Abstract
A simple, sensitive, rapid and eco friendly micellar liquid chromatographic method was developed for the detection of banned color methyl orange in counterfeit saffron and prepared foodstuffs. Methyl orange (p-[[p-(dimethylamino) phenyl] azo] benzenesulfonic acid sodium salt) is a hazardous dye used in titration and is known to be used as common adulterant in counterfeit saffron and cooked foodstuffs like Jalebi (Indian sweet), mango shake, Namkeen (salted snacks), tomato ketchup and ice candy etc., due to its pleasant orange color. In the present work, methyl orange was detected in various food samples using direct injection micellar liquid chromatography without any pretreatment step. A C18 column with an optimum micellar mobile phase containing 0.05-M sodium dodecyl sulfate (SDS), 2 % pentanol buffered to pH 7 was used. Detection was carried out at 458 nm. The retention time was 3.7 min without showing any matrix effect. Linearity (r > 0.9999), intraday and interday precision RSD (%) was less than 1.00 in micellar media. Limit of detection and quantification was found to be 0.05 mg/kg and 0.10 mg/kg, respectively. Robustness study was also included as a part of method validation. The developed method proved to be reliable and sensitive for determination of methyl orange in real food samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term color additive can be applied to any dye, pigment, or other substance made or obtained from a vegetable, animal, or mineral origin that is capable of coloring food (Branen et al. 1989). Food colorants, natural or synthetic, are commonly added to foodstuffs in order to compensate for the loss of natural color and also to provide a desired appearance to it. Among the synthetic food colors, the number of permitted food colorants has been reduced for food safety reasons in recent years. But mockingly, various types of restricted synthetic food colorants are still being widely used all over the world in food products. Methyl orange (p-[[p-(dimethylamino) phenyl] azo] benzenesulfonic acid sodium salt), (Fig. 1) is a toxic dye, usually used in titration (Wikipedia 2010), and is banned for use in food products (Manual of methods of analysis of food additives, Lab manual 8 2005a). But recently, it has been observed that it is being used in number of food products especially in developing countries like India, Bangladesh, Nepal, and Pakistan etc. As far as health is concerned, regular consumption of this dye could be lethal and may cause serious health hazards (Spectrum 2014). The continuous usage of these non-permitted colors has major economic consequences on world-wide food industries as well as on public health and society, so the use of synthetic colorants in food is strictly controlled by Prevention of Food Adulteration Act (PFA) of India. But to the surprise, methyl orange is still being used by manufacturers because of its color, efficacy, and low cost.
Saffron is one of the most expensive spices available in the market. It is derived from the dried stigmas of flowers of Crocus sativus (Esfanjani et al. 2015; Khazaei et al. 2016). It’s a labor-intensive crop, which means that saffron commands a high price; each crocus produces just three stigmas, which are hand-picked and then dried, and it takes thousands of stigmas to make just one ounce of the spice. Hence, it is not very cost-efficient for the local or small scale manufacturers. To manage the demands and minimize the manufacturing cost, they use dried stigmas of some different flowers and add artificial colors to make them appear as the original ones. Methyl orange was found to be the most common artificial color that is added to impart orange color in stigmas of different flowers. Further, the essence of saffron is also added to those stigmas for aroma and genuine appearance. Methyl orange is not only used in the production of counterfeit (replica) saffron; it is also reported to be used in some prepared foodstuffs like Jalebi (an Indian sweet made by deep-frying a wheat flour or maida flour batter in circular shapes, which are then soaked in sugar syrup), etc. (Chemical land 2014; Sforza 2012).
In developing countries like India, where there are no proper rules and regulations for the sale of adulterated and unhealthy edible items and also lack in strict law to govern the marketing of poor quality food, it is necessary to develop a rapid, inexpensive, eco friendly, and reliable technique for determination of methyl orange in counterfeit or adulterated saffron and prepared foodstuffs for assurance of food quality and safety. Various analytical techniques have been reported for the detection and identification of different non-permitted or banned food colors in foodstuffs such as thin layer chromatography (TLC). HPTLC and spectroscopy were used for the detection of adulteration in spices (Mohammad et al. 2015; Sabinto et al. 2011; Djozan et al. 2014; Khazaei et al. 2014; Mehrnia et al. 2016), liquid chromatography mass spectroscopy (LC-MS) in red chili and other prepared foodstuffs (Yoshioka and Ichihashi 2007; Minioti et al. 2007), and high performance liquid chromatography (HPLC) coupled with UV or diode array detector in spices and other spice mixtures (Ertas et al. 2007; Botek et al. 2007; Ishikawa 2007). In spite of the above mentioned techniques, it was established that there was no method reported for detection of methyl orange in foodstuffs. So in the present work, a fast, cost-efficient, and reliable method was developed to detect methyl orange in foodstuffs using micellar liquid chromatography.
