Abstract
In this study, bis(tetraoxacalix[2]arene[2]triazine) modified silica gel was successfully prepared and used as an efficient sorbent for solid-phase extraction. Coupled with ultra-high pressure liquid chromatography (UHPLC), the extraction performance of the sorbent was evaluated by using three flavonoids as model analytes. Main parameters, which affecting extraction efficiency were carefully optimized. The results showed that multiple intermolecular interactions were involved in the sample pretreatment procedure, including π–π, hydrophobic and hydrogen bonding interactions. Under the optimal conditions, the proposed method was applied for the analysis of three flavonoids in grape juice. Satisfactory linear ranges for flavonoids were obtained in the range of 5–200 ng mL−1 for quercetin, 1–200 ng mL−1 for luteolin, and 2–200 ng mL−1 for kaempferol, with good correlation coefficients (>0.9996). Limits of detection (LODs) were in the range of 0.5–2 ng mL−1, and the LOQs were between 1 and 5 ng mL−1. The recovery values of spiked grape juice ranged from 97 to 106% with relative standard deviations (RSDs) less than 4.7% (n = 3). This method exhibited the advantages of simplicity, rapidity, and low solvent consumption, and was promising for the separation and determination of flavonoids in grape juice and other matrixes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flavonoids are a large class of polyphenolic compounds with diphenylpropane skeletons, of which can be divided into four major classes, including 4-oxoflavonoids (flavones, flavonols, etc.), anthocyanins, isoflavones, and the flavan-3-ol derivatives (catechin andtannins) (Miean and Mohamed 2001). Flavonols such as quercetin, myricetin, and the corresponding favones are found to have the activity of anti-aging, anticancer, antioxidant, anti-inflammatory, antibacterial, and antiviral effects (Alvarez-Suarez et al. 2012; M Alvarez-Suarez et al. 2013). These compounds are distributed in medicinal plants, veggies, fruit juices, and honey (Liu et al. 2016; Wang et al. 2015). As the particular importance of their biological activity, the development of analytical methods with simplicity, rapidity, and sensitivity for flavonoids is necessary.
Various analytical methods have been adopted for flavonoid determination, such as spectrophotometer (Falkova et al. 2014), capillary electrophoresis (Memon et al. 2017; Xu et al. 2016), electrochemical (Liao et al. 2015), high-performance liquid chromatography (HPLC) (Liu et al. 2016; Yang et al. 2017), and LC-MS (Li et al. 2016). The spectrophotometry and electrochemical method is simple and low cost, but it has difficulty in the analysis of trace level flavonoids with complex sample matrices. Capillary electrophoresis has the advantages of speed, high separation efficiency, and low cost, but the stability and repeatability are not so good. The LC-MS is high cost and cannot be easily adopted by nonspecialized laboratories. On the contrary, HPLC has good precision, high resolution, and a simple analysis procedure. Meanwhile, the flavonoids in real samples are usually of trace level with complex sample matrices. Thus, it is desired to select a sample pretreatment method before chromatographic analysis.
Until now, many extraction methods have been conducted for the extraction and enrichment of trace-level flavonoids from the complex samples, including decocting extraction (Tan et al. 2012), refluxing extraction (Xu et al. 2016), supercritical fluid extraction (Pereira et al. 2016), microwave-assisted extraction (Hussein et al. 2015), ultrasonic extraction (Lu et al. 2011), and field-assisted extraction methods (He et al. 2016). Though, these methods were common and easy to operate, they are usually time consuming and labor intensive. Solid-phase extraction (SPE) (Aresta et al. 2016; Arvand et al. 2015; Liu et al. 2016; Mnayer et al. 2015; Zhou et al. 2014) has emerged as an attractive alternative for sample preparation owing to its simplicity, high enrichment factor, rapid separation, and low solvent consumption. Therefore, SPE has been developed rapidly for the trace analysis of complex sample matrices (Andrade-Eiroa et al. 2016; Płotka-Wasylka et al. 2016; Wasik et al. 2016). The type of sorbent material is the main factor affected the selectivity, sensitivity, and recovery of the SPE determining method (Płotka-Wasylka et al. 2016). So far, various SPE sorbents have been successfully developed and applied in many analytical fields. Silica C18 is the most widely used sorbent in conventional SPE methods due to its excellent performance in the extraction of hydrophobic compounds. However, the main interaction of silica C18 is hydrophobicity, which may demonstrate indiscriminate adsorption effects, so that a large amount of matrix interferences are extracted simultaneously with the analytes, which may lead to the inaccuracy of the determination. So it is desired to develop an efficient sample pretreatment method for the determination of the flavonoids in complex sample matrices.
