Abstract
In this research, a reliable and rapid chemiluminescent ELISA (CL-ELISA) determination procedure was developed and validated for the detection of deoxynivalenol (DON) in rice. With the help of chemiluminescent substrate, this developed protocol exhibited a high-sensitive character with a limit of detection (LOD) value of 0.94 ng/mL while it was 6.12 ng/mL in our previous report with the conventional colorimetric ELISA procedure by using the same monoclonal antibody (mAb). The mAb used in this research was proved to tolerate no more than 20 % methanol. Therefore, after the extraction procedure with 20 % methanol, samples could be proceeded to take the detection steps directly without dilution by PBS. In spiked rice samples, mean recoveries ranged from 91.40 to 93.48 % with intra-day and inter-day relative standard deviation (RSD) less than 10.62 and 12.41 %, respectively. The results demonstrated that this developed high-sensitive CL-ELISA immunoassay was suitable for screening of DON in rice samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deoxynivalenol (DON), one of type B trichothecenes, was firstly isolated and characterized from moldy barley in Japan. And in the USA, DON was considered to be the emetic factor associated with swine (Coppock et al. 1985). DON is mainly produced by Fusarium graminearum and Fusarium culmorum. There is a common epoxide structure between the C12 and C13 positions of DON, which is responsible for the toxicological activity (Sudakin 2003). In previous reports, there were numerous reports about DON naturally occurring in cereals, including maize, wheat, corn, rye, rice barley, and so on (Bensassi et al. 2010; Birzele et al. 2000; González-Osnaya et al. 2011; Moazami and Jinap 2009). Recently, it was found that DON exhibited serious toxic effects to animals and human beings, such as feed refusal, weight loss, cardiotoxicity, teratogenicity (Prelusky et al. 1997), immunotoxicity (Bondy and Pestka 2000), and apoptosis in vitro without significant dose–effect relationship (Meky et al. 2001).
In order to prevent the toxic effect of DON, maximum residue limits (MRLs) have been settled in different matrices (Commission Regulation 2006). In order to control the contaminant of DON in cereals, analytical methods for DON detection have been developed, such as thin-layer chromatography (TLC) (Schaafsma et al. 1998), high-performance liquid chromatography (HPLC) (Brenn-Struckhofova et al. 2007; Pussemier et al. 2006), gas chromatography (GC) (Tanaka et al. 2000; Valle-Algarra et al. 2011), and liquid chromatography tandem mass spectrometry (LC-MS/MS) (De Boevre et al. 2012; Wang et al. 2012). Although these chromatographic techniques exhibited sensitive and selective characters, they are time consuming and cannot fulfill the demands for rapid screening.
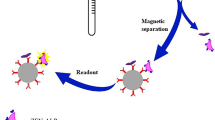
Considering the shortcomings of these instrument techniques, chemiluminescent competitive enzyme-linked immunosorbent assay (CL-ELISA) was proved to be a good alternative method because of its simple, rapid, and sensitive characteristics with wide linear working range. Compared to the traditional colorimetric analysis, CL-ELISA offers the possibility of improving the sensitivity with the enhanced chemiluminescent reaction (ECR) (Li et al. 2012b). Till now, investigations for veterinary drug residue analysis applied CL-ELISA methods have been reported (Chuanlai et al. 2006; Zhang et al. 2006). However, there are no related reports about DON detection applying CL-ELISA method. In this research, a highly sensitive and reliable method was developed for the determination of DON in rice based on CL-ELISA. The diagrammatic sketch of this developed method was shown in Fig. 1. As far as we know, it is the first report of CL-ELISA method for the detection of DON, which will contribute to the control of DON contamination in rice.
Materials and Methods
Chemicals and Reagents
Anti-DON monoclonal antibody (mAb) (no. 4A4) and coating antigen (no. 1103) were obtained from China Agricultural University. DON was purchased from Fermentek biotechnology (Israel). The peroxidase-conjugated goat anti-mouse IgG was obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). SuperSignal chemiluminescence substrate solution was purchased from Thermo Fisher (USA). Water was purified using a Milli-Q Synthesis system from Millipore (Bedford, MA, USA). Other reagents were analytical grade purchased from Beijing Reagent Corp. (Beijing, China).
