Abstract
A high-performance liquid chromatography-based method following the simultaneous analysis of 11 purine and pyrimidine compounds associated with freshness (K value for fish or Xt ratio for marine invertebrates) and umami taste in marine products was developed. This method, which separated 11 compounds in a 55-min cycle using a gradient of pH 3.8 phosphate buffer, acetonitrile, and water as the mobile phase, showed sufficient reproducibility and quantitative ability. Using this method, increases in the K value of an amberjack (Seriola purpurascens) and the Xt ratio of a squid (Todarodes pacificus) because of post-mortem deterioration were evaluated. Furthermore, high- and low-grade lavers (Porphyra tenera) known for their substantial guanosine 5′-monophosphate (an umami taste component) content, were compared using this method. Moreover, other nucleotide 5′-monophosphates, which are also umami components, from canned crab (the snow crab Chionoecetes opilio) and dried shiitake (Lentinula edodes), which is a mushroom containing a lot of guanosine 5′-monophosphate and was used as positive control for analysis by developed method, were successfully analyzed using this method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine products contain various extractable components, including organic acids, amino acids, and peptides. Particularly, purine and pyrimidine compounds are associated with the quality of marine products. In post-mortem marine products, adenosine 5′-triphosphate (ATP) gradually decomposes into adenosine 5′-diphosphate (ADP), adenosine 5′-monophosphate (AMP), inosine 5′-monophosphate (IMP), inosine (HxR), and hypoxanthine (Hx). Saito et al. (1959) used this ATP decomposition pathway to define the K value as an indicator of initial freshness in fish. The K value was defined using the equation below.
Marine invertebrates such as cephalopods (squids and octopi) and crustaceans (shrimp and crabs) have different pathways of ATP decomposition. Cephalopods have a decomposition pathway that proceeds via adenosine (AdR) rather than IMP (Arai and Saito 1961), and crustaceans have both the decomposition pathways proceeding via IMP and via AdR (Arai and Terasaki 1966). In crustaceans, Hx decomposes into xanthine (Xt) (Matsumoto and Yamanaka 1992). Therefore, other indicators are used when the freshness of these marine invertebrates is evaluated, such as the arranged K value, which replaces IMP with AdR and Xt ratio, as defined by the equation below (Yokoyama et al. 1994).
Nucleotide 5′-monophosphates are important because they contribute to the umami taste of marine products. IMP and guanosine 5′-monophosphate (GMP) have umami tastes (Kuninaka 1960). AMP and cytidine 5′-monophosphate (CMP) have no umami taste by themselves, but when the disodium form of these phosphates coexists with glutamic acid, they synergistically enhance the umami taste of glutamic acid (Terasaki et al. 1965).
As mentioned above, purine and pyrimidine compounds have a great influence on the quality of marine products, and many methods for their analysis have been reported. With regard to the HPLC methods, different methods are reported according to analytical objects: methods for freshness levels of vertebrate (fish) (Lee et al. 1982; Özogul et al. 2000; Ryder 1985), methods for freshness of invertebrate (Yokoyama et al. 1994), and methods for umami taste (Hosokawa et al. 1990; Yamasaki 1994). When purine and pyrimidine compounds in marine products are analyzed to evaluate freshness and umami taste, the analysis requires a great deal of time and equipment because the analysis conditions need to be changed according to the analytical objects and samples.
In this study, we developed an HPLC method for the simultaneous analysis of 11 purine and pyrimidine compounds that are associated with the freshness and umami tastes of marine products. Using this method, we evaluated the freshness of amberjack (vertebrate) and squid (invertebrate) as well as nucleotide 5′-monophosphate contents, which are umami taste components, of some foods.
Materials and Methods
Chemicals
Phosphoric acid, 70 % perchloric acid, potassium hydroxide, and disodium hydrogenphosphate dodecahydrate, which were JIS special grade, triethylamine, and anhydrous sodium dihydrogen phosphate, which were Wako special grade, and acetonitrile, which was HPLC grade, were obtained from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). CMP, guanine, Hx, Xt, IMP, HxR, AMP, ADP, AdR, ATP (Sigma Aldrich Japan, Ltd., Tokyo, Japan), and GMP (Wako Pure Chemical Industries, Ltd., Tokyo, Japan) were used as standard compounds.