Micellar liquid chromatography (MLC) is an alternative to conventional reversed-phase liquid chromatography (RPLC) with aqueous organic mobile phases (Berthod and Alvarez 2000). The most important advantage of MLC is that it does not require any prior sample pretreatment. It joins the advantages of both micellar media with the separation capability of liquid chromatography. Other remarkable advantages of MLC include low cost and toxicity, biodegradability of the solvents used, and the easy dissolution of analytical samples. MLC has proved itself to be a useful technique in the determination of diverse group of compounds. This technique was successfully applied for determination of dyes, drugs, pesticides, toxicants, and other complex compounds (Ashok et al. 2014; Bose et al. 2005; Agrawal et al. 2014; Ashok et al. 2015). The stable and reproducible behavior of micellar mobile phases allows accurate prediction of solute retention with a model that can also be used to optimize the separation of mixtures.
The aim of present study is to develop a simple and rapid method for detection and quantification of methyl orange in foodstuffs using direct injection eco friendly MLC technique.
Material and Methods
Apparatus and Instrumentation
The liquid chromatographic system used was of Shimadzu Corporation (Kyoto, Japan) equipped with isocratic pump LC-20 AT having isocratic flow (flow rate 1.0 ml/min), an auto sampler SIL-20 AC (20-μl injection volume), which was coupled to a diode array detector SPD-M20A, having a range of 190–800 nm (was set at 458 nm). The column employed was Princeton C-18 column (250 mm × 4.6 mm, 5-μm particle size) from Princeton Chromatography INC, (New Jersey, USA). pH of the mobile phase was measured by a 101 E pH meter from EI products (Parwanoo, India). The balance used was CY204 Digital Balance, Citizen Scale Pvt. Ltd. (Metuchen, NJ). The vortex shaker was manufactured by Electrolab India (Mumbai, India), and the sonification unit was from Citizen (I) Private limited (Mumbai, India).
Data Analysis
The chromatographic data was acquired and treated with the system software (Shimadzu workstation LC solution software), version 1.22 SP1, which was used for integration of peaks, to obtain dead time, retention time, efficiency, and asymmetry. The data obtained was further computed with Microsoft Office Excel Software version 2007 (Microsoft Corporation, Redmond, Washington).
Chemicals and Reagents
Sodium dodecyl sulfate (SDS) and sodium dihydrogen phosphate were purchased from Himedia Laboratories Pvt. Ltd. (Mumbai, India). Hydrochloric acid, sodium hydroxide, methanol, pentanol, and water (HPLC grade) from Rankem, Ranbaxy Fine Chemicals Ltd. (New Delhi, India). Methyl orange (p-[[p-(dimethylamino) phenyl] azo] benzenesulfonic acid sodium salt) in pure form was purchased from Himedia Laboratories Pvt. Ltd. (Mumbai, India). 0.45 μm nylon membrane filter was purchased from Whatman International Ltd. (Maidstone, England).
Mobile Phase Preparation
Micellar mobile phases were prepared by weighing an appropriate amount of sodium dodecyl sulfate (SDS) and sodium di-hydrogen phosphate. The reagents were dissolved in HPLC grade water, and the pH of the solution was adjusted to desired pH (3, 5, or 7) either by adding few drops of NaOH or HCL. Finally, a suitable volume of modifier (propanol, butanol, or pentanol) was added.
Sample Procurement
Ten different saffron samples of various non-registered trademarks were procured from local sweet shops, general stores, and local markets for determining the adulteration of methyl orange. All above mentioned samples were specifically collected from Bhopal and Saugar cities (M.P.) and Jhansi city (U.P.), India.
Indian sweet (Jalebi) samples were collected from different sweet shops in rural areas near the city, namkeen (salted snacks) samples were purchased from different general stores, samples of mango shake were procured from local juice centers, tomato ketchup samples were procured from local cafeterias and restaurants, and ice candy samples were collected from neighboring mobile ice cream hawkers/vendors. All the above samples were procured from Saugor and Jhansi cities in India. Five different samples of each foodstuff were collected from different areas. Salted snack samples (name withheld) of various registered and non-registered trademarks were purchased from local market. Indian sweet (Jalebi), mango shake, tomato ketchup, and ice candy samples were all freshly prepared snacks and refreshments and did not have any brand name.
