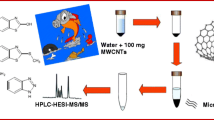
Abstract
A modified Quick, Easy, Cheap, Effective, Rugged and Safe (QuEChERS) method based on the dispersive solid-phase extraction (dSPE) combined with high-performance liquid chromatography (HPLC) was developed for the determination of thiabendazole (TBZ) in environmental and food samples. Hydroxyl functionalized multiwalled carbon nanotube (MWCNT-OH) was used as dSPE material for the preconcentration of analyte. Several experimental parameters, including the amount of sorbent, extraction time, the pH and ionic strength of sample solution, and desorption conditions, were evaluated. Under optimal experimental conditions, good linearity was observed in the range of 10–1000 ng mL−1 with the correlation coefficient of 0.9962. The limit of detection and quantification were 2.6 and 8.7 ng mL−1, respectively. The present method was applied to the analysis of different environmental and food samples, and the recoveries of TBZ obtained were in the range of 92.9–103.9 % with the relative standard deviations lower than 6.5 %. The results showed that the proposed method was a rapid, convenient, and feasible method for the determination of TBZ in environmental and food samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thiabendazole [2-(4-thiazolyl) benzimidazole (TBZ)] is used as an antihelminthic in humans and animals. As an anthelmintic and antifungal agent, it is employed to treat roundworm infections such as threadworm, hookworm/creeping eruption, and visceral larva migrans (toxocariasis) (Ames et al. 1963; Cuckler 1961; Satou et al. 2001). Additionally, TBZ is one of the most commonly used fungicides to control postharvest diseases such as mold, rot, blight, and stain caused by fungi in various fruits and vegetables (Llorent-Martínez et al. 2012). So, TBZ is used as an agricultural antifungal and food preservative. The use of TBZ pesticides provides benefits in providing a supply of high-quality crops, but their incorrect application can leave harmful residues, which involve a potential risk for human health. The use of TBZ is approved with relatively high tolerance levels, which vary from 5 to 10 mg kg−1 depending on the country (Japan, USA, Europe) (García-Reyes et al. 2006; Lemairea et al. 2004; Muela et al. 2010; Blazková et al. 2010). Moreover, several studies have been performed to demonstrate residues in edible parts of raw fruits (García-Reyes et al. 2006; Ito et al. 2003; Veneziano et al. 2004; Zamora et al. 2004) and in fruit juices. TBZ is associated with a host of adverse effects including nephrotoxicity, hepatotoxicity, carcinogenicity, and teratogenicity (Jamieson et al. 2011). TBZ can accumulate in the human body, and its potentially toxic residues in edible animal-origin products may pose a great threat to human health through the food chain. The persistence of TBZ residues in agricultural products destined to human consumption is important problem. Besides, the residues in surface water, groundwater, and soils may also cause potentially environmental problems. To protect consumers from risks related to TBZ in food and environment water, the development of a simple, rapid, and reliable method for the enrichment and recovery of TBZ is of great importance.
The vegetables and fruits determination of pesticide residues and environmental water samples is a difficult task, not only because of the low concentration levels typically found but also because of the complexity of the matrix. The need for efficient methods for pesticide residues concentration and cleanup in environmental analysis is constantly growing. Solid-phase extraction (SPE) has achieved widespread application because of its simple procedure, high preconcentration factor, rapid phase separation, and combination with different detection techniques. Carbon nanotubes (CNTs) have been used as a sorbent material in SPE for the determination of sulfonamides (Fang et al. 2006), atrazine (Zhou et al. 2006a, b), chlorophenols (Cai et al. 2005), and dichlorodiphenyltrichloroethane (Zhou et al. 2006a, b) in different sample matrices. CNTs, ever since their discovery, have attracted extensive attention due to their unique physicochemical and electrical properties. CNTs, which are considered to be extremely superior adsorbents due to their high specific surface area and large micropore volume, have been utilized for the sorption of a number of organic and inorganic pollutants (Wu 2007; Lu et al. 2005; Chin et al. 2007; Duran et al. 2009; Tuzen et al. 2008a, b; Tuzen and Soylak 2007).