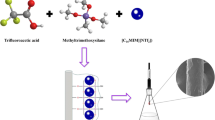
Calixarenes, as cavity-shaped cyclic molecules, are a typical representative of the third-generation host molecules. Nowadays, by modifying calixarenes onto silica gel, calixarenes stationary phases with variety of separation mechanisms have been widely studied (Chelvi et al. 2014; Deng et al. 2014, 2016; Ding et al. 2007; Hu et al. 2012, 2013, 2015, 2016; Śliwka-Kaszyńska and Ślebioda 2014; Sokoließ et al. 2000). Nowadays, a novel kind of heterocyclic calixarene, which made up of benzene and triazine rings linked by an oxygen or nitrogen atom, was emerged with excellent recognizing efficiency and selectivity (Wang 2012). Recently, by bonding bis(tetraoxacalix[2]arene[2]triazine) onto silica gel, a new separation material with multiple-function and mixed-mode separation mechanisms has been synthesized in our lab (Hu et al. 2014). Separation mechanism investigation shows that hydrophobic, π-π, hydrogen bonding and anion exchange interactions, were demonstrated to be involved in the elution process. In this case, a bis(tetraoxacalix[2]arene[2]triazine)-modified SPE sorbent (BTO-SPE) was prepared using similar preparation procedures as its stationary phase, utilized as new SPE material for the enrichment of flavonoids in complex sample matrices.
In the present work, considering the polyphenolic structure of flavonoids, a novel silica-based sorbent functionalized by bis(tetraoxacalix[2]arene[2]triazine) was designed and synthesized. Unlike the main interaction of silica C18 is hydrophobicity, the benzene rings and triazine rings, the rigid cavity may serve to improve the selectivity of SPE with multi-interaction, including π-π, hydrophobic, and hydrogen-bond interactions. Moreover, the presence of oxygen bridges on oxa-calixarene not only provides dipole-dipole interaction but also enhances the conjugation effect with adjacent aromatic rings; therefore, the increased polarity effect and π-π interactions toward flavonoids could be expected. Three flavonoids were selected as model analytes to evaluate the extraction performances of the sorbents by using UHPLC analysis. All the main factors in the SPE process were optimized. Under the optimal conditions, the proposed method was effectively applied for the analysis of flavonoids in fruit juice. The results obtained by using the developed UHPLC-DAD method based on BTO-SPE indicated that it was suitable for the determination of flavonoids in complex sample matrices.
Materials and Methods
Standard, Reagents, and Chemicals
The reference standards of quercetin, luteolin, and kaempferol were purchased from the Aladdin Chemical Reagent Co. Ltd. (Shanghai, China) (Fig. 1S). All other chemicals and solvents used in this study were of analytical grade unless specially mentioned. Deionized water was purified by a Milli-Q system from Millipore (Bedford, MA, USA). Polypropylene SPE tube (6 mL) and PE Frit (20 μm) that was used for SPE were bought from the ANPEL Laboratory Technologies (Shanghai, China). The SPE procedure was performed on an auto SPE instrument that was purchased from the Hanon Instrument Corporation (Jinan, China).
Instruments and Measurement
UHPLC was performed by using an Agilent 1290 series system equipped with a G4220B model binary pump, a DAD detector, a G1316C model thermostatic column compartment, and a G4226A sampler. Elemental analysis and IR spectra analysis of the new prepared BTO-SPE material were performed on a Flash EA 1112 elemental analyzer (Thermo Electron Corporation) and a Bruker Vector 22 instrument. A centrifuge (refrigerated centrifuge 3–18 k, Sigma, German) was employed for the centrifugal separation. A vortex mixer (Shanghai Jingke Industrial Ltd., Shanghai, China) was used for thorough mixing of solutions.