Standard solutions of purine and pyrimidine compounds were prepared by dissolving the purine and pyrimidine compounds in 20-mM phosphoric acid buffer (pH 7.5). These solutions included 0.4281 μmol mL−1 CMP, 0.7559 μmol mL−1 guanine, 0.8601 μmol mL−1 Hx, 0.3809 μmol mL−1 Xt, 0.4415 μmol mL−1 GMP, 1.0293 μmol mL−1 IMP, 0.7728 μmol mL−1 HxR, 0.3820 μmol mL−1 AMP, 0.4406 μmol mL−1 ADP, 0.3320 μmol mL−1 AdR, and 0.3667 μmol mL−1 ATP.
HPLC System
Purine and pyrimidine compounds were analyzed using an HPLC system that included a PU-1580 pump, a DG-980-50 degasser, a CO-965 column oven (40 °C), a UV-970 UV–VIS detector (wavelength, 270 nm) (JASCO Corporation, Tokyo, Japan), a DMC675 Mixer (GLscience, Tokyo, Japan), and a 7725i Rheodyne-sampling injector connected to a 20-μL sample loop (Rheodyne, Rohnert Park, CA, USA). This system was equipped with a CAPCELL PAK UG120 column (250 nm × 4.6 mm, 5-μm particle size, Shiseido, Tokyo, Japan) connected to a Guard-Pak guard column containing Nova Pak-C18 (Waters, Milford, MA, USA). The mobile phase consisted of solutions A [water: triethylamine: phosphoric acid, 950:10:5 (v/v); pH 3.8] and B [water: acetonitrile, 90:10 (v/v)] were used. The gradient employed was 0–20 min, isocratic at 0 % B; 20–35 min, 0 % B to 35 % B; 35–45 min, isocratic at 35 % B. The flow rate was 1.0 mL/min. New samples were injected at intervals of 55 min. HPLC was conducted at room temperature.
Method Validation
Validation of the HPLC method included precision and specificity, linearity, range, and limit of detection and quantitation, recovery.
For the precision and specificity tests, analytical solutions were prepared by diluting standard solution 50-fold 20 mM phosphate buffer (pH 7.5). Five separate 20-μL aliquots of each analytical solution were injected and analyzed using HPLC. The retention time (tR), peak area, capacity factor (K′), resolution factor (Rs), and separation factor (α) were estimated using the results of these five replicate analyses. The parameters were defined using the following standard formulas: K′ = (tR n − tR0) / tR0, Rs = 2(tR n+1 − tR n ) / (W n+1 + W n ), α = K′ n+1/K′ n , where tR n is retention time of each peak, tR0 is the retention time of the first solvent peak (tR0 = 2.60 min), W n is the width of the peak at baseline. The subscripts n refer to the order of purine and pyrimidine compounds elution.
To test the linearity, range, limit of detection (LOD), and limit of quantification (LOQ), analytical solutions were prepared by diluting standard solutions in gradations from 2-fold to 800-fold with 20 mM phosphate buffer (pH 7.5). A 20-μL aliquot of each solution was injected and analyzed using HPLC. The linearity, range, LOD, and LOQ were estimated using the results of these analyses. LOD was calculated as the concentration at which the purine and pyrimidine compounds peaks could be detected without any baseline noise disturbances (>3 times the baseline noise). LOQ was calculated as the concentration at which the analytes’ responses were >10 times the baseline noise.