The “Quick, Easy, Cheap, Effective, Rugged and Safe” (QuEChERS) sample preparation method for determining pesticides in foods was first introduced in 2003 (Anastassiades et al. 2003). The method involved miniaturized extraction with acetonitrile, liquid–liquid partitioning, and a cleanup step which was carried out by mixing the acetonitrile extract with loose sorbents. Because the sorbent is added to the bulk solution or matrix containing the analytes, the possible matrix interferences/components are retained onto it. Using the cleanup step, various elution solvents could be selected. Finally, the sorbent was discarded and the elution solvent was analyzed. Therefore, dSPE can be used with the aim of trapping the target analytes which are later eluted or desorbed with an appropriate solvent (Su et al. 2011; Zhao et al. 2012; Herrera-Herrera et al. 2012).
In this paper, hydroxyl functionalized multiwalled carbon nanotube (MWCNT-OH) was used as the sorbents in dSPE for extracting and preconcentration trace amount of TBZ from samples. After extraction by dSPE, the TBZ was desorbed using “green solvent” ionic liquid as elution. The conditions for separating TBZ were studied and optimized. To the best of our knowledge, this is the first demonstration for MWCNT-OH as dSPE sorbents to extract TBZ and ionic liquid as elution solvent. Moreover, the adsorptive properties and mechanism of MWCNT for TBZ were discussed in detail. The objective of the experiment was not only to know the interaction between TBZ and MWCNT-OH but also to find simple shortcut method for separating TBZ from real samples. Finally, the method was successfully applied to wastewater samples and fruit juices.
Experimental
Reagents and Materials
The analytical standard TBZ (Chemical reference substance, 98.0 % purity) was purchased from HEOWNS Biochemical Technology Co., Ltd (Tianjin, China). The molecular structure of TBZ is as follows:

A 1000-mg L−1 stock standard solution of TBZ was prepared by dissolving 255.1 mg TBZ in 250 mL ethanol in a brown volumetric flask to 250 mL. The standard stock solutions of TBZ were diluted successively to the required concentration with deionized water in the experiment and kept in dark below 4 °C. Chromatographic grade methanol was purchased from Fisher Corporation (Pittsburgh, PA, USA). Analytical grade hydrochloric acid (HCl), sodium hydroxide, sodium chloride, ethanol, acetonitrile, and acetone were purchased from Beijing Chemical Factory (Beijing, China). Pure water was obtained with a Milli-Q water system (Millipore, Billerica, MA, USA). All the solvents and solutions were passed through a 0.45-μm nylon filter before used. 1-Hexyl-3-methylimidazolium hexafluorophosphate ([C6MIM][PF6]) was purchased from Chengjie Chemical Co., Ltd. (Shanghai, China).
Two MWCNTs (95 % purity) (pristine [MWCNT], hydroxyl functionalized [MWCNT-OH]) were purchased from Chengdu Organic Chemicals Co., Ltd, China. Each of the two MWCNTs has a specific surface area above 500 m2 g−1 and an outer diameter below 8 nm. Pore distributions (pore volume with pore diameter in parentheses) of MWCNT used as adsorbent throughout the experiment are as follows: 0.085 m3 g−1 (0–20 nm), 1.839 m3 g−1 (20–50 nm). All these physical parameters of MWCNTs were provided by the manufacturer.
Apparatus and Chromatographic Conditions
UV-Vis-NIR Cary 5000 (Varian Co., USA) was used to record the absorption spectra of TBZ. A S-3C Model pH meter (Shanghai Precision Scientific Instrument Co., China) was used for measuring the pH of solutions. A model TDL80-2B centrifugal machine (Shanghai Anting Scientific Instrument Co., China) was used for sample treatment. The functional groups of MWCNT surface were detected by AVATAR 330 Fourier transform infrared spectroscopy (FTIR) (Nicolet Co., USA). Malvern Zetasizer Nano-ZS particle analyzer (Malvern, UK) was used to determine the zeta potential of adsorbent.
An Agilent 1100 HPLC (Palo Alto, CA, USA) equipped with an automatic sampler and diode array detector was used for the chromatographic analysis. Chromatographic separation of target analytes was performed on a Zorbax SB-C18 column (150 mm × 4.6 mm I.D., 5 μm) (Agilent, Palo Alto, CA, USA). The mobile phase was a mixture of methanol-water (50:50, v/v). The flow rate and column temperature were set at 1.0 mL min−1 and 30 °C, respectively. The detection wavelength for TBZ was set at 298 nm. The injection volume of sample solution was 20 μL.