The UHPLC separations were performed on an Agilent Eclipse plus C18 (4.6 × 100 mm, 1.8 μm) with 60% (0.1% phosphoric acid water, v/v) and 40% methanol at a flow rate of 0.3 mL min−1. The analysis was performed at 30 °C with an injection volume of 3 μL. DAD detection was performed at a wavelength of 360 nm.
Synthesis of Bis(Tetraoxacalix[2]Arene[2]Triazine) SPE Sorbent (BTO-SPE)
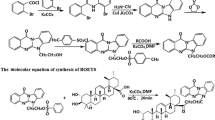
As illustrated in Fig. 2S, BTO-SPE sorbent was prepared by a two-step modification process as previously described (Hu et al. 2014). Firstly, 3-aminopropyl-bonded silica gel (APS) was synthesized and used as a precursor in the following reaction. Then, BTO-SPE was obtained by the reaction of APS and bis(tetraoxacalix[2]arene[2]triazine) in anhydrous tetrahydrofuran under nitrogen atmosphere. Being different from previous work, the silica gel used in the preparation of BTO-SPE was with the particle size of 40–60 μm and specific surface area of 500 m2 g−1. The BTO-SPE was characterized by FT-IR analysis and elemental analysis.
Preparation of Standard Solutions and Sample Collection
Stock solutions of quercetin, luteolin, and kaempferol were prepared by dissolving the accurately weighed reference substance in methanol at concentrations of 0.2 mg mL−1 and stored at 4 °C, respectively. Afterwards, in the optimization of extraction parameters section, working solutions at different concentrations were all obtained by diluting the stock solutions with ultrapure water. The pH of working solution is adjusted by H3PO4 or NaOH.
The grape juice drink was purchased from the local supermarket (Zhengzhou, China), degassed by ultrasound, filtered through a 0.22 μm filter, and then stored at 4 °C before use.
SPE Procedures
Before the SPE procedures, 500 mg of BTO-SPE sorbent was packed into a 6-mL SPE cartridge equipped with two PE Frits (20 μm). As shown in Fig. 1, after the cartridge was preconditioned with 3 mL methanol and 3 mL water, working solution (20 mL, 100 ng mL−1, pH 4) was loaded at a flow rate of 1.5 mL min−1. The cartridge was then washed with 3 mL 5% methanol-water (v/v) and eluted with 6 mL 1% acetic acid-methanol (v/v) at a flow rate of 0.5 mL min−1. The eluent was evaporated at 40 °C under a gentle stream of nitrogen until dryness and redissolved by 1 ml mobile phase. Each sample was filtered through a 0.22-μm Nylon filter (Agilent, USA) prior to UHPLC–DAD analysis.
Results and Discussion
Characterization of BTO-SPE Sorbents
Infrared spectrum analysis was conducted for both APS and BTO-SPE by using a Bruker Vector 22 instrument. A comparison of the IR spectra (Fig. 3S) of APS and BTO-SPE showed that new absorptions appeared at 1597, 1502, and 1453 cm−1, which correspond to the stretch vibration adsorption of benzene rings (C-C). These differences confirmed the successful immobilization of bis(tetraoxacalix[2]arene[2]triazine) onto silica gel.
Quantitative determination of the sorbent material was carried out by means of elemental analysis. Elemental analysis results showed that the contents of C, H, and N in APS were 4.72, 1.15, and 1.29%, respectively; the C, H, and N in BTOSP were 12.87, 1.38, and 5.35%, respectively. The bonding amount of bis(tetraoxacalix[2]arene[2]triazine) was about 323 μmol g−1 based on the change of carbon content.
SPE Optimization
In this section, the influence of different factors (sample pH, flow rate of the sample, composition and volume of washing solution, and eluent volume) on the SPE recoveries (n = 3) of flavonoids were investigated with the aim of obtaining the maximum enrichment efficiency.
