Abstract
Theobromine (TB) is one of important natural methylxanthine alkaloids in plants and their products, but there were few reports on the electrochemistry of TB, especially electrochemical measurements using chemically modified electrode owing to its poor detectability. In this work, a simple and sensitive method for the voltammetric analysis of TB in green tea, chocolate, and coffee samples was successfully realized using nanobiohybrid sensor based on glassy carbon electrode (GCE) modified by both carboxyl-functionalized multiwalled carbon nanotubes (fMWCNTs) and soluble biopolymer sodium salt of carboxymethylcellulose (CMC). Water-dispersible nanonbiohybrids with fMWCNTs were successfully prepared using CMC assist, and CMC-fMWCNTs were characterized by scanning electron microscopy, Fourier transform infrared spectroscopy, transmission electron microscope, electrochemical impedance spectroscopy, and cyclic voltammetry. CMC-fMWCNTs/GCE showed enlarged electrochemically active surface area, good electrode stability, and enhanced electrocatalytic activity. The voltammetric behavior of TB demonstrated an irreversible electrochemical oxidation reaction involving two electrons and two protons, which could detect TB in a wider linear range from 0.5 to 80 μM with a lower limit of detection (LOD) of 0.21 μM. The developed method displayed a high sensitivity, low LOD, good sensing stability, remarkable feasibility, and satisfactory practicality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Theobromine (TB), a class of alkaloid molecules known as xanthine derivatives, is an important methylxanthine found in products of the cocoa tree, Theobroma cacao. TB also exists in the tea and chocolate-related products. In general, chocolate contains 5–7.5 mg g−1 TB (Baggott et al. 2013), and tea contains TB in concentration range of 1.2–4.4 mg cup−1 (Blauch and Traka 1983). TB is a white crystalline powder with bitter tasting, slightly soluble in water or ethanol, soluble in acids and alkalis, and its chemical properties are similar to caffeine (Vinjamuri 2008). The salts of TB are very easy to be decomposed into free acid and base in aqueous solution, while the free base is more stable (Scheller and Schubert 1991; Lind et al. 1999). Moreover, TB is a weak phosphodiesterase inhibitor and adenosine receptor blocker (Sugimoto et al. 2016); it has diuretic, cardiac excitation, vasodilatation, and smooth muscle relaxation effects (Sharma et al. 2005). Therefore, it is an important and effective component for cardiovascular and renal diseases in clinical treatment.
To date, different methods have been exploited for the measurements of TB, such as ultraviolet spectrophotometry (Schack and Waxler 1949), near-infrared spectroscopy (Álvarez et al. 2012), high-performance liquid chromatography (Thomas et al. 2004), and kinetic spectrophotometry (Xia et al. 2013). However, the sensitivity and accuracy of these methods are not high. Although liquid chromatography with tandem mass spectrometry and amperometric measurements are generally accurate for measuring real samples (Serra et al. 2011; Meyer et al. 1996), there are some disadvantages such as the complicated sample pretreatment, usage of expensive instruments, time-consuming and laborious process, and well-controlled experimental conditions. The electrochemistry is a powerful analytical method due to its merit of fast analysis, low cost, simple operation, and high sensitivity. However, there are few reports for electrochemical measurements of TB. Hansen and Dryhurst reported the electrochemistry of TB at the stationary pyrolytic graphite electrode (Hansen and Dryhurst 1971). Subsequently, Spataru et al. studied the electrochemical oxidation of TB including other xanthine derivatives using the boron-doped diamond electrode and its analytical application in three commercially available real products (Spataru et al. 2002). Vinjamuri prepared molecularly imprinted polypyrrole modified electrode for the electrochemical detection of TB, but its sensing performance is very bad (the measurement is only in concentration range from 1 to 20 mM) (Vinjamuri et al. 2008). However, the electrochemical measurement of TB was the least using chemo/biosensors based on chemically modified electrode in comparison with other xanthine derivatives like hypoxanthine, caffeine, and theophylline owing to its bad detectability.