Sample Preparation
Standard Methyl Orange
Stock solution of standard methyl orange was prepared by dissolving 10-mg methyl orange in 100-ml HPLC grade water (100 mg/kg) filtered through 0.45-μm nylon membrane filter and stored at 4 °C. It was further diluted at different concentrations for studying linearity.
Real samples
Ten dissimilar samples of saffron namely A, B, C, D, E, F, G, H, I, and J were procured, and each was analyzed five times during present study. The saffron samples were dissolved in equal amount (w/v) of HPLC grade water and kept overnight for extraction of color. Further, the extracted color was filtered through 0.45-μm nylon membrane filter and stored at 4 °C.
Indian sweet (Jalebi) samples were kept overnight in equal amount of HPLC grade methanol (w/v), and the extract was filtered before injection. Namkeen (salted snack) samples were mashed and left overnight in HPLC grade methanol (w/v) for extraction of color. Further, the extract was filtered. Since mango shake, tomato ketchup, and ice candy samples were already in liquid form, they were simply diluted with equal amount of HPLC grade water (v/v) and filtered. All above solutions were filtered through 0.45-μm nylon membrane filter, stored at 4 °C and were brought at room temperature before injection. Five different samples of each prepared foodstuffs were analyzed five times during present study, and the mean was calculated.
Method Development and Optimization
Selection of Wavelength
For the selection of optimum wavelength, the stock solution of standard methyl orange was diluted up to 10 ppm and a spectrum was taken using a Shimadzu UV–vis spectrophotometer UV-210 (Kyoto, Japan). Two prominent absorption maxima were observed when a scan was plotted between 190 to 800 nm. 458 nm showed maximum absorbance, while the second absorption was seen at 252 nm, which was almost 50 % weaker than the spectra obtained at 458 nm. As yellow-orange color falls in 435–490 nm range of visible region of electromagnetic spectrum, the obtained wavelength for methyl orange also corresponds to this range. Based on the above mentioned data and reported literature (Elsalamony and Ghoneim 2013), 458 nm was selected as optimum wave length for chromatographic detection of methyl orange throughout the study.
Selection of Mobile Phase
C-18 column was selected for the present study. The mobile phase was prepared using SDS as surfactant, because it offers a high degree of selectivity, sensitivity, desorption, and the cleanup process is also easy to perform. For pH selection, three values falling in the working range of column i.e., pH (3, 5, and 7) were selected. No change in the retention time was observed using the selected pH range, but other chromatographic parameters (efficiency and asymmetry factors) were comparatively better while using pH 7. Based on the above chromatographic parameters, pH 7 was chosen as the optimum pH. After selecting column, surfactant, and buffer pH, several injections were performed to find out the best composition of mobile phase. Methyl orange was chromatographed using different SDS concentrations (0.05, 0.10, and 0.15 M), and retention times obtained were 4.8, 5.6, and 6.2 min, respectively. It is evident that 0.05 M SDS gave best results as the retention time was minimum, but other parameters like efficiency and asymmetry were not satisfactory. In order to improve the peak shape and asymmetry, it was decided to introduce an alcohol in the mobile phase. In the mobile phase, the concentration of SDS was fixed at 0.05 M and three different alcohols (%, v/v) i.e., 12.5 % for propanol, 7 % for butanol, and 2 % for pentanol, were mixed in the mobile phase. The retention time then obtained were 5.1, 4.2, and 3.7 min for propanol, butanol, and pentanol, respectively. Efficiency and asymmetry were also improved when the chain length of alcohol was increased.
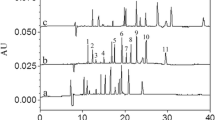
Comparing the results obtained using different mobile phase combinations, and based on chromatographic parameters, it was concluded to use 0.05 M SDS, 2 % pentanol buffered to pH 7 as the optimum mobile phase. The chromatogram of standard methyl orange using optimum mobile phase is given in Fig. 2.
Results
Method Validation
The MLC method developed herein was essentially developed for detection of methyl orange in uncooked as well as prepared foodstuffs. The developed method was validated according to the Food and Drug Administration (FDA) validation guidelines (Manual of methods of analysis of food additives, Lab manual 8 2005b). Linearity, limit of detection (LOD), limit of quantification (LOQ), precision, accuracy, and robustness were the different parameters that were validated.
