Abstract
A microwave-assisted extraction (MAE) procedure to obtain phenolic compounds from carob bark was optimized by using response surface methodology. A four-factor, three-level Box–Behnken design with five central points was used to evaluate the influence of temperature, solid-liquid ratio, ethanol concentration, and time in carob bark extraction in terms of antioxidant activity (DPPH) and total extraction yield. Optimal extraction conditions were found using 80 °C, 35% (v/v) ethanol, a ratio of 35 mL/g, and 29.5 min. Total phenolics content (TPC), antioxidant activity (DPPH, FRAP, ABTS), carbohydrate content, and main polyphenols composition (HPLC) were determined at optimal conditions. An experimental total yield of 66.5% was obtained with a TPC value of 33.6 mg GAE/g DW and polysaccharides content of 345.4 mg glucose/g DW. A high antioxidant activity was also shown by the three methods tested. The results showed the potential of carob pods skin as a natural source of phenolic compounds, in particular gallic acid, and the effectiveness of MAE as extraction technique for the revalorization of this agro-food waste.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, the packaging sector is requesting the use of polymer materials of renewable origin due to the great awareness of existing environmental problems. Therefore, the number of both natural and recyclable biopolymer materials that are suitable for their use in the packaging sector is gaining a greater relevance (Kumar et al. 2017; TĂNASE et al. 2016; Tang et al. 2012). This type of biopolymers is intended to be combined with active ingredients that may have antioxidant effect for the development of active packaging systems to improve the shelf-life of foods (Arrieta et al. 2017). It is also aimed that these compounds could be obtained from agro-food waste (Di Donato et al. 2017; Manousaki et al. 2016), with the aim of increasing the added value of industrial by-products and reducing the total price of the final packaging system. Antioxidants obtained from agro-food waste could greatly enhance the preservation of packaged food in a remarkable way increasing the benefits of this type of products. In this sense, the carob tree fruit is a characteristic product of the Mediterranean area that is currently discarded which is known to have good antioxidant properties, being its valorization very interesting (Kumazawa et al. 2002).
Carob tree (Ceratonia siliqua L.) is one of the most useful native Mediterranean trees. In producing countries, carob pods have been traditionally used as human and animal food, but currently, their main use is related to the extraction of rubber from their seeds (Owis and El-Naggar 2016). Carob trees grow around the Mediterranean basin and countries such as Portugal, Spain, Italy, Greece, Turkey, and Morocco and several regions of North America. In 2010, world carob production was 163 kt, a considerably low amount due to the fact that it is an abandoned plantation. In 2011, the production was doubled reaching 310 kt worldwide, with Spain being the main producer of carob with 135 kt, followed by Italy, Portugal, and Morocco (Vourdoubas and Skoulou 2017).
Carob pods are characterized by their high content in water-soluble sugars (approximately 40–50%, mainly sucrose) and low content in proteins (3–4%) and lipids (0.4–0.8%). Carob pods also contain considerable amounts of polyphenols (Hadrich et al. 2017; Sęczyk et al. 2016), in particular gallic acid (Hadrich et al. 2017). Polyphenols have a wide range of biological properties and, among them, the antioxidant activity is the best known, which can prevent oxidative damage of some biomolecules. Polyphenols have received much attention in recent years due to their ability to act as powerful antioxidants. Polyphenolic compounds are found in the tissues of plants and are important for their growth and development as they provide a defense mechanism against infections and injuries (Karakaya et al. 2001). In addition, carob pod extracts have been reported to exhibit a very strong antimicrobial activity against several bacteria and fungi (Meziani et al. 2015).
For the determination of the main polyphenols present in the carob pod and the evaluation of potential applications of these compounds, the optimization of the extraction process of phenolic compounds must be previously carried out (Bai et al. 2010). For this purpose, microwave-assisted extraction (MAE) shows several advantages over conventional extraction techniques such as the reduction of solvent volume and energy consumption used for extraction, obtaining high recoveries and good reproducibility (Filip et al. 2017; Valdés et al. 2015). Nowadays, this technique has been successfully used for the extraction of different food waste matrices such as peanut (Ballard et al. 2010) and tangerine (Hayat et al. 2009) skins and tomato (Pinela et al. 2016), and other wastes with high added value (Ferreres et al. 2017; Moreira et al. 2017; Rosa et al. 2017). Regarding the extraction of carob pods, some studies have analyzed the antioxidant capacity of the obtained extracts but in very few cases, MAE has been used (Kumazawa et al. 2002; Makris and Kefalas 2004; Piñeiro et al. 2017).
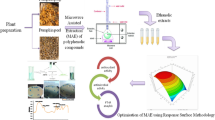
The objective of the present study is to revalorize carob bark as a main source of polyphenols giving an added value to this waste. For this purpose, carob beans not suitable for consumption or sale were selected and the seeds were manually extracted and discarded, in order to use as residue only the bark part of the pod. In this work, the optimal MAE conditions to extract polyphenol compounds were determined by using response surface methodology in terms of total yield and antioxidant capacity (DPPH). Independent variables studied included temperature, time, liquid:solvent ratio, and ethanol concentration. Total phenolics (TPC) and carbohydrates content, antioxidant capacity by using FRAP, DPPH, and ABTS methods, total yield, and quantification of main polyphenols were also determined in the extracts obtained at optimal conditions.