For the recovery tests, fortified samples were prepared by adding a 1-mL aliquot of standard solution (original), solution diluted twofold with 20 mM phosphate buffer (pH7.5) (half of original), and solution diluted fourfold with 20 mM phosphate buffer (pH7.5) (quarter of original) to the meat of an amberjack (Seriola purpurascens). According to the method of Hu et al. (2013), four pieces of amberjack muscle (1 g each) were weighed into separate 50-mL plastic centrifuge tubes, then a 1-mL aliquot of standard solution (original, half of original, quarter of original, 20-mM phosphate buffer) was added to each. Then, 10 mL of a 1-mol L−1 perchloric acid solution was added to each tube. These samples were homogenized and left for 10 min at room temperature. The samples were centrifuged at 1500 g for 10 min, and the supernatants were transferred to 15-mL plastic test tubes. These sample solutions were mixed with 9.8 mL of 1 mol L−1 potassium hydroxide and left in a refrigerator for 30 min. Then, the samples were separated by centrifugation at 1500 g for 10 min. The supernatants were poured into another set of four plastic test tubes and diluted to a total volume of 20 mL with water. Each of these solutions was filtered through a 0.45-μm filter (Millex-LH 0.45 μm, Merck Millipore Ltd., Darmstad, Germany) and 4 mL of the filtered solution was added to 1 mL of 100 mM phosphate buffer (pH 7.5). These solutions were analyzed with HPLC. The recovery and the relative standard deviation (RSD) were estimated using the results of the analysis.
Evaluation of Amberjack Freshness
The purine and pyrimidine compounds content and K value of amberjack samples (n = 6) of various freshness levels were analyzed. Each amberjack was bled by cutting its gills, and its internal organs were removed. The dressed fish was cut into loins and then into fan-shaped blocks (weight, ca. 50 g; thickness, 20 mm) from the dorsal muscle. The experimental protocol was approved by the Institutional Animal Care and Use Committee, University of Miyazaki. The fan-shaped blocks were stored in a refrigerator at 5 °C for 96 h and were taken from the refrigerator at each time point (24, 48, 72, and 96 h). Purine and pyrimidine compounds were extracted from the muscle using the method of recovery test. The extracts were analyzed using HPLC and the contents of each component and K value were evaluated.
The Purine and Pyrimidine Compound Content of Foods
The purine and pyrimidine compound content of foods was analyzed using an HPLC method. Squid (Todarodes pacificus), canned crab (the snow crab Chionoecetes opilio), and dried shiitake (Lentinula edodes) were purchased at a local supermarket. The squid’s mantle muscle was divided into two pieces, and one piece was treated immediately. The other piece was left for 24 h at room temperature and then treated. Two different quality lavers (Porphyra tenera) were gifted from the Ariake fishermen’s cooperative, Saga Pref., Japan. These samples were cultured and manufactured for dried laver sheets in the Ariake Sea in Japan in 2007–2009 and were organoleptically judged by expert panels of the fishermen’s cooperative according to taste, color, and flavor of each sample. The samples were then classified by high or low grades. Purine and pyrimidine compounds were extracted from squid, laver, canned crab, and shiitake using the method of recovery test. The extracts were analyzed using HPLC.
Results and Discussion
Method Validation
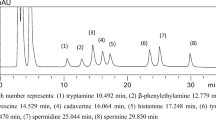
A typical chromatogram of mixed purine and pyrimidine compounds is shown in Fig. 1. Six purine and pyrimidine compounds used to estimate the K value were measured on reversed-phase C18 columns using a mobile phase of phosphate buffers (Ryder 1985). However, under these conditions, all peaks without HxR were close to each other so that the purine and pyrimidine compounds related to the freshness of marine invertebrates (Xt and AdR) and those related to umami taste (CMP and GMP) could not be separated simultaneously. To circumvent this issue, Yokoyama et al. (1992) changed the mobile phase to a mixture of citric acid, acetic acid, and triethylamine and lowered its pH to 4.8. Consequently, the six purine and pyrimidine compounds related to the K value and Xt were separated simultaneously. However, CMP and GMP could not be separated using this method. In this study, the six compounds used to estimate the K value were separated from other compounds (Xt, CMP, and GMP) by lowering the pH of the mobile phase to pH 3.8, but ADP, AdR, and ATP could not be eluted in 100 min under these conditions. Özogul et al. (2000) could determine the K value within a short time using a gradient system with acetonitrile based on Ryder’s method (1985). Following this, we attempted to use a gradient system with a mixture of acetonitrile and water as the mobile phase. As a result, 11 purine and pyrimidine compounds were separated with good specificity in a 55-min cycle. This included not only the six compounds used to estimate the K value, but guanine, Xt, AdR, CMP, and GMP as well.