Carbon nanomaterials for the development of electrochemical bio/chemosensors is nowadays one of the most attractive research fields because of their excellent detectability, superior electrocatalytic ability, high adsorption capacity, tremendously small size, and specific surface (Llobet 2013; Serp and Figueiredo 2009; Lukaszewicz 2006). Carbon nanotubes (CNTs), an extraordinary carbon material with tubular nanostructure, displayed excellent properties including high conductivity, large active surface area, readily modifiable surface, high electrocatalytic activity, excellent chemical stability, and good biocompatibility; this can forecast their prospective utilization in analytical chemistry for fabricating CNT-based electrochemical sensors (Wang 2005; Jacobs et al. 2010; Gooding 2005). Hydrophilic groups like carboxyl, hydroxy, and amino-functionalized carbon nanotubes (fCNTs) have obtained much concern in recent years owing to their ability to reinforce properties, enhance interfacial interactions, and improve the solubility (Gao et al. 2012). Biopolymers can enhance adhesion between the substrate electrode and the coating material, improve water processibility, enhance water stability, and more (Sahoo et al. 2010). Carboxymethyl cellulose (CMC), an excellent environment-friendly biopolymer with water solubility, satisfactory biocompatibility, good hydrophilicity, superior film-forming ability, synergistically electrocatalytic ability, and good adhesive property, could well improve or solve problems mentioned above, which were widely utilized in our previous work (Lu et al. 2015; Zhang et al. 2015a, b).
In this work, carboxyl-functionalized multiwalled carbon nanotubes (fMWCNTs) nanobiohybrids with CMC-modified glassy carbon electrode (GCE) have been successfully fabricated for the trace measurement of TB using linear sweep voltammetry (LSV). The CMC-fMWCNTs/GCE for the electrooxidation mechanism and voltammetric measurement of TB in food samples was investigated in detail (Scheme 1).
Experimental
Chemicals
TB was bought from Aladd in Reagent Co., Ltd. CMC was obtained from TCI Development Co., Ltd. fMWCNTs was obtained from Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences. Phosphate buffer (0.1 M) with various pH were prepared from aqueous solutions of 0.1 M Na2HPO4, NaH2PO4, NaOH, and H3PO4. Other reagents were of analytical reagent grade and employed without further purification, and double-distilled water was used in all experiments.
Apparatus
Cyclic voltammetry (CV) and LSV were implemented using CHI660E electrochemical workstation (Chenhua Instrument Co., Shanghai, China) in electrochemical cell with a three-electrode system. Three-electrode system includes a saturated calomel electrode (SCE) as the reference electrode, a GCE or the modified GCE as the working electrode, and a platinum (Pt) wire as the auxiliary electrode. The pH was measured by CT-6023 portable pH meter (Shanghai Lin Yu Trading Co., Ltd.).
Preparation of CMC-fMWCNTs/GCE
The GCE was carefully polished with chamois leather containing 0.05 μm alumina slurry until a mirror-shine surface appeared and was ultrasonically cleaned with deionized distilled water, absolute ethanol, and deionized distilled water for 5 min, respectively, and afterwards dried in air. CMC was ultrasonicated until completely dissolved in distilled water (0.3 mg mL−1). fMWCNTs were dispersed by sonication in CMC aqueous solution and ultrasonication until completely dispersed. The CMC-fMWCNTs/GCE was fabricated by drop-coating 5 μL water-dispersible CMC-fMWCNTs on the GCE surface, and dried in infrared lamp.
Measurements of TB
Five microliters of phosphate buffer solution containing TB was added into the sealed cell by Finnpipette, and then, the three-electrode system was placed into the cell. Prior to electrochemical tests, the chemically modified electrode was placed into phosphate buffer (pH 7.0) with the stirring time of 60 s. The CV and DPV were used for the measurement of TB, and experiments were performed to determine TB at a scan rate of 50 mV s−1 in phosphate buffer solution (PH 7.0). All experiments were performed at room temperature.