Materials and Methods
Raw Material and Chemical Reagents
Carob pods were obtained from the European carob tree Ceratonia silicua of the variety “ramillete,” which is original of Alicante and Murcia regions (Spain). The obtained bark (Fig. 1a) was oven-dried for 48 h at 40 °C (MCP Vacuum Casting System, Lubeck, Germany). As a first step, carob bark was manually crushed and then introduced into a centrifugal mill (Maype, Manises, Spain). The obtained powder (Fig. 1b) was oven-dried at 40 °C for 12 h and grinded in a Mill ZM 200 centrifugal mill (Retsch, Düsseldorf, Germany) at 12000 rpm. The milled sample was passed through a 0.25-mm sieve. This residue (Fig. 1c) was oven-dried again during 24 h at 40 °C.
Quercetin, sodium carbonate, Folin−Ciocalteu reagent (2 N), 2,2-diphenyl-1-picrylhydrazyl (DPPH), (±) 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), ethanol (96%), ethanol (absolute grade), methanol (HPLC grade), acetonitrile (HPLC grade), 2,2′azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid (ABTS), potassium persulfate, and calcium chloride were purchased from Sigma–Aldrich (Madrid, Spain).
Microwave-Assisted Extraction (MAE) of Polyphenols: Experimental Design
Extraction of polyphenols was carried out by using a commercial microwave oven (Milestone flexiWAVE, Shelton, Connecticut, USA). Two grams of sample was introduced and the microwave agitator was set at 300 rpm during the extraction process. Response surface methodology (RSM) was used to determine the optimal extraction conditions of polyphenols from carob bark. A Box–Behnken design (BBD) was used to determine the effect of four variables which may affect phytochemical contents in plants (Dai and Mumper 2010): extraction temperature, liquid:solid ratio, ethanol concentration, and extraction time. Table 1 shows the selected variables and levels which were set according to experimental limitations and related bibliography (Yang and Zhai 2010). This design consisted of 29 experiments including five central points to estimate the model’s pure error. All experiments were carried out in random order and were performed once. The responses obtained from the experimental design were evaluated in terms of overall yield and antioxidant activity (DPPH method).
After extraction, extracts were centrifuged at 5300 rpm and 4 °C (Digicen 21, Ortoalresa, Ajalvir, Spain) for 15 min to separate the remaining solid residues from the extraction. The supernatant obtained from the centrifugation process was oven-dried (J.P Selecta S.A, Spain) at 40 °C for 24 h and then re-dissolved in 20 mL ethanol 50% (v/v) and frozen at − 20 °C until analysis was performed. Due to some precipitation after this preservation process, samples were centrifuged again at 5300 rpm and 4 °C for 15 min before analysis to separate any precipitated solid residues.
A multiple linear regression analysis was performed to obtain the regression coefficients following a second-order polynomial model:
where Y is the predicted response, X represents the variables of the system, i and j are design variables, β0 is a constant, βi is the linear coefficient, βii is the quadratic coefficient, and βij is the interaction coefficient of variables i and j.
The extracts obtained at optimal conditions were also analyzed to evaluate the antioxidant performance by FRAP and ABTS methods, total phenolics (TPC), and carbohydrates content. Main polyphenols present in carob extracts were also monitored by HPLC analysis.
Total Extraction Yield
The total yield after each extraction was determined as follows:
where Wext is the mass of the dried extract (g) and W0 is the mass of dried carob bark used for extraction (g).
Samples were weighed by means of an Atilon ATL-224-I analytical balance (Acculab, Bradford, England).
Determination of Antioxidant Capacity
DPPH Assay
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was performed according to the methodology described by Sengul Uysal et al. (Uysal et al. 2016). A solution of DPPH 10−4 M was prepared by dissolving 0.0039 g of DPPH radical in 100 mL of absolute ethanol. This stock solution was daily prepared. One milliliter of the extract was mixed with 4 mL of DPPH ethanolic solution and kept in darkness conditions at room temperature for 30 min. The decrease in absorbance was determined at 517 nm using a Biomate-3 UV-VIS spectrophotometer (Thermospetronic, Mobile, AL, USA).
The antioxidant capacity of the extracts was expressed as percentage of DPPH inhibition by using the following equation:
where A0 is the absorbance of the control and A1 is the absorbance of the extract after 30 min.