Real Sample Pretreatment
Wastewater Samples
Wastewater samples include industrial wastewater and synthetic samples. Industrial wastewater was taken from a municipal wastewater treatment plant (Shenyang, China). The synthetic water samples were prepared by adding 1 × 10−2 mol L−1 Na+, K+, Ca2+, Mg2+, NO3 −, CO3 2−, HCO3 −, SO4 2−, SO3 2−, PO4 3− ions, because these inorganic minerals usually contained in fruits and vegetables. The spiked wastewater samples were prepared by spiking the working solutions into 10 mL of sample. Then, the resulting solution was referred to as sample solution, filtered through 0.45-μm filters and then stored at 4 °C.
Fruit Juice Samples
Fruit juices were commercial samples obtained from local supermarkets. For recovery studies, samples were used without any pretreatment. The spiked juice samples were prepared by spiking a known amount of TBZ working solutions into 10 mL of sample. Then, the resulting solution was referred to as sample solution, filtered through 0.45-μm filters and then stored at 4 °C. The natural pH of juice samples was close to 6.0, and the sample solution was used directly without any pH adjustment.
dSPE Procedure
Ten milliliters of spiked sample was transferred to a flask containing 7 mg MWCNT-OH. The mixture was extracted for 4 min under stirring at 1000-rpm rate at 25 °C. Subsequently, it was centrifuged for 5 min at 5000 rpm. The upper water phase was discarded. Then, the mixture was ultrasonically desorbed for 10 min by 1.0 mL of methanol with 200 μL of [C6MIM][PF6] and centrifuged for 5 min at 5000 rpm. Finally, the resulting solution was referred to as analytical solution, filtered through a 0.45-μm filter membrane and was analyzed by HPLC.
Results and Discussion
Optimization of Dispersive Solid-Phase Extraction
The parameters that affect the extraction efficiency, including the type of sorbent, the amount of sorbent, extraction time, the pH and ionic strength of solution, and desorption conditions, were investigated. All the experiments were performed in triplicate, and the concentration of TBZ in the spiked samples was 100 ng mL−1.
Selection and Characterization of Sorbents
Two MWCNTs (pristine [MWCNT], hydroxyl functionalized [MWCNT-OH]) were chosen to test their adsorbability of TBZ. In order to make their adsorption efficiency for TBZ as high as possible, the adsorption conditions (solution pH, sorbent amount) of each sorbent were optimized. The adsorption of TBZ on different types of MWCNTs was studied and the UV–vis absorption spectrogram of TBZ solutions is shown in Fig. 1a. MWCNT-OH showed higher adsorption efficiency for TBZ than MWCNT. Functional groups can change the wettability of CNT surfaces and, consequently, make CNT more hydrophilic (Fig. 1b) and helpful for the adsorption of relatively low-molecular-weight and polar compounds (Cho et al. 2008; Liao et al. 2008; Onyestyak et al. 2004). The main limitation, when MWCNTs are used as sorbent materials, is their aggregation tendency which reduces the active surface, thus affecting their effectiveness. Therefore, MWCNT-OH was used as adsorbent for TBZ in the experiment.
a Effect of different MWCNTs on adsorption behavior of TBZ (the concentration of TBZ in the spiked samples was 20 μg mL−1). b Dispersed state of nanotubes in aqueous solutions: a, the sample after 30 min; b, the same sample after 1 day. c Zeta potential of MWCNT under various pH; D. the FTIR spectra of MWCNT
The values of zeta potential of MWCNT and MWCNT-OH suspensions were also determined under various pH (Fig. 1c), and the point of zero charge (PCZ) for MWCNT and MWCNT-OH were found to be about 3.0 and 3.5. As compared with the MWCNT, the surface charge of MWCNT-OH is more positive for pH <3.5 but is more negative for pH ≥3.5. This could be the presence of more oxygen-containing functional groups on the surface of MWCNT-OH (Fig. 1d), which enhances the deposition of H+ for pH <3.5 and improves the deposition of OH− for pH ≥3.5.