Preparation of Real Samples
The green tea, chocolate, and coffee were purchased from the local supermarket. The green tea sample was obtained by cutting them into small pieces, using a mortar and pestle to crush. Sample sizes were 1 g tea, 6.5 g chocolate, and 1 g coffee, put into 100 mL boiling water for 30 min, respectively. After filtration, the green tea sample was diluted 1000-fold, the chocolate sample was diluted 200-fold, and the coffee sample was diluted 500-fold; all sample solutions were adjusted to pH 7.5 with phosphate buffer.
Results and Discussions
Preparation of CMC-fMWCNTs
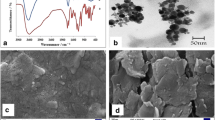
Solution-dispersible materials are often more favorable for the advanced material processing; thus, the solution processability of advanced materials is very necessary. Moreover, water-dispersible material can also reduce the cost. Figure 1 shows the water dispersibility of fMWCNTs (I) and CMC-fMWCNTs (II) with ultrasonic treatment after 20 min. Obviously, fMWCNTs could not fully dispersed in aqueous solution (Fig. 1a (I)), and they were deposited progressively in the bottom of bottles owing to their bad water processability (Fig. 1a (I)). After 1 h of static cultivate, there were massive sediments into the bottom of bottles, and only a very small amount of fMWCNTs was dispersed in water (Fig. 1b (I)), which were attributable to their carboxyl group. While CMC-fMWCNTs were thoroughly dispersed in aqueous solution after ultrasonic treatment for 20 min (Fig. 1a (II)), even CMC-fMWCNTs still were dispersed stably after 60 days of stationary cultivation (Fig. 1b (II)), indicating that CMC as an extraordinary dispersant and stabilizing agent accelerated the interaction with fMWCNTs.
Characterization of CMC-fMWCNTs
SEM
The surface morphological structures of advanced materials directly affect the performance of sensing devices. SEM was utilized to study surface morphological structures of original fMWCNTs and CMC-fMWCNTs. Figure 2a presents the pristine CMC; it was a regular and homogeneous structure with smoothness and compactness, which was in accordance with previous reports (Lu et al. 2015; Zhang et al. 2015a, b), while fMWCNTs were bended or entangled with each other and had an inattentive filamentous structure (Fig. 2b). When fMWCNTs were added into CMC aqueous solution, fMWCNTs were embedded/incorporated into CMC (Fig. 2c); this resulted in the formation of water-dispersible CMC-fMWCNTs.
TEM
TEM images of the water-dispersible CMC-fMWCNTs with different magnifications are presented in Fig. 3. As reported in previous reports, the pure MWCNTs tended to form aggregates in bundles (Saleh et al. 2008). It could be seen that CMC promoted the water dispersibility of fMWCNTs, and the formation of fMWCNT network embedded/incorporated by CMC, indicating that fMWCNTs containing CMC possessed the ability to mutually enhance their own water dispersibility.
FTIR
FTIR spectra of raw fMWCNTs (a), CMC (b), and CMC-fMWCNTs (c) are presented in Fig. 4. It could be seen from the spectrum of CMC that there were two peaks around 1108 and 1030 cm−1 for the stretching vibrations of C–O–C, while the bands around 1600 and 1427 cm−1 were attributed to stretching vibrations of the carbonyl group. The existence of the peak about 2900 cm−1 was ascribed to the stretching vibration of C–H, and a broad peak presented at 3100–3600 cm−1 was assigned to the stretching frequency of the –OH group. As for fMWCNTs, there was a stretch vibration of C–O at 1086 cm−1 and the C–O stretching vibration around 1637 cm−1 was ascribed to the carbonyl group; the broad peak at 3434 cm−1 belonged to O–H stretching vibrations. For the spectrum of CMC-fMWCNT composite, the characteristic absorption peak of both CMC and fMWCNTs became weaker and appeared a new peak, indicating that there was a strong interaction between CMC and fMWCNTs, this interaction was likely to promote the aqueous dispersion of fMWCNTs in the presence of CMC.