FRAP Assay
The ferric ion reducing antioxidant power (FRAP) was also determined as described by Sengul Uysal et al. (Uysal et al. 2016). The FRAP reagent was prepared freshly by mixing 300 mM acetate buffer (pH = 3.6), 10 mM/TPTZ, and 20 mM ferric chloride in a mixing ratio of 10:1:1 (v/v/v), respectively. Then, 2 mL of reagent and 200 μL of the sample solution were mixed and incubated at 30 °C for 30 min. The absorbance was then determined at 593 nm. Trolox was used as standard and the results were expressed as milligrams trolox equivalents per gram dry weight (mg TE/g DW). All samples were analyzed in triplicate.
ABTS Assay
The antioxidant capacity against 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) was also measured according to the method described by S. Uysal et al. (2016). ABTS radical cation was produced directly by reacting 7 mM ABTS solution with 2.45 mM potassium persulfate and allowing the mixture to stand for 12–16 h in darkness conditions at room temperature. Prior to the assay, the solution was diluted with ethanol to an absorbance of 0.700 ± 0.02 at 734 nm. One milliliter of extract was then added to 2 mL of ABTS solution and mixed at room temperature for 30 min. The absorbance was determined at 734 nm. ABTS scavenging activity was also expressed as mg TE/g DW and measurements were carried out in triplicate.
Total Phenolics Content (TPC)
Total phenolics content was determined according to the Folin-Ciocalteu colorimetric assay following the method described by Meziani et al. [13]. 0.5 mL of extract was mixed with 0.9 mL of Folin-Ciocalteu reagent and 3.6 mL of sodium carbonate solution at a concentration of 75 g/L. The test tubes were stored in darkness conditions at room temperature for 30 min. Absorbance was determined at 765 nm and the total phenolics content of samples were expressed as milligrams of gallic acid equivalents per gram dry weight (mg GAE/g DW). All samples were analyzed in triplicate.
Phenol-Sulfuric Acid Method
The basic principle of this method is that carbohydrates, when dehydrated by reaction with concentrated sulfuric acid, can produce furfural derivatives. The subsequent reaction between furfural derivatives and phenol results in a compound with a detectable color. Two milliliters of the carbohydrate solution (extract) was mixed with 1 mL of 5% aqueous solution (v/v) of phenol in a test tube. Subsequently, 5 mL of concentrated sulfuric acid were rapidly added to the mixture. After 10 min, the mixture was vortexed for 30 s and placed for 20 min in a water bath at room temperature for color development. Then, absorbance at 490 nm was recorded. Reference solutions were identically prepared using a calibration curve between 40 and 180 ppm. The results were expressed in mg glucose/g DW.
Main Polyphenols Profile by HPLC Analysis
Prior to the quantification of polyphenols, these were verified using an Agilent high-performance liquid chromatograph model 1100 Series trailer at the same time a variable wavelength UV-visible detector and analyzer of ion trap mass spectrometer. The wavelength used was 280 nm and mass spectrometric detection was performed in the negative ionization mode (ESI). After passing through the flow cell of the diode array detector, the column eluate was split (1:10) and the sample was directed to an LCQ ion trap mass spectrometer fitted. Scan range was 50–900 m/z and scan rate, 1 scan/s. The desolvation temperature was 350 °C. High spray voltage was set at 4500 V. Nitrogen was used as the dry gas at a flow rate of 10 mL/min.
The identification and quantification of main polyphenols present in carob bark extracts was carried out following the method proposed by SuJung et al. (Hu et al. 2016) with some modifications. Thirty-four milligrams of dried carob extract was diluted with 50 mL of ultrapure miliQ water. The diluted solution (1:100 v/v) was analyzed for polyphenols content using an Agilent 1260 infinity HPLC system coupled with DAD detector. A BRISA LC2 C18 column (250 mm × 4.6 mm × 5 μm, Teknokroma) at 30 °C was used. The mobile phase consisted of two solvents: 0.1% acetic acid (A) and 100% acetonitrile (B). The gradient used was 100% A (1 min) linearly decreasing to 60% A at 25 min (5 min). The flow rate was 0.5 mL/min and the injection volume was 20 μL. Quantification of polyphenols was based on retention times compared with corresponding standards (Sigma–Aldrich, Germany) and their verification were done with Agilent high-performance liquid chromatograph model 1100 Series trailer at the same time a variable wavelength UV-visible detector and Analyzer of ion trap mass spectrometer (Agilent model 1100 Series LC/MSD Trap SL). Analysis was performed in triplicate and mean values were reported. Polyphenols content was calculated from mean peak areas using external calibration method and expressed as mg/g DW.
Statistical Analysis
Analysis of variance (ANOVA) was carried out from experimental data and mean values were compared at confidence level of 95% (p < 0.05) using the Tukey test.
Statgraphics Centurion XVI (Statistical Graphics, Rockville, MD, USA) was used to generate and analyze the results of the BBD. A graphic analysis of the main effects and interactions between the variables was used for results interpretation. Response surface methodology (RSM) was used to determine optimal extraction conditions. Multiple linear regression was performed to obtain regression coefficients of the quadratic polynomial model previously described. Lack of fit, determination coefficient (R2) and F test of the model were evaluated from analysis of variance (ANOVA) at confidence level of 95% (p < 0.05).