Apparatus
White opaque 96-well polystyrene microtiter plates were purchased from Costar (Costar Inc., Milpitas, CA, USA). SpectraMax M5 microplate reader was purchased from Molecular Devices (CA, USA). MIKRO 22R centrifuge from Hettich Laborapparate (Tuttlingen, Germany) was obtained for this study.
Principle and the Procedure of the Chemiluminescent ELISA
The 96-well white opaque polystyrene microtiter plates were coated with 100 μL of coating antigen (no. 1103) in 50-mM carbonate (pH 9.6). After incubation for 2 h at 37 °C, each well was washed with washing buffer (phosphate-buffered saline (PBS) containing 2 % tween-20, pH 7.4) for three times and then adding 150 μL of 5 % BSA in PBS (0.01 M, pH 7.4) for 2 h at 37 °C for blocking. After these washing steps, sample or DON standard solution was added to each well at 50 μL/well. And then, 50 μL/well of purified mAb was added immediately for competition reaction at 37 °C for 30 min. Following the washing procedure, 100 μL/well of peroxidase-conjugated goat anti-mouse IgG was added and incubated at 37 °C for 30 min. After these immunological steps, SuperSignal chemiluminescence substrate solution was added and measured by SpectraMax M5 as soon as the addition. Each well of chemiluminescence intensities was analyzed individually.
Standard Curve
Calibration curves were fitted by 14 concentration levels of 0.01; 0.1; 0.5; 1.0; 2.0; 5.0; 10; 20; 50; 100; 200; 500; 1,000; and 10,000 ng/mL at triplicate each. Standard curves were evaluated by plotting absorbance against the logarithm of the each concentration and fitted to a four-parameter logistic equation using Origin version 7.5 (OriginLab, Northampton, MA, USA).
A stands for the asymptotic maximum (chemiluminescence intensity in the absence of analyte); B is the curve slope at the inflection point; C is the X value at the inflection point, which produces 50 % of the maximum absorbance; and D is the asymptotic minimum (background signal).
Methanol Tolerance Test
The tolerance of organic solvent for this very anti-DON mAb was tested with methanol. In this research, a series of different proportions of methanol in PBS (including PBS, 10, 20, 40, and 60 % methanol in PBS) were applied for organic solvent tolerance investigation. The developed CL-ELISA protocol was used for the methanol tolerance test. And the result was evaluated by IC50 of each standard curve in different proportions of methanol in PBS.
Sample Pretreatment
Two-gram samples were weighed into 50-mL polypropylene centrifuge tubes, and 20 mL of methanol/H2O (20:80, v/v) was added to each sample for extraction. Then, the mixture was vortexed for 5 min and centrifuged at 9,000g for 15 min at 4 °C. For analysis, 50-μL aliquots of the supernatant of each sample were added to the 96-well plate for this developed CL-ELISA procedure.
Accuracy and Precision
Negative cereal samples were spiked with DON at concentrations 0.5, 1.0, and 2.0 mg/kg. The analysis was performed at six replicates for each concentration (n = 6). The recoveries were calculated on the basis of the standard curve.
Results and Discussion
Methanol Tolerance Test
Standard curves in different proportions of methanol were prepared according to the developed CL-ELISA protocol (Fig. 2). From the figure, there was no apparent difference among all the standard curves when the methanol is no more than 20 %. This indicated that the anti-DON mAb used in this research could tolerate no more than 20 % methanol.