EIS
EIS is a powerful tool to reveal the interface characteristic of different modified electrodes; it was performed at the open circuit potential of 0.17 V and the frequency extent from 0.1 Hz to 100 kHz in a redox probe of 5 mM [Fe(CN)6]3−/4− containing 0.1 M KCl. It was well known that the semicircle diameter of Nyquist plots was used to estimate the electron transfer resistance (R ct) of different modified electrode. As can be seen from Fig. 5, the semicircle diameter of Nyquist plots increased when CMC (curve b) was modified onto the GCE surface in comparison with the impedance of the GCE (curve a); the main reason was that the electron transport was hindered by CMC owing to its nonconductivity, and made it difficult to transfer the interfacial charge. Nevertheless, the semicircle diameter of Nyquist plots decreased quickly when CMC-fMWCNTs (curve c) was drop-coated onto the GCE surface in comparison with the impedance of CMC/GCE, indicating that high conducting fMWCNTs accelerated the conduction path between the fMWCNTs/GCE and the supporting electrolyte, and enhanced the [Fe(CN)6]3−/4− diffusion towards the electrode surface due to high conductivity. More interestingly, the introduction of CMC prominently decreased the semicircle diameter of Nyquist plots of fMWCNTs/GCE; this phenomenon was in accordance with previous reports (Zhang et al. 2016; Chang et al. 2016). The main reason is that CMC promoted good dispersion of fMWCNTs network (Fig. 1), which increased the specific surface area or decreased the aggregation of fMWCNTs network due to homogeneous dispersion of fMWCNTs network (Figs. 1 and 2). They are likely to improve the electron transfer and electroconductivity (Zhang et al. 2016; Chang et al. 2016). In addition, CMC as a surfactant also accelerated probably the electron transfer and electroconductivity (Zhang et al. 2015a).
CVs
Cyclic voltammetric tests were implemented in 5 mM [Fe(CN)6]3−/4− with 0.1 M KCl (Fig. 5 (inset)). A pair of well-defined anodic and cathodic peaks was observed on different modified electrodes; the influence of the peak-to-peak separation between cathodic peak potential and the anodic peak potential of all modified electrodes was very close to the bare GCE. Moreover, it could be seen that peak currents of fMWCNTs/GCE and CMC/GCE were lower than the GCE, but peak currents of fMWCNTs-CMC/GCE were higher than the GCE, which was due to the fast electron transfer of high conducting f-SWCNT and the poor effect of CMC, which was in accordance with results of EIS.
Electrode Stability
The stability and adhesion of film electrode was very important in the application in electrochemistry of chemically modified electrode. Therefore, the electrode stability of CMC-fMWCNTs/GCE was studied in 5 mM [Fe(CN)6]3−/4− with 0.1 M KCl by performing 50 cycles of CVs tests at a scan rate of 50 mV s−1. The relative standard deviation (RSD) of redox peak currents were 0.87 and 1.18%, respectively; this result revealed that the CMC-fMWCNTs/GCE has a better cycle stability and a higher repetitive charge/discharge reversibility.
Electrochemically Active Surface Area
The electrochemically active surface area of CMC-fMWCNTs/GCE was estimated by CVs using 5 mM K3[Fe(CN)6] containing 0.1 M KCl as a probe at different scan rates (Fig. 6). For a reversible process,
where A, C 0, n, D, v, and I p stand for the electrochemically active surface area of electrode (cm−2), the concentration of redox species (mol cm−3), the electron number of redox species, the diffusion coefficient of 5 mM K3[Fe(CN)6] with 0.1 M KCl (7.6 × 10−6 cm s−2), scan rates (V s−1), and the redox peak current (A). The A of the CMC-fMWCNTs/GCE was estimated to be approximately 0.22 cm−2, which significantly enlarged the A of GCE and provided a favorable platform for electrochemically sensing of analytes.