Effect of the Amount of Sorbent
The amount of the sorbent has a direct effect on the extraction of TBZ. Different amounts of the sorbent ranging from 3 to 20 mg were applied to extract TBZ from sample solution. The results indicated that 7 mg sorbent was enough for the extraction with the recoveries ranging from 75.59 to 99.58 %. Further increasing the amount of the adsorbents gave no significant improvement for the recoveries of TBZ. Therefore, 7 mg was selected as the amount of the adsorbents.
Effect of Extraction Time
The extraction recovery is highly dependent on the mass transfer of analyte from sample solution to the sorbent. As a result, the effect of the extraction time from 1 to 15 min on the recoveries of TBZ was investigated. The results indicated that the recoveries of TBZ increased by extending the extraction time from 1 to 4 min, until equilibrium was rapidly attained around 4.0 min. The high surface area of MWCNT-OH along with homogeneous distribution of the nanosorbent throughout the sample could be the possible reasons for achieving such a fast extraction process. This is a superior advantage over the conventional SPE and other microextraction techniques, which usually need more than 30–60 min to reach the equilibrium. Therefore, 4 min was chosen as the extraction time in this study.
Effect of pH
The effect of pH was examined and the results are shown in Fig. 2. The adsorption maxima generally occurred between pH 4.0 and 12.0 (TBZ: pK a1 = 4.73; pK a2 = 12.0), but when pH < 4.73 or pH > 12.0, the adsorption percentages were decreased. As can be seen from Fig. 2, there was no observable effect on the adsorption of TBZ on MWCNT with an increase of pH from 4.0 to 12.0. With the increase of pH (pH >12.0), the adsorption ratio decreased. In general, the natural pH of TBZ solution was close to 5.73. In this work, the TBZ solution was used directly without any pH adjustment with HCl or NaOH.
Effect of Ionic Strength
Generally, NaCl has a “salting-out” effect on the adsorption of hydrophobic compounds (Xie et al. 1997). On the other hand, the increase of ionic strength might also alter the aggregation state of MWCNT, the aggregates of MWCNT would be more compact (squeezing-out) (Zhang et al. 2010). A highly compacted aggregation structure of MWCNT is unfavorable for TBZ adsorption. Therefore, the TBZ solution without adjusting ionic strength was adopted in this experiment.
Desorption Conditions
The desorption of TBZ from the adsorbents was studied with different organic solvents, including ethanol, ethyl acetate, methanol, and acetonitrile. The best recovery was only 41 % when 1 mL of methanol was used. In order to increase the recovery and decrease the volume of elution solvent, high elution capability solvent ionic liquid (Talebpour et al. 2012) was added in the experiment. Moreover, different volumes of ionic liquid ranging from 0.1 to 0.5 mL were also investigated. The results shown in Fig. 3 indicated that 0.2 mL of ionic liquid with 1 mL of methanol was enough to desorb TBZ from the sorbent perhaps because of the properties of IL, which have good solubility and extractability for various organic compounds. Therefore, 1 mL of methanol with 200 μL of [C6MIM][PF6] was used to desorb GEN from the sorbent.
The effect of desorption time from 1 to 10 min was investigated. A desorption time of 5 min appeared to be sufficient for complete desorption. Finally, 5 min was selected as the optimum desorption time.