Linearity
Linearity study was carried out using different matrices in a series of seven different concentrations of methyl orange i.e., 0.05, 0.10, 0.25, 0.50, 1.0, 2.50, and 5.0 mg/kg and injected in triplicate using optimum micellar mobile phase. Quantification of methyl orange in samples was carried out on the basis of peak areas and comparison with a calibration curve obtained with the corresponding standards. Linearity was calculated (r > 0.9999).
Limit of Detection and Limit of Quantification
Limit of detection (LOD) for standard methyl orange was determined using 3 s criterion involving a series of five solutions containing a low concentration of methyl orange. The results were based on the standard deviation of the response and also on the slope of a specific calibration curve containing methyl orange. LOD and LOQ for standard methyl orange were calculated to be 0.05 and 0.10 mg/kg, respectively (Table 1).
Interday and Intraday Precisions
Intraday and interday precisions of the developed method were determined by analyzing methyl orange at three different concentrations, i.e., 0.5, 1.0, and 2.5 mg/kg, in standard form, saffron samples, and prepared foodstuffs. Intraday precision was determined by assaying these three solutions five times on the same day, and interday precision was evaluated using the same samples over five different days. Intra and interday precision RSD (%) in standard methyl orange was found to be in the range of 0.51–0.80 and 0.56–0.92 %, in saffron samples 0.58–0.85 and 0.65–0.90 %, and in prepared foodstuffs 0.70–0.92 and 0.81-0.99 %, respectively. The low variability and high precision of the results obtained in different days and using different concentration are quite evident, and this indicates the usefulness of the method.
Recovery
The recovery of methyl orange from saffron samples were determined by spiking the dye-free saffron samples, diluted with HPLC grade water and known amount of the methyl orange at three different concentrations within the calibration range. The spiked samples were processed and analyzed by five replicates with the developed procedure. The relative recovery was calculated by comparing the concentration obtained from dye supplemented sample with the actual added amounts. The results obtained show satisfactory recoveries of methyl orange in the range of 98.75–99.23 %.
Robustness
The developed method was found to be robust based on the fact that the small deliberate changes in the method conditions did not have any significant effect on the chromatographic behavior of methyl orange. The developed method was also tested on different instruments and other counterfeit saffron samples and was found to be precise. The chromatographic conditions such as the flow rate, percentage of the organic modifier, strength of SDS, and pH of mobile phase were altered. Variation in flow rate had stronger influence on the retention time of the studied compound than any other selected parameters. However, variations in all the above mentioned parameters had no significant effect on retention time and other chromatographic parameters of methyl orange (Table 2).
Application of Developed MLC Method
Analysis of Methyl Orange in Saffron Samples
The developed MLC method was applied to various saffron samples procured from the market. The saffron samples were labeled as A, B, C, D, E, F, G, H, I, and J. Each sample of saffron was analyzed five times, and the mean value was considered. Among these 10 samples screened in the laboratory, 7 samples (A, C, D, F, G, H, and J) showed the presence of methyl orange: 8.05, 3.41, 4.11, 5.23, 6.79, 7.75, and 6.91 mg/kg, respectively. Sample B, E, and I gave negative result for methyl orange in saffron samples. Table 3 demonstrates the concentration of methyl orange detected in these samples and their standard deviation. Quantification of methyl orange in counterfeit saffron samples was performed using calibration curves and confirmed by comparing absorption spectra of the samples with that of standard. The low detection limits allowed the accurate detection of dye in food materials comparative to other techniques (Falcon and Gandara 2005; Rajabi et al. 2015; Kobra et al. 2015; Shukla and Iqbal 2015; Gonzalez et al. 2003; Harp et al. 2012; Sarfarazi et al. 2015; Kiseleva et al. 2003). Figure 3 shows the selected chromatogram of counterfeit saffron sample A, which was found positive for presence of methyl orange.
Analysis of Methyl Orange in Prepared Foodstuffs
Five different samples (A, B, C, D, E) of each of Indian sweet, salted snack, mango shake, tomato ketchup, and ice candy were analyzed using developed MLC method. Four out of 5 samples of Indian sweet, 3 out of 5 samples of salted snack, 2 out of 5 samples of mango shake, and 3 out of 5 samples of tomato ketchup were found positive for the presence of methyl orange. Table 4 shows the amount of methyl orange detected in these samples and their standard deviation. All above samples were analyzed five times to obtain the mean value with the help of MS-Excel. Selected chromatograms are given. Figure 4 illustrates the chromatogram of Jalebi sample A.