Sample Pretreatment
Based on the structure, there are three hydroxyl radicals in DON, which is related to the hydrophilic character. Acetonitrile/water (85:15, v/v) is the most common extraction solvent for DON in different matrices in the previous reports (Anselme et al. 2006; Pussemier et al. 2006). Besides, acetonitrile, methanol, and ethyl acetate were also used for the extraction. However, on the basis of our previous studies (Li et al. 2012a, b), it was found that a high percentage of organic solvent is not suitable for immunoassay, and it can result in distinct matrix effects. Moreover, it was also found that 20 % methanol could be applied for the extraction in color metric ELISA procedure. On the other hand, 20 % was the highest percentage of methanol that the anti-DON mAb could tolerate. Therefore, 20 % methanol was tested in the developed CL-ELISA protocol, and the recoveries were satisfactory.
Standard Curves
The principle of the developed CL-ELISA protocols was exhibited in Fig. 1. Calibration curves were prepared using serious concentration levels of the target analyte to evaluate parameters of each protocol. Standard curves were obtained by plotting absorbance against the logarithm of the concentrations and fitted to a four-parameter logistic equation (Fig. 3). The standard curves and correlation coefficient (R) were 0.9988.
Sensitivity
The sensitivity of the developed CL-ELISA was evaluated with IC50 and limit of detection (LOD), measured by IC10). The regression analysis was obtained from the sigmoidal standard curve with four-parameter equations (Fig. 3). From the figure, IC50 and LOD for DON in CL-ELISA procedure were 12.98 and 0.94 ng/mL, respectively. Compared to our previous report of conventionally colorimetric ELISA protocol with the same mAb (IC50 62.38 ng/mL, LOD 6.65 ng/mL) (Li et al. 2012a, b), the CL-ELISA protocol exhibited a more sensitive result with a much lower LOD and IC50 value.
Accuracy and Precision
Accuracy and precision were evaluated by determining recoveries of DON in fortified samples with six replicates on three validation days. Close agreements between measured values and spiked values were obtained at each spiked levels. Mean recovery values ranged from 91.40∼93.48 % with intra-day and inter-day relative standard deviation (RSD) less than 10.62 and 12.41 %, respectively (Table 1).
Conclusion
For the first time, an ELISA method based on the chemiluminescent reaction was described for the determination of DON in rice. The proposed format offers the advantages of simplicity, rapidity, and cost-effectiveness. With respect to its overall sensitivity and specificity, the CL-ELISA method is superior to the conventional colorimetric ELISA protocol by using the same mAb. This developed CL-ELISA method could be a suitable tool for rapid screening of DON in rice samples.
References
Anselme M, Tangni EK, Pussemier L, Motte J, Van Hove F, Schneider Y, Van Peteghem C, Larondelle Y (2006) Comparison of ochratoxin A and deoxynivalenol in organically and conventionally produced beers sold on the Belgian market. Food Addit Contam 23:910–918
Bensassi F, Zaied C, Abid S, Hajlaoui MR, Bacha H (2010) Occurrence of deoxynivalenol in durum wheat in Tunisia. Food Control 21:281–285
Birzele B, Prange A, KrÄmer J (2000) Deoxynivalenol and ochratoxin A in German wheat and changes of level in relation to storage parameters. Food Addit Contam 17:1027–1035
Bondy GS, Pestka JJ (2000) Immunomodulation by fungal toxins. J Toxicol Env Heal B 3:109–143
Brenn-Struckhofova Z, Cichna-Markl M, Böhm C, Razzazi-Fazeli E (2007) Selective sample cleanup by reusable sol–gel immunoaffinity columns for determination of deoxynivalenol in food and feed samples. Anal Chem 79:710–717
Chuanlai X, Cifang P, Kai H, Zhengyu J, Wukang W (2006) Chemiluminescence enzyme immunoassay (CLEIA) for the determination of chloramphenicol residues in aquatic tissues. Luminescence 21:126–128
Commission Regulation (EC) No 1881/2006 of 19 December (2006) Setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union, L 364/17