Mechanistic Aspects
The variation in pH cannot only affect the protonation–deprotonation transition of functional groups on MWCNT, but also results in a change in chemical speciation for ionizable organic compounds. Furthermore, TBZ could have different charges on different sites depending on solution pH. When solution pH is below 4.73, TBZ exists as a cation (TBZH3 2+), due to the protonation of organic nitrogen atom. At pH between 4.73 and 12.0, TBZ will exist as a neutral molecule (TBZH0). At solution pH greater than 12.0, TBZ exists as anion (TBZ−) from the loss of protons from the nitrogen atom of benzimidazole moiety. TBZ adsorption is significantly impeded when pH < pKa1 or pH > pKa2. The ionized form of TBZ is the predominant fraction at pH <4.73 or pH >12.0, and the hydrogen bonding and hydrophobic interactions between MWCNT-OH and ionized TBZ are much weaker than that between MWCNT-OH and nonionized TBZ. Besides, both TBZ and MWCNT-OH are positive charge or negatively charged and the electrostatic repulsion between them can also weaken their adsorption to some degree. While TBZ showed high adsorption under conditions with 4.0 < pH < 12.0, on this condition, nearly all of TBZ molecules carry no net electrical charge, which makes them hardly have electrostatic attraction or repulsion with MWCNT-OH. The increase of pH from 4.0 to 12.0 had no significant effect on the adsorptive affinity of TBZ in the experiment. Therefore, the adsorption mechanism is probably the π-π stacking interactions between bulk π systems on MWCNT-OH surface and TBZ molecules. In addition, strength of hydrogen bonds was the primary cause of the enhanced adsorption for reasons. Hydrogen bond interactions were formed between the –N atom of TBZ and the –OH groups of MWCNT-OH, and the benzene ring on MWCNT-OH surface might also act as H-bond donor and form H-bonds with –N atom on TBZ. Different mechanisms (hydrogen bonding interaction between and π-π stacking interaction) may act simultaneously and respond differently to the change of pH value; thus, the prediction of TBZ adsorption on MWNT-OH is not straightforward.
Regeneration of the MWCNT-OH
In order to evaluate the stability and possibility of reuse of MWCNT-OH sorbent, repeating application of MWCNT-OH experiments were performed. Removal efficiencies of repeating application of MWCNT-OH are shown in Fig. 4a. It was stable for up to five adsorption recycles without obvious decrease in the removal efficiency for TBZ. The adsorption efficiency could be still above 85 % in the final recycle, which indicated that there were no irreversible sites on the surface of the adsorbent.
In the FTIR spectra of MWCNT-OH (Fig. 4b), MWCNT-OH showed some apparent characteristic bands of TBZ after adsorption, which were aromatic –C = C– bonds (1600–1400 cm−1), benzene ring –C–H bonds (900–650 cm−1), phenolic = C–N– band (1463 cm−1), C–S band (600–700 cm−1), and N–H bonds (3460 cm−1), while MWCNT-OH displayed no significant bands before adsorption. It can also be seen from Fig. 4b (d) that the main functional groups of TBZ disappeared after regeneration. FTIR of MWCNT-OH after the five recycle did not show evident change as compared to that of the first recycle.
Analysis of Samples
Under the optimal conditions, the proposed method was evaluated. Pure water samples spiked at different concentrations of TBZ (10–1000 ng mL−1) were used. For each concentration level, three replicate analyses were performed. The relationship exhibited good linearity with correlation coefficient (r) of 0.9962. The limit of detection (LOD) was determined based on the signal to noise (S/N) ratio of 3 and found to be 2.6 ng mL−1.
To evaluate the applicability of the proposed method, some real samples, including industrial wastewater, synthetic samples, and juice (orange, apple and pear) samples, were analyzed. The sample solutions were used without any pretreatment. The spiked samples were prepared by spiking the TBZ working solutions into 10 mL of the sample. Then, the resulting solution was referred to as sample solution, filtered through 0.45-μm filters and then stored at 4 °C. The typical chromatograms of the blank and spiked orange juice sample are shown in Fig. 5. As can be seen, no significant interference peaks are found at the retention time of TBZ.
To evaluate precision and accuracy of the proposed method, the spiked samples (20, 100, and 200 ng mL−1) were analyzed. Precision was evaluated by measuring intra-day and inter-day relative standard deviations (RSDs). The intra-day and inter-day precision of the method were evaluated by analyzing the spiked samples at three concentration levels on the same day and the five consecutive days, respectively. The results obtained are shown in Table 1, and the results indicate that the present method has good repeatability. The RSDs and recoveries are in the range of 1.8–6.5 and 92.9–103.9.0 %, respectively. It can be considered that the present method provides acceptable recoveries for the determination of TBZ in real samples.
Conclusions
In this work, a simple and rapid method for analysis of TBZ in environmental and food samples has been developed based on dSPE coupled with HPLC determination. MWCNT-OH was successfully used as adsorbent in dSPE for the extraction and preconcentration of TBZ. The results demonstrated that the proposed method gives good recoveries and reproducibilities. The proposed method promises simplicity, less organic solvent consumption and high sensitivity. Additional work is in progress on evaluating the performance of proposed method for the determination of other pesticide residue in various matrices.