Electrochemical Behaviors of Theobromine
Figure 7 shows CVs of 80 μM TB at bare GCE, CMC-fMWCNTs/GCE in phosphate buffer. There is no reduction peak in the reverse scan (Fig. 8), indicating that the electrochemical response was an irreversible process. No obvious oxidation peak for TB was observed at bare GCE. However, when fMWCNTs-CMC was modified on the electrode surface, a remarkable oxidation peak of TB appeared at approximately 1.34 V. The result clearly demonstrated that CMC-fMWCNTs had electrocatalytic ability towards the oxidation of TB. Also, this result indicated that CMC-fMWCNT film could provide more effective active sites than bare GCE.
CVs of 80 μM TB at CMC-fMWCNTs/GCE in phosphate buffer (pH 7.5) at scan rates from a to h with 0.025, 0.05, 0.075, 0.10, 0.15, 0.20, 0.25, and 0.3 V s−1, respectively (a). Plots of anodic peak currents vs. scan rates (b). Plots of anodic peak currents vs. the square root of scan rates (c). Plots of anodic peak potentials vs. lnv for CMC-fMWCNTs/GCE (d)
Optimization of Parameters
Influence of Scan Rates
The influence of potential scan rates on voltammetric responses of TB at CMC-fMWCNTs/GCE was given by CVs (Fig. 8a). The anodic peak currents (I pa) increased linearly with the increasing scan rates in ranges from 25 to 300 mV s−1 (Fig. 8b), suggesting that an irreversible electrode reaction for TB at CMC-fMWCNTs/GCE was a typical adsorption-controlled process (Gosser 1993). From Fig. 8c, it could be also seen an upwards bending characteristic curve of I pa vs. v 1/2, indicating that there was no linear relationship between I pa and v 1/2. However, I pa had a linear relationship with v (Fig. 8b), manifesting that the electrochemical oxidation of TB at CMC-fMWCNTs/GCE was an adsorption-controlled electrode process. There was a good linear relationship between the anodic peak potential (E pa) and lnv (Fig. 8d); this was obtained from the Laviron equation (Laviron 1974):
where α is the coefficient of charge transfer, E 0 is the formal standard potential, n is the electron number, T, R, and F have their convention meanings (T = 298 K, R = 8.314 J mol−1 K−1, and F = 96,485 C mol−1). Thus, the αn value was calculated from the slope (0.031) of E p vs. lnv. In most systems, α could usually be approximated 0.5. The αn value was calculated as 1.24, and n was calculated approximately to be 2.
Influence of pH
Figure 9a shows the influence of pH on electrochemical behaviors of TB. The voltammetric response of 80 μM TB was examined over the pH range from 5.5 to 8.5 by CVs at a scan rate of 50 mV s−1. The relationship between the anodic peak current (I pa) and pH value is shown in Fig. 9b. I pa of TB at CMC-fMWCNTs/GCE increased gradually with the increase of pH value until it acquired a maximal value of pH 7.5, then reduced quickly with the increase of pH again. Considering the sensitivity of the as-fabricated electrode for the detection of TB, the optimal pH of 7.5 was used for the voltammetric detection of TB. Besides, the relationship between anodic oxidation peak potentials (E pa) and pH value was also presented in Fig. 9c, E pa shifted negatively with the increasing pH, suggesting that the proton was participated in the electrochemical process of TB. The relationship between E pa and pH was given as follows:
where m is the proton number taking part in the electrochemical process, which was obtained from slopes of E pa vs. pH. As can be seen from Fig. 9c, the slope of dE pa/dpH plots was 0.054. So, the m/n ratio of TB was about 1, implying that the number of electron and proton was the same. Thus, the electrode reaction of TB at CMC-fMWCNTs/GCE involved a two-proton and two-electron process (Spataru et al. 2002) (Scheme 1).