Results
MAE Optimization
The influence of several factors on the MAE extraction efficiency of polyphenols from carob bark, such as extraction time (min), ethanol concentration (% v/v), liquid:solid ratio (mL/g), and extraction temperature (°C), was studied in terms of total yield (%) and DPPH inhibition (%) using surface response methodology. The experimental values selected for each variable in the Box–Behnken design and the responses obtained are given in Table 2.
Figure 2 shows Pareto charts and significant effects at 95% confidence obtained for the studied responses. The total yield obtained from carob bark ranged from 57.9 to 67.5%. As it can be seen in the Pareto chart, the yield was significantly affected by ethanol concentration and liquid:solid ratio. The ethanol concentration used was the main significant variable influencing extraction yield with a negative effect. So, the yield was increased within the studied levels with the decrease of ethanol concentration. The solvent constitution is one of the most important factors in an extraction process as it could influence the polarity of solvent, being crucial for the solubility of the extracted compounds (Zhao et al. 2018). The presence of water in the solvent could facilitate an increase in extraction yield by improving the swelling of the plant material, which is favorable to increase the surface area of contact between the matrix and the solvent (Hayat et al. 2009; Pan et al. 2003). A high concentration of ethanol could interrupt the extraction of phenolic compounds possibly due to a lower penetration of ethanol into the plant matrix through the protective function and a lower solubility of some extractable phenolic compounds (Tóth et al. 2014) The increase in water content in the solvent composition was statistically influential to improve the overall extraction yield (Spigno et al. 2007). In this sense, with decreasing ethanol concentration and adding water, the polarity of the solvent is increased and not only polyphenols could be extracted but also other polar compounds such as fructose, glucose, or sucrose (present in carob pods) which may result in increasing the total yield (Biner et al. 2007). It was also observed that an increase in the liquid:solid ratio resulted in increasing the total yield. A high solvent volume could accelerate substance transfer and promote solubility by increasing the contact surface of the plant material with the solvent, improving the extraction efficiency (Zhao et al. 2018).
Regarding antioxidant capacity, a higher extraction temperature improved the antioxidant capacity which ranged from 68 to 89% of DPPH inhibition. A high temperature could speed up intermolecular interactions and facilitate molecular motion, which could increase the solubility of the solutes into the solution (Zhao et al. 2018). A significant negative interaction was also observed between time and ethanol concentration, with a lower concentration of ethanol generally improving the antioxidant activity of the extracts. Ethanol changes the dielectric properties of the solvent towards microwave heating and the degree of microwave absorption usually increases with the dielectric constant. The decrease in ethanol results in an increase in the dielectric constant of the system due to higher water content, increasing the absorption of more microwave energy by the system and increasing the temperature inside the sample leading to the rupture of cells (Chemat and Cravotto 2012). Some studies have shown that small amounts of water in the extracting solvent during MAE make possible the diffusion of water into the cells of the matrix, leading to better heating and thus facilitating the transport of compounds into the solvent at higher mass transfer rates (Huma et al. 2018). Finally, a significant positive interaction between time and liquid:solid ratio also affected the antioxidant capacity, increasing DPPH inhibition with higher extraction times and solvent volume.
As a result of the analysis performed, it was decided to focus on the antioxidant capacity as this variable is more directly correlated with the extraction of phenolic compounds compared with the total yield that could be more influenced by the co-extraction of different compounds together with the target polyphenols.
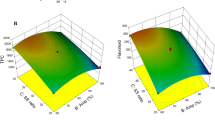
A graphical analysis in terms of response surfaces was performed to study the interactions present in the antioxidant capacity of the obtained extracts (Fig. 3). These surface graphics represent in a visual way the interactions between the different factors, giving very valuable information for the analysis and optimization of the extraction. The interaction between time and ethanol concentration is plotted in Fig. 3a. As it can be seen, the best results for antioxidant capacity were obtained for a high time and a low ethanol concentration (approaching a 90% inhibition of DPPH) or for a low time and a high ethanol percentage.
Figure 3b shows the interaction between extraction time and liquid:solid ratio, where it can be appreciated that high time values and an intermediate liquid:solid ratio favors obtaining a high percentage of DPPH inhibition. Regarding extraction temperature, the effect of this variable was similar when studying the interaction between the other studied variables, showing an increase in temperature a significant improvement in the antioxidant capacity of the obtained extracts (Fig. 3c, e, f). In particular, the interactions between temperature and liquid:solid ratio or ethanol concentration showed that an intermediate value of these variables together with a high temperature could remarkably improve the antioxidant capacity.
Finally, it can be seen in Fig. 3d the interaction between liquid:solid ratio and ethanol concentration with no significant effect on the extraction process under the conditions studied to improve the antioxidant capacity.
The experimental data obtained from all 29 combinations for DPPH was fitted to a second-order polynomial model giving the following equation:
where X1, X2, X3, and X4 correspond to extraction time, ethanol concentration, liquid:solid ratio, and extraction temperature, respectively.