To confirm the precision and specificity of the method developed here, tR, peak area, K′, Rs, and α were investigated; the results are displayed in Table 1. The RSDs of the tRs for all the purine and pyrimidine compounds were less than 0.20 %. The RSDs of the peak areas for all purine and pyrimidine compounds, except AdR and ATP, were less than 2.00 %, and the RSDs of the peak areas of AdR and ATP were 2.266 and 3.538 %, respectively. The separation of each purine and pyrimidine compound exhibited good specificity. The RSDs of the peak areas obtained using the HPLC method developed here were less than 4 % for each compound, which suggests that the method developed here exhibits high reproducibility. The linearity, range of detection, LOD, and LOQ were investigated; the results are shown in Table 2. The correlation factor for each of the purine and pyrimidine compounds was more than 0.999. This suggests the method developed here exhibits good linearity. The linear range varied depending on the purine and pyrimidine compound species. The LODs and LOQs for the various purine and pyrimidine compounds were found to be from 0.8624 to 5.0253 and from 2.8748 to 16.7511, respectively. The mean recovery for each of the purine and pyrimidine compounds was investigated; the results are shown in Table 3. The recoveries of all the purine and pyrimidine compounds, except IMP, from fish were 74.09–120.59 %. The recoveries of IMP ranged from 104.93 to 242.97 %. The average recoveries obtained using the method of Ryder (1985) ranged from 94.2 to 100.1 %, depending on the compound. In our developed method, the average recoveries of all compounds except IMP ranged from 84.28 to 104.52 %, indicating that the method has the sufficient quantitative ability. When a lower concentration of a standard solution was added to the sample, the recovery increased. This tendency is consistent with the method of Ryder (1985). Concerning IMP, when the concentration of the added standard was original or half of the original, the recovery was 107.37 or 104.93 %, respectively. When the added standard solution was a quarter of the original, the recovery was 242.97 %. The reason or this sharp increase might be that the peak area of the added IMP was too small compared with that of the natural IMP. This may occur because the absorption of 270-nm lights on IMP is lower than that on the other compounds, but dead fish have very large amounts of IMP.
Evaluation of Amberjack Freshness
The purine and pyrimidine compounds contents and K values of amberjack of varying freshness during cooled storage at 5 °C are shown in Table 4. Typical chromatograms from high freshness-level fish and low-freshness-level fish are shown in Fig. 2a, b, respectively. The content of CMP was low, and Xt was not detected from 0 to 96 h. The small content of AdR were detected at 0, 24, and 48 h but then vanished at 72 h. The contents of AMP, ADP, and ATP varied with each time point. The content of IMP changed, but there was no significant difference. The contents of Hx did not change between 0 and 24 h. The content of HxR at 0 h was higher than that of 24 h, but there were no significant differences between them. Then, the contents of Hx and HxR increased gradationally from 24 to 96 h. Consistent with this, the K value of amberjack did not also change between 0 and 24 h and then increased gradationally to 96 h. The GMP content was analyzed simultaneously with the other purine and pyrimidine compounds associated with the K value. The GMP content changed very little from 0 to 48 h (approximately 0.25 mg 100 g−1 muscle), increased at 72 h (0.67 ± 0.53 mg 100 g−1 muscle), and then decreased at 96 h (0.41 ± 0.18 mg 100 g−1 muscle).
HPLC chromatograms of purine and pyrimidine compounds in high-freshness- and low-freshness-level amberjacks. a High-freshness-level amberjack (before storage). b Low-freshness-level amberjack (after storage at 5 °C for 96 h). A number of each chromatogram represents each compound: 1, CMP; 2, guanine; 3, Hx; 4, Xt; 5, GMP; 6, IMP; 7, HxR; 8, AMP; 9, ADP; 10, AdR; 11, ATP
The K value is commonly used as an indicator of the initial freshness of fish. Many methods of K value determination have been studied. In particular, many HPLC methods have been reported (Lee et al. 1982; Özogul et al. 2000; Ryder 1985). In this study, the K values of amberjack stored at 5 °C for 96 h were measured using the method developed here. The K value before storage was 8.69 ± 0.88 %. It increased to 26.25 ± 1.89 % after 72 h and reached 30.78 ± 2.22 % after 96 h. This suggests that the method developed here can analyze the K value of fish sufficiently.