In ice candy that is readily available in local market and mainly consumed by children, 5 out of 5 samples were found adulterated with methyl orange. Quantity of methyl orange detected is given in Table 4.
Discussion
The results demonstrated that the method was suitable for routine analysis of methyl orange in raw as well as cooked food samples. The specificity studies have shown that the analyte can be detected without interferences in complex matrices i.e., cooked foodstuffs, despite the presence of endogenous compounds. The sensitivity and the calibration range are useful to detect the analyte at the levels that can be found in cooked foodstuffs. The low variability and high precision of the results obtained in different days are evident, which indicate the usefulness of the method. The developed method was found to be robust as the small deliberate changes in the method conditions did not have any significant effect on the chromatographic behavior of the analyte. The usefulness of the method has been assessed by the quantification of methyl orange in real samples from counterfeit saffron and cooked foodstuffs, no interference has been observed, and the levels of methyl orange have been determined with adequate precision.
Conclusion
It is a pioneering method to detect methyl orange in edibles. The MLC method developed here is comparatively less time-consuming, less toxic, and cost-effective in comparison to the reported liquid chromatographic techniques in different matrices and does not require pretreatment steps even while analyzing prepared foodstuffs. The developed MLC method can be used for detection of the methyl orange in raw as well as prepared foodstuffs. The low detection limit obtained allows the accurate determination of methyl orange in foodstuff at low concentrations. The developed method can be easily used by law enforcement agencies and food testing laboratories that perform analyses to ensure adulteration of foodstuffs and for quality control of food products. It is a fast, simple, and readily available analytical method to evaluate a product’s authenticity.
References
Agrawal N, Peiro SM, Romero JE, Durgbanshi A, Bose D, Vicente JP, Broch SC (2014) Determination of paroxetine in blood and urine using micellar liquid chromatography with electrochemical detection. J Chromatogr Sci 20:1–7
Ashok V, Agrawal N, Durgbanshi A, Romero JE, Bose D (2014) Determination of adulteration of malachite green in green pea and some prepared foodstuffs by micellar liquid chromatography. J AOAC Inter 97:1387–1392
Ashok V, Agrawal N, Durgbanshi A, Romero JE, Bose D (2015) A novel micellar chromatographic procedure for the determination of metanil yellow in foodstuffs. Anal Methods 7:9324–9330
Berthod A, Alvarez CG (2000) In: Cazes J (ed) Micellar liquid chromatography. Marcel-Dekker, New York
Bose D, Durgbanshi A, Dominguez AM, Capella Peiro ME, Broch SC, Romero JE (2005) Rapid determination of acetaminophen in physiological fluids by liquid chromatography using SDS mobile phase and ED detection. J Chromatogr Sci 43:313–318
Botek P, Poustka J, Hajslova J (2007) Determination of banned dyes in spices by liquid chromatography mass spectrometry. Czech J Food Sci 25:17–24
Branen AL, Davidson PM, Salminen S (1989) Food Additive. Marcel Dekker Inc, New York, pp 139–193
Chemical land (2014) Methyl Orange. http://chemicalland21.com/specialty chem/finechem/ methylorange.html Accessed 08 November 2014
Djozan D, Karimian G, Jouyban GA, Iranmanesh F, Gorbanpour H, Akbar A (2014) Discrimination of saffron based on thin-layer chromatography and image analysis. J Planer Chromatogr 4:274–280
Elsalamony RA, Ghoneim SA (2013) Tio2 anatase nano-powder prepared by a gamma ray irradiation and photocatalytic activity. 2nd International Conference on Energy Systems and Technologies; Cairo, Egypt
Ertas E, Ozer H, Alasalvar C (2007) A rapid HPLC method for determination of sudan dyes and para red in red chili pepper. Food Chem 105:756–760
Esfanjani AF, Jafari SM, Assadppor E, Mohannadi A (2015) Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. J Food Enging 165:149–155
Falcon MS, Gandara SJ (2005) Determination of food dyes in soft drinks containing natural pigments by liquid chromatography with minimal cleanup. Food Control 16:293–297