The ANOVA was used for the statistical testing of the model. The non-significance of the lack-of-fit tests (p = 0.2069) verified the suitability of the selected model. The R2 value obtained was 0.69, indicating a certain degree of correlation between experimental and predicted values. The optimal extraction conditions predicted by the model to optimize the extraction of carob bark in terms of antioxidant capacity are a high extraction time (29.5 min), a low ethanol percentage (35%), an elevated temperature (80 °C), and an intermediate liquid:solid ratio of 35 mL/g. These conditions will be tested to verify the degree of confidence of the model.
Chemical Analysis of Extracts at Optimal Conditions
The selection of optimal conditions was considered taking into account the conditions predicted by the model and the behavior observed from the response surface plots. As a result, it was decided to maintain the extraction conditions already predicted but modifying the value of the extraction time (Table 3), which was previously established in 29.5 min (a high value of time). So, it was intended to analyze in an experimental way if the application of a shorter time with the same conditions for the rest of the studied variables could imply some improvement in antioxidant activity. The results obtained for total yield, antioxidant activity (FRAP, DPPH, and ABTS methods), TPC, and polysaccharides are shown in Table 4.
Antioxidant Capacity
Table 4 shows the results obtained for antioxidant activity at the different extraction conditions, A and B, which were significantly different for all methods analyzed, obtaining the best results for the extraction performed at B conditions by using a longer extraction time, as predicted by the model. The extended time of microwaves irradiation also resulted in some increase in the extraction yield, obtaining 66.5% for B extraction which could be considered a high level of extraction capacity at an industrial level for the revalorization of carob waste. It has been reported that, in some cases, polyphenols recovery could be affected by the extension of microwaves extraction time due to some degradation of phenolic compounds (Song et al. 2011). In this study, however, an increase in extraction time was directly related to an improvement in the antioxidant capacity (Karami et al. 2015), in particular from the results obtained for ABTS and DPPH methods. The used conditions seemed to significantly improve the antioxidant capacity compared to similar carob bean reported extractions (Roseiro et al. 2013b).
The efficacy of MAE was compared with a conventional Soxhlet extraction (cold water and allowed to stand for 12 h at 3 °C and boiled for 10 min with stirring). With this method, a maximum of 20 to 60% DPPH inhibition was obtained by using final concentrations of extracts of 10 μg/mL and 50 μg/mL, respectively (Kumazawa et al. 2002). Compared to Soxhlet extraction, MAE cost less solvent and required lower extraction temperature and time.
The variety of the tree is a relevant issue when evaluating the antioxidant capacity. In this sense, no previous study has been found in the literature dealing with the antioxidant capacity of carob pods from the Alicante region of Spain. Worse antioxidant capacities but more polyphenols has been obtained with other pod species. Furthermore, carob pods as industrial product could be a potential source of polyphenol which can possibly substitute the use of synthetic antioxidants, thus improving the functional properties of a range of food products. Studies carried out on carob polyphenols have shown good antioxidant potential in microsomal lipid peroxidation and erythrocyte ghost systems (Huma et al. 2017)
The experimental results have demonstrated that MAE allows obtaining high values of antioxidant capacity. Even more, it has to be taken into account the origin of the carob pod, and especially its quality, as only poor quality pods and their bark, considered as waste, were used for this study. So, the antioxidant capacity results obtained could be considered very positive for the use of this type of residue as a source of antioxidant compounds.
Total Polyphenols Content (TPC)
Table 5 shows the results obtained for total polyphenols content (TPC) at different extraction conditions. As it can be seen, only by varying the extraction time from 5 to 29.5 min the amount of polyphenols obtained from the residue was improved by more than 30%, demonstrating also the extraction of phenolic compounds from carob at tested conditions by MAE. A high content of polyphenols were obtained for both extractions when compared to conventional extractions of carob beans previously reported, showing an average of around 20 mg GAE/g (Turhan et al. 2006). Comparing the obtained results by MAE with other extraction methods such as ultrasound-assisted extraction (UAE) and supercritical fluid extraction (SFE), the improvement obtained in this work for TPC was around 100% and 20%, respectively (Roseiro et al. 2013a).
In relation to other similar extractions by microwaves, the values obtained have remained below due to the selected conditions. For TPC optimization conditions, values of 70 mg GAE/g were obtained for a time of 4.5 min and an ethanol concentration of 45% (Huma et al. 2018). This reduction in the TPC values is due to the selection of the factors for a better obtaining of antioxidant properties instead of a greater amount of TPC. If we compare the results obtained with other residues, and in particular the use of the MAE, we can see how the residue studied has very good TPC values. Values of 15.1 mg GAE/g have been obtained for red grape peel and 20.6 mg GAE/g for olive leaves (Makris et al. 2007).