Contents of Purine and Pyrimidine Compounds in Foods
Purine and pyrimidine compound contents of foods were investigated using the method developed here (Table 5). Typical chromatograms of high-freshness- and low-freshness-level squids are shown in Fig. 3a, b, respectively. High-freshness-level squid was found to have lower amounts of CMP, Hx, and Xt and higher amounts of HxR, AdR, and ATP than low-freshness-level squid. The Xt ratios of high-freshness- and low-freshness-level squids were 1.77 ± 0.37 and 102.13 ± 2.74‰, respectively. Yokoyama et al. (1994) defined the Xt ratio as an indicator of freshness for marine invertebrates and evaluated the freshness of squid, which could not be measured using the K value. In this study, the Xt ratio of squid was measured using the method developed here. Using this method, the Xt ratios of high-freshness- and low-freshness-level squids could be easily distinguished. Therefore, the freshness of marine invertebrates such as squid, shrimp, and crab can be evaluated using the method developed here.
HPLC chromatograms of purine and pyrimidine compounds in high-freshness- and low-freshness-level squids. a High-freshness-level squid. b Low-freshness-level squid. A number of each chromatogram represents each compound: 1, CMP; 2, guanine; 3, Hx; 4,Xt; 5,GMP; 6, IMP; 7, HxR; 8, AMP; 9,ADP; 10, AdR; 11, ATP
Typical chromatograms of high- and low-grade lavers are shown in Fig. 4a, b, respectively. High-grade laver was found to have greater amounts of GMP and IMP than low-grade laver. Noda et al. (1975) measured the GMP content of laver (Porphyra spp.) and found that natural laver contained 5.7–12.5 mg GMP per 100 g of sample. In this study, high-grade laver was found to contain 7.56 ± 0.77 mg GMP per 100 g of sample, which is consistent with the levels measured by Noda et al. (1975).
Typical chromatograms of canned crab are shown in Fig. 5a. Canned crab was found to contain large amounts of GMP and IMP and some amount of AMP. Canned crab did not contain the other purine and pyrimidine compounds. Canned crab was found to have little purine and pyrimidine compounds but large amounts of GMP, IMP, and AMP, which are related to the umami taste. Mouri et al. (1965) measured the purine and pyrimidine compounds content of crab at each stage (raw, during treatment for canning, and after treatment for canning). As a result, the content of most compounds decreased, but the AMP content increased during the canning process. Results obtained using the method developed here agreed with those of Mouri et al. (1965). The high GMP and IMP contents are thought to be from the umami seasoning. This suggests that the method developed here can analyze a food additive.
Finally, to confirm the applicability of the method for terrestrial foods, shiitake, which contains large amounts of nucleotide 5′-monophosphates, was analyzed using the method developed here. Typical chromatograms of shiitake are shown in Fig. 5b. Shiitake was found to contain some amounts of CMP, GMP, and AMP. GMP, in particular, was found in large quantities. Sasaki et al. (2000) measured the GMP content of dried shiitake and found it to be 10.72 g 100 g−1. In this study, the GMP content of dried shiitake was found to be 9.07 ± 0.26 g 100 g−1, which agrees with the result of Sasaki et al. (2000)). Kasuga et al. (1999) also measured the nucleotide content of dried shiitake and reported that dried shiitake contained some amount of CMP. In our study, a small amount of CMP was detected in dried shiitake, which agrees with the result of Kasuga et al. (1999).
These results demonstrate that the HPLC method developed here is useful for the analysis of purine and pyrimidine compounds in foods associated with the umami taste.
Conclusion
The results of this study show that the method developed here separates more kinds of purine and pyrimidine compounds than the usual methods, but require less equipment and time. Moreover, the method developed here shows sufficient reproducibility and quantitative ability.
Using this method, compounds associated with the freshness of marine vertebrates and invertebrates as well as compounds associated with the umami taste can be analyzed simultaneously. Therefore, the method developed here would be applicable to the evaluation of correlations between freshness and umami taste for a wide range of marine products as well as the evaluation of umami taste for terrestrial foods.