References
Ames ER, Cheney JM, Rubin R (1963) The efficacy of thiabendazole and bephenium hydroxynaphthoate against Ostertagio ostertagi and Cooperia oncophora in experimentally infected calves. Am J Vet Res 24:295–299
Anastassiades M, Lehotay S, Stajnbaher D, Schenck F (2003) Fast andeasy multiresidue method employing acetonitrile extraction/partitioning and “Dispersive Solid-Phase Extraction” for the determination of pesticide residues in produce QuEChERS method. J Aoac Int 86:412–431
Blazková M, Rauch P, Fukal L (2010) Strip-based immunoassay for rapid detection of thiabendazole. Biosens Bioelectron 25:2122–2128
Cai Y, Mou S, Lu Y (2005) Multi-walled carbon nanotubes as a solid-phase extraction adsorbent for the determination of chlorophenols in environmental water samples. J Chromatogr A 1081:245–247
Chin CJM, Shih LC, Tsai HJ, Liu TK (2007) Adsorption of o-xylene and p-xylene from water by SWCNTs. Carbon 45:1254–1260
Cho HH, Smith BA, Wnuk JD, Fairbrother DH, Ball WP (2008) Influence of surface oxides on the adsorption of naphthalene onto multiwalled carbon nanotubes. Environ Sci Technol 42:2899–2905
Cuckler AC (1961) Thiabendazole, a new broad spectrum anthelmintic. J Parasitol 47:S36–S37
Duran A, Tuzen M, Soylak M (2009) Preconcentration of some trace elements via using multiwalled carbon nanotubes as solid phase extraction adsorbent. J Hazard Mater 169:466–471
Fang GZ, He JX, Wang S (2006) Multiwalled carbon nanotubes as sorbent for on-line coupling of solid-phase extraction to high-performance liquid chromatography for simultaneous determination of 10 sulfonamides in eggs and pork. J Chromatogr A 1127:12–17
García-Reyes JF, Llorent-Martinez EJ, Ortega-Barrales P, Molina-Diaz A (2006) Determination of thiabendazole residues in citrus fruits using a multicommuted fluorescence-based optosensor. Anal Chim Acta 557:95–100
Herrera-Herrera A, Ravelo-Pérez L, Hernández-Borges J, Afonsob M, Palenzuela J, Rodríguez-Delgado M (2012) Oxidized multi-walled carbon nanotubes for the dispersive solid-phase extraction of quinolone antibiotics from water samples using capillary electrophoresis and large volume sample stacking with polarity switching. J Chromatogr A 1218:5352–5361
Ito Y, Goto T, Oka H, Matsumoto H, Miyazaki Y, Takahashi N, Nakazawa H (2003) Simple and rapid determination of thiabendazole, imazalil, and o-phenylphenol in citrus fruit using flow-injection electrospray ionization tandem mass. J Agric Food Chem 51:861–866
Jamieson JD, Smith EB, Dalvie DK, Stevens GJ, Yanochko GM (2011) Myeloperoxidase-mediated bioactivation of 5-hydroxythiabendazole: a possible mechanism of thiabendazole toxicity. Toxicol in Vitro 25:1061–1066
Lemairea G, Delesclusea C, Pralavorioa M, Lediraca N, Lescab P, Rahmani R (2004) The role of protein tyrosine kinases in CYP1A1 induction by omeprazole and thiabendazole in rat hepatocytes. Life Sci 74:2265–2278
Liao Q, Sun J, Gao L (2008) The adsorption of resorcinol from water using multi-walled carbon nanotubes. Colloid Surf A 312:160–165
Llorent-Martínez EJ, Fernández-de Córdova ML, Ruiz-Medina A, Ortega-Barrales P (2012) Fluorimetric determination of thiabendazole residues in mushrooms using sequential injection analysis. Talanta 96:190–194
Lu C, Chung YL, Chang KF (2005) Adsorption of trihalomethanes from water with carbon nanotubes. Water Res 39:1183–1189
Muela S, Escalera B, Peńa M, Bustamante P (2010) Influence of temperature on the solubilization of thiabendazole by combined action of solid dispersions and co-solvents. Int J Pharm 384:93–99
Onyestyak G, Otvos Z, Valyon J, Kiricsi I, Rees LVC (2004) Acetylene sorption dynamics in carbon nanotubes. HeIv Chim Acta 87:1508–1514
Satou T, Koga M, Koike K, Tada I, Nikaido T (2001) Nematocidal activities of thiabendazole and ivermectin against the larvae of Strongyloides ratti and S. venezuelensis. Vet Parasitol 99(4):311–322