Gonzalez M, Gallego M, Valcarcel M (2003) Determination of natural and synthetic colorants in prescreened dairy samples using liquid chromatography-diode array detection. J Anal Chem 75:685–693
Harp BP, Bermudez EM, Baron CI, Richard GI (2012) Qualitative identification of permitted and non-permitted colour additives in food products. Food Addit Contam Part A 29:886–896
Ishikawa F (2007) Analysis of food dyes in food: confirmation of the presence of non permitted dyes by LC-MS and structural analysis of unknown dyes by NMR. Foods Food Ingred J Jpn 212:968–976
Khazaei KM, Jafari SM, Ghorbani M, Kakhki AM (2014) Application of maltodextrin and gum Arabic in microencapsulation of saffron petal’s anthocyanins and evaluating their storage stability and color. Carbohydr Polym 105:57–62
Khazaei KM, Jafari SM, Ghorbani M, Kakhki AM (2016) Optimization of anthocyanin extraction from saffron petals with response surface methodology. Food Anal Meth 15:1–9
Kiseleva MG, Pimenova VV, Eller KI (2003) Optimization of conditions for the HPLC determination of synthetic dyes in food. J Anal Chem 58:685–690
Kobra H, Mohtasebi SS, Foroughirad A, Varnamkhasti MG, Rafiee S, Rezei K (2015) Detection of adulteration in saffron samples using electronic nose. Int J Food Prop 7:1391–1401
Manual of methods of analysis of food additives, Lab manual 8 (2005a) Directorate General of Health Services. Ministry of Health and Family Welfare, New Delhi, India, pp 75–75
Manual of methods of analysis of food additives, Lab manual 8 (2005b) Directorate General of Health Services. Ministry of Health and Family Welfare, New Delhi, India, pp 86–93
Mehrnia MA, Jafari SM, Zadeh M, Maghsoudlou Y (2016) Crocin loaded nano-emulsions: factors affecting emulsion properties in spontaneous emulsification. Intern J Bio Macro 84:261–267
Minioti KS, Sakellariou CF, Thomaidis NS (2007) Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Anal Chim Acta 583:103–110
Mohammad I, Shukla SK, Shakeel W (2015) Rapid detection of adulteration in indigenous saffron of Kashmir Valley. India Res J Forensic Sci 3:07–11
Rajabi H, Ghorbani M, Jafari SM, Rajabjadeh G (2015) Retention of saffron bioactive components by spray drying encapsulation using maltodextrin, gum Arabic and gelatin as wall materials. Food hydrol 51:327–337
Sabinto L, Scordino M, Gargano M, Bellingo A, Traulo P (2011) HPLC/PDA/ESI-MS evaluation of saffron (Crocus sativus L.) adulteration. Nat Prod Comm 6:1873–1876
Sarfarazi M, Jafari SM, Rajabzadeh G (2015) Extraction optimization of saffron nutraceuticals through response surface methodology. Food Anal Meth 9:2273–2285
Sforza S (2012) Food Authentication using bioorganic molecules. Destech Publications Inc. Lancaster, Pennsylvania, USA, pp 312–312
Shukla SK, Iqbal M (2015) Forensic analysis of the saffron: rapid authenticity testing. Int J Rese Appl Sci Eng Technol 3:139–143
Spectrum Chemical (2014) Methyl Orange Data Sheet http://www.spectrumchemical.com/ msds/m3970.pdf Accessed 04 November 2014
Wikipedia (2010) Methyl Orange. http://en.wikipedia.org/wiki/Methyl_orange.html Accessed 18 August 2014.
Yoshioka N, Ichihashi K (2007) Determination of 40 synthetic food colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode array detection. Talanta 74:1408–1413
Acknowledgments
Vipin Ashok is thankful to the University Grants Commission (MHRD), Government of India, for awarding Rajiv Gandhi National Research Fellowship. The work was carried out as a joint venture between Dept. of Bioanalytical Chemistry, Universitat Jaume I, Castello, Spain and Dept. of Criminology & Forensic Science, Dr. Harisingh Gour University, Sagar (M.P.), India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Vipin Ashok declares that he has no conflict of interest. Nitasha Agrawal declares that she has no conflict of interest. Josep Esteve-Romero declares that he has no conflict of interest. Devasish Bose declares that she has no conflict of interest. Neeti Prakash Dubey declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Ashok, V., Agrawal, N., Esteve-Romero, J. et al. Detection of Methyl Orange in Saffron and Other Edibles Using Direct Injection Micellar Liquid Chromatography. Food Anal. Methods 10, 269–276 (2017). https://doi.org/10.1007/s12161-016-0578-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0578-3