Extraction Materials
It is important to select a SPE sorbent that can adsorb with the target analytes. Firstly, a preliminary test was carried out to evaluate the adsorption efficiency of materials. The results showed that the recoveries of the three selected flavonoids on BTO-SPE sorbents were ranged 50–60%, while the value were 15–25% when proceeded on APS. Considering the difference in the main structure of BTO-SPE and APS, it can be concluded the higher recovery of BTO-SPE sorbents may be attributed to the multiple interactions with the solutes, including hydrophobic, π-π, and hydrogen bonding interaction. Therefore, further optimization experiments were carried out by using BTO-SPE sorbent.
The Influence of pH
Flavonoids were acidic analytes containing phenolic hydroxyl groups, of which the acidities were positively correlated to the number and position of the hydroxyl groups. Thus, the sample solution pH will not only affect the different states of analytes but also influence the charges on the surface of the sorbent. So, the extraction of them may strongly depend on the pH value of the sample solution. In addition, the adjustment of the pH in the extraction process can also affected the solubility of the acidic/basic target analytes. Therefore, the effect of sample pH on the recoveries was investigated in the range of 3 to 8 in the study. As shown in Fig. 2, the recoveries of the flavonoids were greatly influenced by the sample pH within the range of pH examined.
The best result was observed at pH 4, with recoveries of 82.5, 80.8, and 78.0% for quercetin, luteolin and kaempferol, respectively. Therefore, pH 4 medium was selected for the entire extraction process in this work. The obtained results can be explained as this: on the one hand, the flavonoids were in protonated format low pH medium while they were in deprotonated format in high pH medium, the hydrophilic guest (protonated or deprotonated) would not be able to strongly absorbed on the reversed-phase material. Moreover, the hydrogen bonding interaction between bis(tetraoxacalix[2]arene[2]triazine) and the protonated or deprotonated flavonoids will become weaker in lower or higher pH solution. In addition to that, with the increase of the pH value, the surface of the BTO-SPE sorbent may be covered with negative charges, the charge repulsive interaction between the sorbent and the analytes may weaken the adsorption capability. Therefore, pH 4 was the best condition for this extraction process.
Optimization of the Sample Loading Rate
The flow rate of the sample may be another critical factor that affects the purification effect of analytes and the sample pretreatment time. In this section, flow rates ranged from 0.5 to 2 mL min−1 were investigated under controlled negative pressure. As shown in Fig. 3, for the three flavonoids, the extraction recoveries increased with the sample loading rate increasing from 0.5 to 1.5 mL min−1 and then decreased with the flow rate increased up to 2.0 mL min−1. In order to achieve the maximum adsorption, 1.5 mL min−1 was selected as the loading rate of sample passed through the SPE cartridge in the following experiments.
The Washing Step
A proper washing solvent is of great importance to reduce interfering substances and to improve the recovery. Thus, the effect of the composition and volume of the washing solution on the extraction of flavonoids was studied. As the interfering substances in the juice sample were mainly water soluble substances, we selected water and different concentrations of methanol solution (methanol/water, v/v) at the same volume (3 mL) to investigate the influence toward recoveries. The results showed that the recoveries decreased with the concentration of methanol increasing from 5 to 20%, and there was no significant difference observed in the range of 2–5%. Generally, high concentration of methanol in the washing solution would be more effective to reduce interfering substances in the real samples; meanwhile, which may also result in the loss of the target compounds. Therefore, 5% methanol solution was chosen as the optimal washing solution composition.
The volume of 5% methanol solution was optimized from 0.5 to 5 mL, and the result showed that washing with 3 mL of 5% methanol solution yielded higher percentage of recovery. As a result, 3 mL 5% methanol solution was chosen as the washing solution in this study.
Optimization of Eluent Type and Volume
In SPE, the eluting step is very important in order to desorb the retained analytes on sorbents with a suitable solvent. In this study, five eluting types, including 1% acetic acid/methanol (v/v), 1% acetic acid/acetonitrile (v/v), 1% acetic acid/acetone (v/v), ethyl acetate, methylene dichloride, were investigated to obtain the most suitable elution solvent. The eluent solvents containing these analytes were evaporated until dryness and redissolved by 1 ml mobile phase, then loaded on UHPLC–DAD for analysis. As can be seen from Fig. 4, highest extraction recoveries for all the studied flavonoids were obtained with 1% acetic acid–methanol as the elution solvent compared to other solvents. Therefore, 1% acetic acid–methanol was selected as the most effective elution solvent in the following extraction experiments.