In relation with MAE, Huma et al. (2018) said that the improvement with other techniques such as UAE and CSE cannot be excessively high with high extraction times. This factor is due to the degradation of plant cell walls by microwave irradiation as observed through scanning electron microscopy. However, the same quantities are extracted in very short time with MAE in comparison with other techniques.
The extraction of polyphenols by using MAE supposes a great opportunity to extract these compounds from the dried residue of carob bark, in a quick and easy way. Depending on the thermal stability of these compounds, they can be further encapsulating to preserve them for further applications. From an industrial point of view, the revalorization of this waste should be optimized, including extraction time, in order to reach a confidence value to optimize the final process.
Total Polysaccharides Content
Although the main objective of this work was not focused on the determination of the total amount of polysaccharides that could be extracted under the tested conditions, it was decided also to evaluate the amount of total polysaccharides present in the obtained extracts due to the complexity of the sample and the visible co-extraction in the process of other compounds such as carob beam gum. Table 4 shows the results obtained for the total polysaccharides content at different extraction conditions. As it can be seen, no significance differences were observed between the polysaccharides content obtained at A and B conditions.
These results show how this type of waste has a large amount of polysaccharides. When analyzing the chemical composition of carob bean pods, Khlifa et al. showed how the polysaccharide compositions of the carob pod are about 50% of the total amount in its chemical structure (Khlifa et al. 2013). The residue studied has 35% polysaccharides in relation to the dry sample. This value indicates a smaller amount of polysaccharides, which can be positive when it comes to obtaining better results of antioxidant capacity.
From the obtained results, it has been shown that this residue contains a large amount of polyphenols, but it is also known that it is a residue rich in sugars, which could be directly related to the obtained results for the carbohydrate analysis (Biner et al. 2007).
HPLC Analysis of Main Polyphenols
The identification of the type and content of phenolic compounds present in carob bark is essential to determine the potential of the obtained extracts for further applications. Detection and quantification of main phenolic compounds present in carob extract obtained at optimum conditions (80 °C, 29.5 min, 35% ethanol, and 35 mL/g ratio) was performed by HPLC-DAD. Figure 4 shows polyphenols detected, highlighting the presence of gallic acid as the main polyphenolic compound present in carob extract in accordance with previous works (Almanasrah et al. 2015; Bouli et al. 2010; El Ansari Zineb et al. 2017; Papagiannopoulos et al. 2004). Other polyphenols also detected are presented in Table 5. Other authors have reported the presence of other compounds, not detected in this work, such as vanillic acid or rutin (Balaban 2004). This difference in results could be due to many factors such as environmental differences, extraction procedures, genetic characteristics of the plant, and state of maturity (Stavrou et al. 2018).
Chromatogram of main compounds found in carob (Table 5) at 280 nm
Conclusions
Microwave-assisted extraction (MAE) was demonstrated to be an efficient process for the extraction of phenolic compounds with antioxidant activity, in particular gallic acid, from the European carob tree Ceratonia silicua of the variety “ramillete.” A Box–Behnken design was used to optimize the MAE experimental conditions by using RSM, obtaining a value of R2 of 0.69. The best extraction conditions were as follows: 80 °C, 29.5 min, 35% ethanol, and 35 mL/g ratio. The TPC value and overall yield obtained were 33.6 ± 0.4 mg GAE/g DW and 66.5 ± 0.4%, respectively. A high concentration of polysaccharides was also co-extracted at optimized conditions. The obtained results from carob tree will contribute to the revalorization of this type of agro-food waste for different applications in the food industry sector, such as the encapsulation of phenolic compounds for functional food or the development of active films. In conclusion, the results from this work have shown the potential of carob barks as a natural source of phenolic compounds with antioxidant performance and the effectiveness of MAE for the reutilization of this agro-food waste.
References
Almanasrah M, Roseiro LB, Bogel-Lukasik R, Carvalheiro F, Brazinha C, Crespo J, Kallioinen M, Mänttäri M, Duarte LC (2015) Selective recovery of phenolic compounds and carbohydrates from carob kibbles using water-based extraction. Ind Crop Prod 70:443–450
Arrieta M, Sessini V, Peponi L, (2017) Biodegradable poly (ester-urethane) incorporated with catechin with shape memory and antioxidant activity for food packaging. Eur Polym J
Bai XL, Yue TL, Yuan YH, Zhang HW (2010) Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J Sep Sci 33(23-24):3751–3758
Balaban M (2004) Identification of the main phenolic compounds in wood of Ceratonia siliqua by GC-MS. Phytochem Anal 15(6):385–388
Ballard TS, Mallikarjunan P, Zhou K, O’Keefe S (2010) Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem 120(4):1185–1192
Biner B, Gubbuk H, Karhan M, Aksu M, Pekmezci M (2007) Sugar profiles of the pods of cultivated and wild types of carob bean (Ceratonia siliqua L.) in Turkey. Food Chem 100(4):1453–1455
Bouli AA, Hansali M, Owen RW, (2010) Determination of phenolic composition of carob pods grown in different regions of Morocco. J Nat Prod 3
Chemat F, Cravotto G (2012) Microwave-assisted extraction for bioactive compounds: theory and practice. Springer Science & Business Media.