Sensing Performance
Determination of TB
TB with different concentrations were obtained to study the relationship between responsive peak currents and TB concentrations; we investigated it by LSVs at CMC-fMWCNTs/GCE in phosphate buffer with pH 7.5. Responsive peak currents had good linear relationship in TB concentration ranges from 0.5 to 80 μM (Fig. 10). In addition, the limit of detection (LOD) and limit of quantitation (LOQ) were defined as 3 and 10 s/m, respectively, where m is the slope of the calibration curve and s is the standard deviation for the replicate measurement of the sample in the absence of analytes under the same conditions. In this work, the replication determination for ten times was recorded in blank solution using CMC-fMWCNTs/GCE. So, the calculated LOD and LOQ was 0.21 and 0.7 μM, respectively. The sensitivity was defined as m/A, where A stand for the electrochemically active surface area of electrode (cm−2) and the sensitivity was 104.26 μA μM−1 cm−2. As listed in Table 1, the as-developed CMC-fMWCNTs/GCE displayed the better electrochemical sensing performance for the detection of TB due to wider linear range. These also revealed that the developed CMC-fMWCNTs/GCE was a good platform for electrochemically sensing TB. In addition, the stability of successive assays of the sensor based on CMC-fMWCNTs/GCE was evaluated by detecting the peak current of 80 μM TB in phosphate buffer (pH 7.5), and a RSD of 2.21% was obtained for 30 successive assays. Indicating that the CMC-fMWCNTs modified GCE had good repeatability. Six different modified electrodes showed the RSD of 1.94%, revealing that the CMC-fMWCNTs/GCE displayed high reproducibility.
Interferences
The anti-interference of the CMC-fMWCNTs/GCE was investigated by measurement of 8 μM of TB in the presence of different common interferents. Under optimal experimental conditions, different interferents like amino acid, organic acids, vitamins, saccharides, and anion-cations were added. Many of them did not produce any obvious interference towards the current response of CMC-fMWCNTs/GCE because of bad electroactivity, poor interaction, or relatively far away from anodic peak potential of TB. Table 2 lists the LSV of FeCl3, glutamic acid, glucose, and ascorbic acid with different concentrations; they displayed weak interference (the alteration of the anodic oxidation peak currents of TB was not more than 5%), implying that the proposed method has good selectivity.
Practical Application
To evaluate the feasibility of the developed sensor, the CMC-fMWCNTs/GCE was utilized to measure the concentration of TB in chocolate, green tea, and coffee samples by using a standard addition method, respectively. Three different concentrations of TB were added into three samples to measure the recoveries of TB by LSV, respectively. The obtained results are listed in Table 3; the range of the recovery was 93.5–109.7%, and the range of RSD was 0.18–2.11%, indicating that the described method is adequate for practical application in detecting TB in real samples.
Conclusions
CMC-fMWCNT nanobiocomposites with water dispersibility were successfully prepared, and their structure and properties were characterized. The CMC-fMWCNTs/GCE displayed enlarged electrochemically active surface area, good electrode stability, an irreversible electrochemical reaction towards TB with a well-defined electrooxidation peak at approximately 1.34 V, and enhanced electrocatalytic activity for the electrooxidation of TB, accompanying a two-proton and two-electron process, which could detect TB in a linear range from 0.5 to 80 μM with a low LOD of 0.21 μM, high sensitivity, and good sensing stability; the developed method was employed to measure the content of TB in green tea, chocolate, and coffee samples with good feasibility and practicality. Satisfactory results indicated that CMC-fMWCNT nanobiocomposites will be good and attractive candidates for practical applications in the cocoa, tea, and chocolate-related products.