Su R, Xu X, Wang X, Li D, Li X, Zhang H, Yu A (2011) Determination of organophosphorus pesticides in peanut oil by dispersive solid phase extraction gas chromatography-mass spectrometry. J Chromatogr B 879:3423–3428
Talebpour Z, Taraji M, Adib N (2012) Stir bar sorptive extraction and high performance liquid chromatographic determination of carvedilol in human serum using two different polymeric phases and an ionic liquid as desorption solvent. J Chromatogr A 1236:1–6
Tuzen M, Soylak M (2007) Multiwalled carbon nanotubes for speciation of chromium in environmental samples. J Hazard Mater 147:219–225
Tuzen M, Saygi KO, Usta C, Soylak M (2008a) Pseudomonas aeruginosa immobilized multiwalled carbon nanotubes as biosorbent for heavy metal ions. Bioresource Technol 99:1563–1570
Tuzen M, Saygi KO, Soylak M (2008b) Solid phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubes. J Hazard Mater 152:632–639
Veneziano A, Vacca G, Arana S, De Simone F, Rastrelli L (2004) Determination of carbendazim, thiabendazole and thiophanate-methyl in banana (Musa acuminata) samples imported to Italy. Food Chem 87:383–386
Wu C (2007) Adsorption of reactive dye onto carbon nanotubes: equilibrium, kinetics and thermodynamics. J Hazard Mater 144:93–100
Xie WH, Shiu WY, Mackay D (1997) A review of the effect of salts on the solubility of organic compounds in seawater. Mar Environ Res 44:429–444
Zamora T, Pozo OJ, Lopez FJ, Hernandez F (2004) Determination of tridemorph and other fungicide residues in fruit samples by liquid chromatography–electrospray tandem mass spectrometry. J Chromatogr A 1045:137–143
Zhang S, Shao T, Bekaroglu SSK, Karanfil T (2010) Adsorption of synthetic organic chemicals by carbon nanotubes: effects of background solution chemistry. Water Res 44:2067–2074
Zhao P, Wang L, Luo J, Li J, Pan C (2012) Determination of pesticide residues in complex matrices using multi-walled carbon nanotubes as reversed-dispersive solid phase extraction sorbent. J Sep Sci 35:153–158
Zhou Q, Xiao J, Wang W (2006a) Using multi-walled carbon nanotubes as solid phase extraction adsorbents to determine dichlorodiphenyltrichloroethane and its metabolites at trace level in water samples by high performance liquid chromatography with UV detection. J Chromatogr A 1125:152–158
Zhou Q, Xiao J, Wang W, Liu G, Shi Q, Wang J (2006b) Determination of atrazine and simazine in environmental water samples using multiwalled carbon nanotubes as the adsorbents for preconcentration prior to high performance liquid chromatography with diode array detector. Talanta 68:1309–1315
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC51178212), the Foundation of 211 Project for Innovative Talent Training of Liaoning University, the Foundation for Young Scholars of Liaoning University (No. 2013LDQN13), and the Science and Technology Foundation of Ocean And Fisheries of Liaoning Province (No. 201406; No. 201408).
Conflict of Interest
Xu Xu declares that he has no conflict of interest. Na Long declares that he has no conflict of interest. Junna Lv declares that he has no conflict of interest. Lingling Wang declares that he has no conflict of interest. Minhui Zhang declares that he has no conflict of interest. Xinyu Qi declares that he has no conflict of interest. Lei Zhang declares that he has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, X., Long, N., Lv, J. et al. Functionalized Multiwalled Carbon Nanotube as Dispersive Solid-Phase Extraction Materials Combined with High-Performance Liquid Chromatography for Thiabendazole Analysis in Environmental and Food Samples. Food Anal. Methods 9, 30–37 (2016). https://doi.org/10.1007/s12161-015-0167-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0167-x