The eluent volume was optimized from 0.5 to 9 mL. The recoveries of flavonoids increased as the eluent volume increases from 0.5 to 6 mL and slightly decreased as the eluent volume changed from 6 to 9 mL due to the dilution of sample and the loss in the process of nitrogen-blowing process. Thus, 6 mL 1% acetic acid–methanol was used as eluent solvent for the elution of the flavonoids.
As high elution rate would result in incomplete desorption, the elution rate is another important factor which should be evaluated. The recovery of the tested flavonoids decreased with the elution rate increased from 0.5 to 2 mL min−1. As a result, 0.5 mL min−1 was chosen as the optimal elution rate.
Optimization of the Volume of Sample Loading
The volume of sample loading may be another essential factor for the sample pretreatment step. Finding the maximum value of sample loading is especially necessary for the improvement of limit of quantification. During the optimizing experimental process, the recoveries of the three selected flavonoids decreased greatly when the sample loading rate was larger than 20 mL. Therefore, 20 mL sample was loaded onto the cartridge.
Analytical Performance of the Proposed Method
As a result of the above experiments, the optimized parameters for the extraction of flavonoids using BTO-SPE were as follows: 20 mL sample (pH 4) was loaded onto BTO-SPE sorbent with a flow rate of 1.5 mL min−1, the cartridge was then washed with 3 mL 5% methanol solution and finally eluted with 6 mL 1% acetic acid-methanol at a flow rate of 0.5 mL min−1, and the eluate was evaporated until dryness and redissolved in 1 mL mobile phase prior to the UHPLC-DAD analysis.
To ensure analytical results with the appropriate quality, the developed SPE-UHPLC-DAD method was evaluated by investigating the analytical parameters of flavonoids in water samples under the optimal conditions. The analytical characteristics of the proposed methodology are summarized in Table 1.
The calibration step affects both the final result of a determination and the value of the combined measurement uncertainty; the major aim of which is to minimize analytical measurement uncertainty and the conformance to quality assurance and quality control systems (QA/QC)(Konieczka and Namieśnik 2010). Calibration curves are determined using linear regression and are obtained using standard solutions (quercetin, luteolin, kaempferol) that were tested in triplicate according to the whole SPE procedure and the UHPLC-DAD analysis method described above. As shown in Table 1, all analytes exhibited good linearity with correlation coefficients (r 2) ranging from 0.9996 to 0.9999. The limits of detection (LODs, S/N = 3) were in the range of 0.5–2 ng mL−1, investigated under the optimal chromatographic conditions. The limits of quantification (LOQs, S/N = 3) were found to be 1–5 ng mL−1.
The method detection limits (MDLs) were calculated using the following equation (Xu et al. 2015):
In this equation, S b is the standard deviation of the calibration curve intercept and a represents the slope of the calibration curve. The method quantitation limits (MQLs) were obtained by multiplying the MDL by 3. The MQL values for the analytes were in the ranges of 0.024–0.046 ng mL−1, as shown in Table 1.
The intra-day, inter-day precision, and recoveries of this method were evaluated by determining the different concentrations of flavonoids (10, 50, and 100 ng mL−1) in water samples according to the whole SPE procedure. The intra-day precision of the method was evaluated using three replicates of standard solutions measured in 1 day on the UHPLC-DAD, in terms of relative standard deviation (RSD%) varied between 1.7% and 3.2%. The inter-day precision was analyzed on three consecutive days, and the RSD% was found to be in the ranges of 3.2–4.7%. Good recoveries were obtained by BTO-SPE, ranging from 97.2 to 106.0% (Table 2). These excellent results indicated that the developed method was simple yet provided good qualitative results for the determination of flavonoids at trace levels.