References
Álvarez C, Pérez E, Cros E, Lares M, Assemat S, Boulanger R, Davrieux F (2012) The use of near infrared spectroscopy to determine the fat, caffeine, theobromine and (−)-epicatechin contents in unfermented and sun-dried beans of Criollo cocoa. J Near Infrared Spectros 20:307–315. doi:10.1255/jnirs.990
Baggott MJ, Childs E, Hart AB, De Bruin E, Palmer AA, Wilkinson JE, De Wit H (2013) Psychopharmacology of theobromine in healthy volunteers. Psychopharmacology 228:109–118. doi:10.1007/s00213-013-3021-0
Blauch JL, Traka SM (1983) HPLC determination of caffeine and theobromine in coffee, tea, and instant hot cocoa mixes. J Food Sci 48:745–747. doi:10.1111/j.1365-2621.1983.tb14888.x
Chang J, Xiao W, Liu P, Liao XN, Wen YP, Bai L, Li LJ, Li MF (2016) Carboxymethyl cellulose assisted preparation of water-processable halloysite nanotubular composites with carboxyl-functionalized multi-carbon nanotubes for simultaneous voltammetric detection of uric acid, guanine and adenine in biological samples. J Electroanal Chem 780:103–113. doi:10.1016/j.jelechem.2016.09.013
Gao C, Guo Z, Liu JH, Huang XJ (2012) The new age of carbon nanotubes: an updated review of functionalized carbon nanotubes in electrochemical sensors. Nano 4:1948–1963. doi:10.1039/C2NR11757F
Gooding JJ (2005) Nanostructuring electrodes with carbon nanotubes: a review on electrochemistry and applications for sensing. Electrochim Acta 50:3049–3060. doi:10.1016/j.electacta.2004.08.052
Gosser DK (1993) Cyclic voltammetry: simulation and analysis of reaction mechanisms. VCH: New York.p43
Hansen BH, Dryhurst G (1971) Electrochemical oxidation of theobromine and caffeine at the pyrolytic graphite electrode. J Electroanal Chem 30:407–416. doi:10.1016/0368-1874(71)87024-7
Jacobs CB, Peairs MJ, Venton BJ (2010) Review: Carbon nanotube based electrochemical sensors for biomolecules. Anal Chim Acta 662:105–127. doi:10.1016/j.aca.2010.01.009
Laviron E (1974) Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J Electroanal Chem 52:355–393. doi:10.1016/S0022-0728(74)80448-1
Lind T, Siegbahn PEM, Crabtree RH (1999) A quantum chemical study of the mechanism of tyrosinase. J Phys Chem B103:1193–1202. doi:10.1021/jp982321r
Llobet E (2013) Gas sensors using carbon nanomaterials: a review. Sens Actuat B 179:32–45. doi:10.1016/j.snb.2012.11.014
Lu S, Bai L, Wen YP, Li MF, Yan D, Zhang R, Chen KJ (2015) Water-dispersed carboxymethyl cellulose-montmorillonite-single walled carbon nanotube composite with enhanced sensing performance for simultaneous voltammetric determination of two trace phytohormones. J Solid State Electrochem 19:2023–2037. doi:10.1007/s10008-014-2695-5
Lukaszewicz JP (2006) Carbon materials for chemical sensors: a review. Sens Lett 4:53–98. doi:10.1166/sl.2006.020
Meyer A, Ngiruwonsanga T, Henze G (1996) Determination of adenine, caffeine, theophylline and theobromine by HPLC with amperometric detection. Fresenius J Anal Chem 356:284–287. doi:10.1007/s0021663560284
Sahoo NG, Rana S, Cho JW, Li L, Chan SH (2010) Polymer nanocomposites based on functionalized carbon nanotubes. Prog Polym Sci 35:837–867. doi:10.1016/j.progpolymsci.2010.03.002
Saleh NB, Pfefferle LD, Elimelech M (2008) Aggregation kinetics of multiwalled carbon nanotubes in aquatic systems: measurements and environmental implications. Environ Sci Technol 42:7963–7969. doi:10.1021/es801251c
Schack JA, Waxler SH (1949) An ultraviolet spectrophotometric method for the determination of theophylline and theobromine in blood and tissues. J Pharmacol Exp Ther 97:283–291
Scheller F, Schubert F (1991) Biosensors. Elsevier Science Publishers B.V, Amsterdam
Serp P, Figueiredo JL (2009) Carbon materials for catalysis. John Wiley & Sons, Hoboken
Serra A, Macià A, Romero MP, Piñol C, Motilva MJ (2011) Rapid methods to determine procyanidins, anthocyanins, theobromine and caffeine in rat tissues by liquid chromatography-tandem mass spectrometry. J Chromatogr B879:1519–1528. doi:10.1016/j.jchromb.2011.03.042