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15(10):7313–7352
Di Donato P, Taurisano V, Tommonaro G, Pasquale V, Jiménez JMS, de Pascual-Teresa S, Poli A, Nicolaus B (2017) Biological properties of polyphenols extracts from agro industry’s wastes. Waste Biomass Valorization, 1-12.
El Ansari Zineb BM, Alain B, Ahmed L (2017) Total polyphenols and gallic acid contents in domesticated carob (Ceratonia siliqua L.) pods and leaves. Int J Pure App Biosci 5(4):22–30
Ferreres F, Grosso C, Gil-Izquierdo A, Valentão P, Mota AT, Andrade PB (2017) Optimization of the recovery of high-value compounds from pitaya fruit by-products using microwave-assisted extraction. Food Chem 230:463–474
Filip S, Pavlić B, Vidović S, Vladić J, Zeković Z (2017) Optimization of microwave-assisted extraction of polyphenolic compounds from Ocimum basilicum by response surface methodology. Food Anal Methods 10(7):2270–2280
Hadrich B, Dimitrov K, Kriaa K, (2017) Modelling investigation and parameters study of polyphenols extraction from carob (Ceratonia siliqua L.) using experimental factorial design. J Food Process Preserv 41(2).
Hayat K, Hussain S, Abbas S, Farooq U, Ding B, Xia S, Jia C, Zhang X, Xia W (2009) Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep Purif Technol 70(1):63–70
Hu S, Kim B-Y, Baik M-Y (2016) Physicochemical properties and antioxidant capacity of raw, roasted and puffed cacao beans. Food Chem 194:1089–1094
Huma ZE, Jayasena V, Nasar-Abbas SM, Imran M, Khan MK, (2017) Process optimization of polyphenol extraction from carob (Ceratonia siliqua) kibbles using microwave-assisted technique. J Food Process Preserv
Huma ZE, Jayasena V, Nasar-Abbas SM, Imran M, Khan MK (2018) Process optimization of polyphenol extraction from carob (Ceratonia siliqua) kibbles using microwave-assisted technique. J Food Process Preserv 42(2):e13450
Karakaya SE, El SN, Taş AA (2001) Antioxidant activity of some foods containing phenolic compounds. Int J Food Sci Nutr 52(6):501–508
Karami Z, Emam-Djomeh Z, Mirzaee HA, Khomeiri M, Mahoonak AS, Aydani E (2015) Optimization of microwave assisted extraction (MAE) and soxhlet extraction of phenolic compound from licorice root. J Food Sci Technol 52(6):3242–3253
Khlifa M, Bahloul A, Kitane S (2013) Determination of chemical composition of carob pod (Ceratonia siliqua L.) and its morphological study. J Mater Environ Sci 4(3):348–353
Kumar N, Kaur P, Bhatia S, (2017) Advances in bio-nanocomposite materials for food packaging: a review.Nutr Food Sci 47(4)
Kumazawa S, Taniguchi M, Suzuki Y, Shimura M, Kwon M-S, Nakayama T (2002) Antioxidant activity of polyphenols in carob pods. J Agric Food Chem 50(2):373–377
Makris DP, Kefalas P (2004) Carob pods (Ceratonia siliqua L.) as a source of polyphenolic antioxidants. Food Technol Biotechnol 42(2):105–108
Makris DP, Boskou G, Andrikopoulos NK (2007) Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J Food Compos Anal 20(2):125–132
Manousaki A, Jancheva M, Grigorakis S, Makris DP (2016) Extraction of antioxidant phenolics from agri-food waste biomass using a newly designed glycerol-based natural low-transition temperature mixture: a comparison with conventional eco-friendly solvents. Recycling 1(1):194–204
Meziani S, Oomah BD, Zaidi F, Simon-Levert A, Bertrand C, Zaidi-Yahiaoui R (2015) Antibacterial activity of carob (Ceratonia siliqua L.) extracts against phytopathogenic bacteria Pectobacterium atrosepticum. Microb Pathog 78:95–102
Moreira MM, Barroso MF, Boeykens A, Withouck H, Morais S, Delerue-Matos C (2017) Valorization of apple tree wood residues by polyphenols extraction: comparison between conventional and microwave-assisted extraction. Ind Crop Prod 104:210–220
Owis AI, El-Naggar E-MB (2016) Identification and quantification of the major constituents in Egyptian carob extract by liquid chromatography–electrospray ionization-tandem mass spectrometry. Pharmacogn Mag 12(Suppl 1):S1
Pan X, Niu G, Liu H (2003) Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem Eng Process Process Intensif 42(2):129–133
Papagiannopoulos M, Wollseifen HR, Mellenthin A, Haber B, Galensa R (2004) Identification and quantification of polyphenols in Carob Fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MS n. J Agric Food Chem 52(12):3784–3791
Piñeiro Z, Marrufo-Curtido A, Vela C, Palma M (2017) Microwave-assisted extraction of stilbenes from woody vine material. Food Bioprod Process 103:18–26