Comparisons Between C18 Material and Methods
To investigate the extraction performance of BTO-SPE, the comparison with commercial available Welchrom C18 Cartridges (Liu et al. 2016) was conducted. Three spiked concentrations (25, 50, and 100 ng mL−1) of flavonoids in water samples were tested. As can be seen from the mean recoveries (n = 3) values shown in Table 1S, the extraction performance of commercial C18 SPE material is apparently inferior to that of BTO-SPE, especially for the analytes in the spiked concentrations of 100 ng mL−1. The high extraction capacity of BTO-SPE is mainly attributed to its multiple-function interactions, including hydrophobic, π-π, hydrogen bonding, and anion-exchange interactions (Hu et al. 2014).
So far, there are many other methods that have been reported for the extraction of flavonoids. A comparative study of our developed method with other reported sample preparation procedures was performed. Both the methods developed by Liu et al. and Wang et al. used smaller amount of sorbents, but high enrichment efficiency was obtained (Liu et al. 2016; Wang et al. 2015). As it can be seen from Table 3, although some shortcomings may exist, the developed method is advantageous in significantly higher recoveries compared with the reported methods. In addition, the time and organic solvent assumption were also lower or at least comparable with the conventional method. In summary, our developed method can be considered as a promising alternative for the determination of flavonoid compounds.
Real Grape Juice Sample Analysis
To demonstrate the applicability of the proposed method for quantitative analysis of flavonoids in real samples, the developed analysis method was applied for the grape juice drink samples. Under the optimal SPE conditions described above, the sample was analyzed by UHPLC-DAD. The optimized chromatogram of grape juice drink sample 1# was shown in Fig. 5, from which we can see that flavonoids were obtained better separation from the matrix. Each component uncertainty was calculated according the method reported and shown in the Table 2S. When the confidence level is 95% and the expansion factor k = 2, the expanded uncertainty was calculated. Concentrations of flavonoids in 12 grape juice samples were determined and listed in Table 3S; the concentrations of quercetin, luteolin, and kaempferol vary greatly with the difference of origin place and brand. As listed in Table 4, in grape juice sample 1#, the analytes of quercetin and luteolin in the grape juice sample were detected; quercetin was quantified to be 11.63 ± 0.81 ng mL−1. The recoveries of the spiked flavonoids in the grape juice drink sample 1# were studied with an addition level at 10, 50, and 100 ng mL−1, which were in the range of 97.5–104.5%; the relative standard deviations (RSDs, n = 5) were in the range of 2.2–4.3%, and the results are shown in Tables 4. Therefore, the results of our study demonstrate the suitability of the proposed method for practical applications.
Conclusion
In this work, the bis(tetraoxacalix[2]arene[2]triazine)-modified silica was successfully synthesized and applied as an adsorbent for SPE. To evaluate the extraction performance of the resultant sorbents, three flavonoids were selected as the model analytes. From the results of comparative experiments, the extraction efficiency of BTO-SPE apparently surpassed that of commercial C18 sorbents. The excellent capability for the extraction of flavonoids was mainly attributed to hydrophobic hydrogen bonding and dipole–dipole and π-π interactions between them. After a series of optimization studies, a method was set up coupled with UHPLC-DAD and successfully applied to the preconcentration and determination of trace-level flavonoids in grape juice sample. The proposed SPE-UHPLC-DAD method offered an interesting and effective option for the analysis of flavonoids in biological samples. Higher recoveries and low detection limits were achieved with BTO-SPE sorbent compared with other cartridges due to its unique characteristics of mixed-mode and multiple interactions. This method is promising to be used in the cleanup and determination of flavonoids in juice samples.