Sharma V, Gulati A, Ravindranath SD, Kumar V (2005) A simple and convenient method for analysis of tea biochemicals by reverse phase HPLC. J Food Compos Anal 18:583–594. doi:10.1016/j.jfca.2004.02.015
Spataru N, Sarada BV, Tryk DA, Fujishima A (2002) Anodic voltammetry of xanthine, theophylline, theobromine and caffeine at conductive diamond electrodes and its analytical application. Electroanalysis 14:721–728. doi:10.1002/1521-4109(200206)14:11<721::AID-ELAN721>3.0.CO;2-1
Sugimoto N, Katakura M, Matsuzaki K, Ohno-Shosaku T, Yachie A, Shido O (2016) Theobromine, the primary methylxanthine found in Theobroma cacao, can pass through the blood-brain barrier in mice. FASEB J.30 (1 Supplement), lb635-lb635
Thomas JB, Yen JH, Schantz MM, Porter BJ, Sharpless KE (2004) Determination of caffeine, theobromine, and theophylline in standard reference material 2384, baking chocolate, using reversed-phase liquid chromatography. J Agr Food Chem 52:3259–3263. doi:10.1021/jf030817m
Vinjamuri AKK (2008) A selectivity study on the use of caffeine and theobromine imprinted polypyrrole surface electrodes Masters Theses:5
Vinjamuri A, Burris SC, Dahl D (2008) Caffeine and theobromine selectivity using molecularly imprinted polypyrrole modified electrodes. ECS Trans 13:9–20. doi:10.1149/1.3002805
Wang J (2005) Carbon-nanotube based electrochemical biosensors: a review. Electroanalysis 17:7–14. doi:10.1002/elan.200403113
Xia Z, Ni Y, Kokot S (2013) Simultaneous determination of caffeine, theophylline and theobromine in food samples by a kinetic spectrophotometric method. Food Chem 141:4087–4093. doi:10.1016/j.foodchem.2013.06.121
Zhang H, Xu JK, Wen YP, Wang ZF, Zhang J, Ding WC (2015a) Conducting poly (3, 4-ethylenedioxythiophene): poly (styrene-sulfonate) film electrode with superior long-term electrode stability in water and synergistically enhanced electrocatalytic ability for application in electrochemical sensors. Synth Met 204:39–47. doi:10.1016/j.synthmet.2015.03.010
Zhang J, Xu JK, Wen YP, Wang ZF, Zhang H, Ding WC (2015b) Voltammetric determination of phytoinhibitor maleic hydrazide using PEDOT: PSS composite electrode.J. Electroanal Chem 751:65–74. doi:10.1016/j.jelechem.2015.05.032
Zhang ZX, Zhang J, Zhang H, Xu JK, Wen YP, Ding WC (2016) Characterization of PEDOT: PSS-reduced graphene oxide@Pd composite electrode and its application in voltammetric determination of vitamin K3. J Electroanal Chem 775:258–266. doi:10.1016/j.jelechem.2016.06.005
Acknowledgements
The authors would like to appreciative of financial support from the National Science Foundation of China (31660492, 51662014), Jiangxi Provincial Department of Education (GJJ150428), China Postdoctoral Science Foundation (2015M571987), Special Funds for Jiangxi Province Postdoctoral Research Funds (2015KY44), and Jiangxi Provincial Innovation Fund of Postgraduates (No. YC2016-S183).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Yingqiong Peng declares that she has no conflict of interest. Wenjuan Zhang declares that she has no conflict of interest. Juan Chang declares that she has no conflict of interest. Yaoping Huang declares that she has no conflict of interest. Li Chen declares that she has no conflict of interest. Hong Deng declares that he has no conflict of interest. Zhong Huang declares that he has no conflict of interest. Yangping Wen declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects. This is an original research article that has neither been published previously nor considered presently for publication elsewhere. All authors named in the manuscript are entitled to the authorship and have approved the final version of the submitted manuscript.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Peng, Y., Zhang, W., Chang, J. et al. A Simple and Sensitive Method for the Voltammetric Analysis of Theobromine in Food Samples Using Nanobiocomposite Sensor. Food Anal. Methods 10, 3375–3384 (2017). https://doi.org/10.1007/s12161-017-0867-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0867-5