Coppock RW, Swanson SP, Gelberg HB, Koritz GD, Hoffman WE, Buck WB, Vesonder RF (1985) Preliminary study of the pharmacokinetics and toxicopathy of deoxynivalenol (vomitoxin) in swine. Am J Vet Res 46:169–174
De Boevre M, Di Mavungu JD, Maene P, Audenaert K, Deforce D, Haesaert G, Eeckhout M, Callebaut A, Berthiller F, Van Peteghem C (2012) Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit Contam A 29:819–835
González-Osnaya L, Cortés C, Soriano JM, Moltó JC, Manes J (2011) Occurrence of deoxynivalenol and T-2 toxin in bread and pasta commercialised in Spain. Food Chem 124:156–161
Li Y, Shi W, Shen J, Zhang S, Cheng L, Wang Z (2012a) Development of a rapid competitive indirect ELISA procedure for the determination of deoxynivalenol in cereals. Food Agr Immunol 23:41–49
Li Y, Zhang Y, Cao X, Wang Z, Shen J, Zhang S (2012b) Development of a chemiluminescent competitive indirect ELISA method procedure for the determination of gentamicin in milk. Anal Methods-UK 4:2151–2155
Meky FA, Hardie LJ, Evans SW, Wild CP (2001) Deoxynivalenol-induced immunomodulation of human lymphocyte proliferation and cytokine production. Food Chem Toxicol 39:827–836
Moazami EF, Jinap S (2009) Natural occurrence of deoxynivalenol (DON) in wheat based noodles consumed in Malaysia. Microchem J 93:25–28
Prelusky DB, Rotter BA, Thompson BK, Trenholm HL (1997) Effect of the appetite stimulant cyproheptadine on deoxynivalenol‐induced reductions in feed consumption and weight gain in the mouse. J Environ Sci Heal B 32:429–448
Pussemier L, Piérard J, Anselme M, Tangni EK, Motte J, Larondelle Y (2006) Development and application of analytical methods for the determination of mycotoxins in organic and conventional wheat. Food Addit Contam 23:208–1218
Schaafsma AW, Nicol RW, Savard ME, Sinha RC, Reid LM, Rottinghaus G (1998) Analysis of Fusarium toxins in maize and wheat using thin layer chromatography. Mycopathologia 142:107–113
Sudakin DL (2003) Trichothecenes in the environment: relevance to human health. Toxicol Lett 143:97–107
Tanaka T, Yoneda A, Inoue S, Sugiura Y, Ueno Y (2000) Simultaneous determination of trichothecene mycotoxins and zearalenone in cereals by gas chromatography–mass spectrometry. J Chromatogr A 882:23–28
Valle-Algarra FM, Mateo EM, Mateo R, Gimeno-Adelantado JV, Jimenez M (2011) Determination of type A and type B trichothecenes in paprika and chili pepper using LC-triple quadrupole-MS and GC-ECD. Talanta 84:1112–1117
Wang Y, Cao X, Li Y, Yang S, Shen J, Zhang S (2012) Simultaneous determination of type-A and type-B trichothecenes in rice by UPLC-MS/MS. Anal Methods-UK 4:4077–4082
Zhang S, Zhang Z, Shi W, Eremin SA, Shen J (2006) Development of a chemiluminescent ELISA for determining chloramphenicol in chicken muscle. J Agr Food Chem 54:5718–5722
Acknowledgments
This work was supported by Development Project of Shandong Province Science and Technology (no. 2013YD15011).
Compliance with Ethics Requirements
Yanshen Li declares that he has no competing financial interests. Gongzhen Liu declares that he has no competing financial interests. Xuejun Fu declares that he has no competing financial interests. Jun He declares that he has no competing financial interests. Zhanhui Wang declares that he has no competing financial interests. Jianhai Hou declares that he has no competing financial interests. Xingyuan Cao declares that he has no competing financial interests. Weimin Shi declares that she has no competing financial interests. Suxia Zhang declares that she has no competing financial interests. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yanshen Li and Gongzhen Liu contributed equally to this study.
Rights and permissions
About this article
Cite this article
Li, Y., Liu, G., Fu, X. et al. High-Sensitive Chemiluminescent ELISA Method Investigation for the Determination of Deoxynivalenol in Rice. Food Anal. Methods 8, 656–660 (2015). https://doi.org/10.1007/s12161-014-9941-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-9941-4