References
Alvarez-Suarez JM et al (2012) Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem Toxicol 50:1508–1516
Andrade-Eiroa A, Canle M, Leroy-Cancellieri V, Cerdà V (2016) Solid-phase extraction of organic compounds: a critical review (part I). TrAC Trends Anal Chem 80:641–654
Aresta A, Di Grumo F, Zambonin C (2016) Determination of major isoflavones in soy drinks by solid-phase micro extraction coupled to liquid chromatography. Food Anal Method 9:925–933
Arvand M, Chaibakhsh N, Daneshvar S (2015) Amperometric determination of quercetin in some foods by magnetic core/shell Fe3O4@ZnO nanoparticles modified glassy carbon electrode. Food Anal Method 8:1911–1922
Chelvi SKT et al (2014) Preparation and characterization of 4-isopropylcalix[4]arene-capped (3-(2-O-β-cyclodextrin)-2-hydroxypropoxy)-propylsilyl-appended silica particles as chiral stationary phase for high-performance liquid chromatography. J Chromatogr A 1324:104–108
Deng ZF et al (2014) Liquid chromatographic behavior of two alanine-substituted calix[4]arene-bonded silica gel stationary phases. J Sep Sci 37:3268–3275
Deng ZF et al (2016) On-cartridge derivatisation using a calixarene solid-phase extraction sorbent for facile, sensitive and fast determination of formaldehyde in beer. Food Chem 211:314–319
Ding C et al (2007) Preparation and characterization of six calixarene bonded stationary phases for high performance liquid chromatography. J Chromatogr A 1170:73–81
Falkova MT, Pushina MO, Bulatov AV, Alekseeva GM, Moskvin LN (2014) Stepwise injection spectrophotometric determination of flavonoids in medicinal plants. Anal Lett 47:970–982
He Y, Xiao X, Cheng Y, Li G (2016) Progress in field-assisted extraction and its application to solid sample analysis. J Sep Sci 39:177–187
Hu K et al (2015) Development of a decaaza-cyclophane stationary phase for high-performance liquid chromatography. J Sep Sci 38:60–66
Hu K et al (2014) Development of a V-shape bis(tetraoxacalix[2]arene[2]triazine) stationary phase for high performance liquid chromatography. Talanta 130:63–70
Hu K et al (2012) Preparation, characterization and application of a new 25,27-bis-[2-(5-methylthiadiazole)thioethoxyl]-26,28-dihydroxy-para-tert-butyl calix[4]arene stationary phase for HPLC. J Sep Sci 35:239–247
Hu K et al (2016) Calixarene ionic liquid modified silica gel: a novel stationary phase for mixed-mode chromatography. Talanta 152:392–400
Hu K et al (2013) Development and application of a new 25,27-bis(l-phenylalaninemethylester-N-carbonylmethoxy)-26,28-dihydroxy-para-tert-butylcalix[4]arene stationary phase. J Sep Sci 36:445–453
Hussein LA, Ghany MFA, Yamani HZ (2015) Development of microwave-assisted extraction of trigonelline biomarker from Trigonella foenum-graecum seeds followed by high-performance thin-layer chromatographic and high-performance liquid chromatographic analyses. Jpc-J Planar Chromatogr-Mod Tlc 28:373–379
Konieczka P, Namieśnik J (2010) Estimating uncertainty in analytical procedures based on chromatographic techniques. J Chromatogr A 1217:882–891
Li Z et al (2016) Simultaneous quantification of hyperin, reynoutrin and guaijaverin in mice plasma by LC-MS/MS: application to a pharmacokinetic study. Biomed Chromatogr 30:1124–1130
Liao Y, Wang N, Ni Y, Xu J, Shao S (2015) Electrochemical sensor based on Nbim/CNT composite for selective determination of luteolin in the flavonoids. J Electroanal Chem 754:94–99
Liu H, Zhang M, Guo Y, Qiu H (2016) Solid-phase extraction of flavonoids in honey samples using carbamate-embedded triacontyl-modified silica sorbent. Food Chem 204:56–61
Lu C et al (2011) Ionic liquid-based ultrasonic/microwave-assisted extraction combined with UPLC for the determination of anthraquinones in Rhubarb. Chromatographia 74:139–144
M Alvarez-Suarez J, Giampieri F, Battino M (2013) Honey as a source of dietary antioxidants: structures, bioavailability and evidence of protective effects against human chronic diseases. Cur Med Chem 20:621–638
Memon AF, Solangi AR, Memon SQ, Mallah A, Memon N, Memon AA (2017) Simultaneous determination of quercetin, rutin, naringin, and naringenin in different fruits by capillary zone electrophoresis. Food Anal Method 10:83–91