References
Arai K, Saito T (1961) Changes in adenine nucleotides in the muscles of some marine invertebrates. Nature 192:451–452
Arai K, Terasaki M (1966) Nucleotides in the muscles of marine invertebrates. Nippon Suisan Gakk 32:174–179
Hosokawa M, Sakakibara H, Yajima I, Hayashi K (1990) Non-volatile flavor components in dorsal, abdominal and red meat parts of dried skipjack (katsuobushi) studies on flavor components of katsuobushi, part II. Nippon Shokuhin Kogyo Gakk 37:856–861
Hu Y, Zhang J, Ebitani K, Konno K (2013) Development of simplified method for extracting ATP-related compounds from fish meat. Nippon Suisan Gakk 79:219–225
Kasuga A, Fujihara S, Aoyagi Y (1999) The relationship between the varieties of dried shiitakemushrooms (Lentinus edodes (Berk.) Sing.) and chemical composition. Nippon Shokuhin Kagaku Kogaku Kaishi 46:692–703
Kuninaka A (1960) Studies on taste of ribonucleic acid derivatives. Nippon Nogeik Kaishi 34:489–492
Lee E-H, Ohshima T, Koizumi C (1982) High performance liquid chromatographic determination of K value as an index of freshness of fish. Nippon Suisan Gakk 48:255
Matsumoto M, Yamanaka H (1992) Post-mortem biochemical changes in the muscle of tanner crab during storage. Nippon Suisan Gakk 58:915–920
Mouri T, Terada J, Aoyama N, Hashida W (1965) Flavor improvement of canned foods with 5′-nucleotides v changes of 5′-nucleotides in fishery canned foods. Canners J 44:62–67
Noda H, Horiguchi Y, Araki S (1975) Studies on the flavor substances of ‘nori’, the dried laver porphyra spp.II free amino acids and 5′-nucleotides. Nippon Suisan Gakk 41:1299–1303
Özogul F, Taylor KDA, Quantick PC, Özogul Y (2000) A rapid HPLC-determination of ATP-related compounds and its application to herring stored under modified atmosphere. Int J Food Sci Tech 35:549–554
Ryder JM (1985) Determination of adenosine triphosphate and its breakdown products in fish muscle by high-performance liquid chromatography. J Agr Food Chem 33:678–680
Saito T, Arai K, Matsuyoshi M (1959) A new method for estimating the freshness of fish. Nippon Suisan Gakk 24:749–750
Sasaki H, Negishi Y, Okuzaki M, Sugahara T (2000) Variation in tasty component of dried shiitake mushroom (Lentinus edodes (Berk) Sing.) under the different storage condition. J Integr Stud Diet Habits 11:289–295
Terasaki M, Wada S, Fujita E, Takemoto T, Nakajima T, Yokobe T (1965) Studies on taste of tricholomic acid and ibotenic acid (part 2) taste intensification with adenine nucleotides, uridylic acid and cytidylic acid. Eiyo Shokuryo 18:222–225
Yamasaki Y (1994) Comparative studies on taste components of katsuobushi and niboshi extracts. J Home Econ Jpn 45:41–45
Yokoyama Y, Sakaguchi M, Kawai F, Kanamori M (1992) Changes in concentration of ATP-related compounds in various tissues of oyster during ice storage. Nippon Suisan Gakk 58:2125–2136
Yokoyama Y, Takahashi S, Sakaguchi M, Kawai F, Kanamori M (1994) Postmortem changes of ATP and its related compounds and freshness indices in spear squid doryteuthis bleekeri muscles. Fish Sci 60:583–587
Acknowledgments
This study was supported by a grant from the University of Miyazaki, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mami Ishimaru declares that she has no conflict of interest. Masato Haraoka declares that he has no conflict of interest. Hideo Hatate declares that he has no conflict of interest. Ryusuke Tanaka declares that he has no conflict of interest.
Ethical Approval
This article contains some studies with fish subjects. All procedures performed in studies involving animals (fish) were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Ishimaru, M., Haraoka, M., Hatate, H. et al. Simultaneous Analysis of Purine and Pyrimidine Compounds Associated with the Freshness and Taste of Marine Foods. Food Anal. Methods 9, 1606–1615 (2016). https://doi.org/10.1007/s12161-015-0341-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0341-1