target recycling. Biosens Bioelectron 78:51–57. https://doi.org/10.1016/j.bios.2015.11.019
Zhang C, Wang S, Fang GZ, Zhang Y, Jiang LQ (2008) Competitive immunoassay by capillary electrophoresis with laser-induced fluorescence for the trace detection of chloramphenicol in animal-derived foods. Electrophoresis 29:3422–3428. https://doi.org/10.1002/elps.200800188
Zhang B, Li HH, Pan WX, Chen QS, Ouyang Q, Zhao JW (2017) Dual-color upconversion nanoparticles (UCNPs)-based fluorescent immunoassay probes for sensitive sensing foodborne pathogens. Food Anal Methods 10:2036–2045. https://doi.org/10.1007/s12161-016-0758-1
Funding
This work was supported by the National Key R and D Program of China (Project No. 2016YFD0401204), the International Science and Technology Cooperation Program of China (Project No. 2014DFR30350).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheng, W., Huang, N., Liu, Y. et al. An Ultrasensitive Fluorescence Immunoassay Based on Magnetic Separation and Upconversion Nanoparticles as Labels for the Detection of Chloramphenicol in Animal-Derived Foods. Food Anal. Methods 13, 2039–2049 (2020). https://doi.org/10.1007/s12161-020-01820-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01820-5