Miean KH, Mohamed S (2001) Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agri Food Chem 49:3106–3112
Mnayer D, Fabiano-Tixier A-S, Petitcolas E, Ruiz K, Hamieh T, Chemat F (2015) Simultaneous extraction of essential oils and flavonoids from onions using turbo extraction-distillation. Food Anal Method 8:586–595
Pereira P, Cebola MJ, Oliveira MC, Bernardo-Gil MG (2016) Supercritical fluid extraction vs conventional extraction of myrtle leaves and berries: comparison of antioxidant activity and identification of bioactive compounds. J Supercrit Fluid 113:1–9
Płotka-Wasylka J, Szczepańska N, de la Guardia M, Namieśnik J (2016) Modern trends in solid phase extraction: new sorbent media. TrAC Trends Anal Chem 77:23–43
Śliwka-Kaszyńska M, Ślebioda M (2014) Polycyclic aromatic hydrocarbons as test probes to investigate the retention behavior of 1,3-alternate calix[4]arene silica-bonded stationary phases. J Sep Sci 37:543–550
Sokoließ T, Menyes U, Roth U, Jira T (2000) New calixarene-bonded stationary phases in high-performance liquid chromatography: comparative studies on the retention behavior and on influences of the eluent. J Chromatogr A 898:35–52
Tan G et al (2012) Detection and identification of diterpenoid alkaloids, isoflavonoids and saponins in Qifu decoction and rat plasma by liquid chromatography-time-of-flight mass spectrometry. Biomed Chromatogr 26:178–191
Tian M, Qiao J, Row KH (2013) Facile preparation of an ionic liquid composite mesoporous polymer as a solid phase extraction adsorbent for the separation and purification of flavonoids from Chamaecyparis obtusa. Anal Lett 46:1331–1341
Wang M-X (2012) Nitrogen and oxygen bridged calixaromatics: synthesis, structure, functionalization, and molecular recognition. Acc Chem Res 45:182–195
Wang N, Liang X, Li Q, Liao Y, Shao S (2015) Nitro-substituted 3,3'-bis(indolyl)methane-modified silica gel as a sorbent for solid-phase extraction of flavonoids. RSC Adv 5:15500–15506
Wasik A, Kot-Wasik A, Namiesnik J (2016) New trends in sample preparation techniques for the analysis of the residues of pharmaceuticals in environmental samples. Cur Anal Chem 12:280–302
Xu J-J, An M, Yang R, Cao J, Ye L-H, Peng L-Q (2016) Trace amounts of poly-beta-cyclodextrin wrapped carbon nanotubes for the microextraction of flavonoids in honey samples by capillary electrophoresis with light-emitting diode induced fluorescence detection. Electrophoresis 37:1891–1901
Xu J-J, Ye L-H, Cao J, Cao W, Zhang Q-Y (2015) Ultramicro chitosan-assisted in-syringe dispersive micro-solid-phase extraction for flavonols from healthcare tea by ultra-high performance liquid chromatography. J Chromatogr A 1409:11–18
Yang P et al (2017) Dispersive liquid-liquid microextraction method for HPLC determination of phenolic compounds in wine. Food Anal Method:1–15
Zhou N, Sang R, Zhu X (2014) Functionalized beta-cyclodextrin polymer solid phase extraction coupled with UV-visible spectrophotometry for analysis of kaempferol in food samples. Food Anal Method 7:1256–1262
Acknowledgments
The authors acknowledge the support of the National Natural Science Foundation of China (21605042, 21275133), Doctoral Research Fund of Henan University of Chinese medicine (BSJJ2014-09), and Provincial Universities Basic Scientific Research Fund of Henan University of Chinese medicine (2014KYYWF-QN05).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies with human or animal subjects.
Conflict of Interest
Kai Hu declares that he has no conflict of interest. Zhifen Deng declares that she has no conflict of interest. Sui Li declares that he has no conflict of interest. Mingxia Wu declares that she has no conflict of interest. Wei Liu declares that he has no conflict of interest. Shusheng Zhang declares that he has no conflict of interest.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Hu, K., Deng, Z., Li, S. et al. SPE-UHPLC-DAD Method for the Simultaneous Determination of Three Flavonoids in Grape Juice by Using Bis(tetraoxacalix[2]arene[2]triazine)-Modified Silica as Sorbent. Food Anal. Methods 10, 3434–3442 (2017). https://doi.org/10.1007/s12161-017-0904-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0904-4