Pinela J, Prieto M, Carvalho AM, Barreiro MF, Oliveira MBP, Barros L, Ferreira IC (2016) Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: a nutraceutical-oriented optimization study. Sep Purif Technol 164:114–124
Rosa R, Tassi L, Orteca G, Saladini M, Villa C, Veronesi P, Leonelli C, Ferrari E (2017) Process intensification by experimental design application to microwave-assisted extraction of phenolic compounds from Juglans regia L. Food Anal Methods 10(3):575–586
Roseiro LB, Duarte LC, Oliveira DL, Roque R, Bernardo-Gil MG, Martins AI, Sepúlveda C, Almeida J, Meireles M, Gírio FM (2013a) Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: effect on the phenolic profile and antiproliferative activity. Ind Crop Prod 47:132–138
Roseiro LB, Tavares CS, Roseiro JC, Rauter AP (2013b) Antioxidants from aqueous decoction of carob pods biomass (Ceretonia siliqua L.): optimisation using response surface methodology and phenolic profile by capillary electrophoresis. Ind Crop Prod 44:119–126
Sęczyk Ł, Świeca M, Gawlik-Dziki U (2016) Effect of carob (Ceratonia siliqua L.) flour on the antioxidant potential, nutritional quality, and sensory characteristics of fortified durum wheat pasta. Food Chem 194:637–642
Song J, Li D, Liu C, Zhang Y (2011) Optimized microwave-assisted extraction of total phenolics (TP) from Ipomoea batatas leaves and its antioxidant activity. Innovative Food Sci Emerg Technol 12(3):282–287
Spigno G, Tramelli L, De Faveri DM (2007) Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng 81(1):200–208
Stavrou IJ, Christou A, Kapnissi-Christodoulou CP (2018) Polyphenols in carobs: a review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem
Tănase EE, Popa VI, Popa ME, Râpă M, Popa O (2016) Biodegradation study of some food packaging biopolymers based on PVA. Bulletin UASVM Animal Science and Biotechnologies 73:1
Tang X, Kumar P, Alavi S, Sandeep K (2012) Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit Rev Food Sci Nutr 52(5):426–442
Tóth ME, Vígh L, Sántha M (2014) Alcohol stress, membranes, and chaperones. Cell Stress Chaperones 19(3):299–309
Turhan I, Tetik N, Aksu M, Karhan M, Certel M (2006) Liquid–solid extraction of soluble solids and total phenolic compounds of carob bean (Ceratonia siliqua L.). J Food Process Eng 29(5):498–507
Uysal S, Zengin G, Aktumsek A, Karatas S (2016) Chemical and biological approaches on nine fruit tree leaves collected from the Mediterranean region of Turkey. J Funct Foods 22:518–532
Valdés A, Vidal L, Beltrán A, Canals A, Garrigós MC (2015) Microwave-assisted extraction of phenolic compounds from almond skin byproducts (prunus amygdalus): a multivariate analysis approach. J Agric Food Chem 63(22):5395–5402
Vourdoubas J, Skoulou VK (2017) Possibilities of upgrading solid underutilized lingo-cellulosic feedstock (carob pods) to liquid bio-fuel: bio-ethanol production and electricity generation in fuel cells-a critical appraisal of the required processes. Studies in Engineering and Technology 4(1):25–34
Yang Z, Zhai W (2010) Optimization of microwave-assisted extraction of anthocyanins from purple corn (Zea mays L.) cob and identification with HPLC–MS. Innovative Food Sci Emerg Technol 11(3):470–476
Zhao C-N, Zhang J-J, Li Y, Meng X, Li H-B (2018) Microwave-assisted extraction of phenolic compounds from Melastoma sanguineum fruit: optimization and identification. Molecules 23(10):2498
Funding
This research was supported by the Spanish Ministry of Science, Innovation and Universities (MICIU) programs MAT2017-84909-C2-2-R and AGL2015-63855-C2-1-R and by the EU H2020 project YPACK (reference number 773872). Quiles-Carrillo wants to thank GV for his FPI grant (ACIF/2016/182) and MECD for his FPU grant (FPU15/03812). Torres-Giner also thank MINECO for his Juan de la Cierva contract (IJCI-2016-29675).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Luis Quiles-Carrillo declares that he has no conflict of interest. Cristina Mellinas declares that she has no conflict of interest. Mari Carmen Garrigos declares that she has no conflict of interest. Rafael Balart declares that he has no conflict of interest. Sergio Torres-Giner declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quiles-Carrillo, L., Mellinas, C., Garrigos, M.C. et al. Optimization of Microwave-Assisted Extraction of Phenolic Compounds with Antioxidant Activity from Carob Pods. Food Anal. Methods 12, 2480–2490 (2019). https://doi.org/10.1007/s12161-019-01596-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01596-3