Abstract
A poly(glutamic acid)-modified glassy carbon electrode (PGA/GCE) for the electrochemical determination of kojic acid was developed. The polymerization mechanism of glutamic acid at the glassy carbon electrode, the electrocatalytic mechanism of the poly(glutamic acid) film toward kojic acid, and the electrochemical behavior of kojic acid at the PGA/GCE were investigated. The studies revealed that the oxidation of kojic acid at the PGA/GCE is greatly facilitated, which is attributed to the weakening of the bond energy between oxygen and hydrogen due to the formation of hydrogen bond between hydroxyl group in kojic acid and the nitrogen atom in poly(glutamic acid). The oxidation of kojic acid at the PGA/GCE is a one proton–one electron process. The catalytic effect was further used to determine kojic acid by cyclic voltammetry. The oxidation peak current shows a linear relationship with the concentrations of kojic acid in the range of 8.00 × 10−6 to 6.60 × 10−4 mol L−1 with the detection limit of 8.00 × 10−7 mol L−1. The modified electrode shows excellent sensitivity, selectivity, and stability and has been applied to detect kojic acid in a variety of food products with satisfactory results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kojic acid, 5-hydroxy-2-(hydroxymethyl)-4-pyrone, is a natural metallic product of several species of the genus Aspergillus (Yabuta 1924). It shows broad properties such as preventing oxidation, scavenging free radicals, chelating metal ions, and inhibiting the catecholase activity of tyrosinase, which is the essential enzyme for melanin formation (Rho et al. 2007; Shih and Zen 1999). Due to its excellent properties, it is widely used in food and cosmetic industries as a preservative and a skin lightening agent. Although consumption of kojic acid at low levels does not present a concern for safety (Burdock et al. 2001), studies show that continuous overuse of kojic acid is carcinogenic and tumorigenic (Chusiri et al. 2011; Fujimoto et al. 1998; Wei et al. 1991). In addition, kojic acid is a by-product of some fermentation and plays an important role in monitoring fermentation process (Futamura et al. 2001; Wang et al. 2011). For example, kojic acid is a by-product in the fermentation process of malting rice for making rice wines, sweet beverages, and distilled liquors (Bentley 2006). Thereby, development of rapid and convenient method for the determination of kojic acid is highly desirable.
Up to now, various methods have been developed for the quantification of kojic acid, such as high-performance liquid chromatography (Higashi and Fujii 2012; Huang et al. 2004, 2012; Kimura et al. 2000; Lin et al. 2007a; Liu 2004; Zhao et al. 2003), ion-pair liquid chromatography (Shih 2002), spectrophotometry (Januario Correr et al. 2005; Piantavini et al. 2010), capillary electrophoresis (Lin et al. 2007b), and electrochemical methods (Liu et al. 2009; Shih and Zen 1999; Shih et al. 2007; Wang et al. 2011, 2012, 2013; Yang and Zhang 2007; Yang et al. 2011). Among these methods, chromatographic methods require expensive equipments, large amount of organic solvents, and are time-consuming. Spectrophotometric methods are susceptible to interference by the turbidity or color of the samples. Capillary electrophoresis method is not very sensitive. Comparing to the above methods, electrochemical methods are convenient, rapid, economical, and sensitive. However, the oxidation of kojic acid at bare glassy carbon electrode is kinetically slow and a relatively high overpotential is required. Thus, various modifications have been made to bare electrode to lower the overpotential of kojic acid oxidation. The reported electrode modifiers include screen printed polypropylene (Shih and Zen 1999), molecularly imprinted polymer (Wang et al. 2011), PVP (polyvinylpyrrolidone) (Yang and Zhang 2007), carbon nanotube/alizarin red S (Liu et al. 2009), reduced graphene sheet (Wang et al. 2012), CuO/Fe2O3 microspheres/chitosan (Yang et al. 2011), graphene–Pt nanocomposite (Wang et al. 2013), and carbon nanotube (Shih et al. 2007).
In recent years, a new class of biocompatible polymer, poly(amino acid) has attracted extensive attention for the fabrication of biosensors. Amino acids are biologically important organic compounds that contain amine and carboxylic acid functional groups and possess many unique properties. Amino acids are easily available and can be easily electropolymerized onto electrode surface. The thickness of the poly(amino acid) can also be well controlled by adjusting the deposition cycles. The electrocatalytic activity of poly(amino acid) film toward many biomolecules resides in the formation of hydrogen bond between nitrogen atoms in amino acid and hydroxyl group in biomolecules. Kojic acid is such a molecule that contains hydroxyl group and we expect poly(amino acid) can catalyze the oxidation of kojic acid at an electrode surface. To the best of our knowledge, the electrocatalytic activity of poly(glutamic acid) towards the oxidation of kojic acid has never been studied.
In this study, a poly(glutamic acid)-modified glassy carbon electrode (PGA/GCE) was fabricated. The catalytic properties of poly(glutamic acid) towards kojic acid and electrochemical behaviors of kojic acid at the PGA/GCE were presented. Using the fabricated electrode, a method for the determination of kojic acid was developed. The method has been fully validated in terms of linearity, recovery, reproducibility, and robustness. The applicability of the developed method has been successfully demonstrated in the determination of kojic acid in a variety of food products such as bean paste, rice wine, etc.
Materials and Methods
Reagents and Materials
Kojic acid was purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China). Glutamic acid was purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). All other chemical reagents (analytical-reagent grade) were obtained from Beijing Chemical Reagent Company (Beijing China). Kojic aicd standard solutions (8.0 × 10−4 and 8.0 × 10−3 mol L−1) were prepared by dissolving kojic acid in double-distilled water. Glutamic acid solution (2.5 × 10−3 mol L−1) was prepared by weighing 0.0367 g of glutamic acid to 100.00 mL of double-distilled water. Phosphate buffer solutions (PBS) were prepared by mixing stock solutions of 0.2 mol L−1 Na2HPO4 and 0.1 mol L−1 citric acid. Acetate buffer solutions (HAc–NaAc, pH 2.6 to 5.8) were prepared by mixing 0.3 mol L−1 acetic acid and 0.2 mol L−1 sodium acetate. All aqueous solutions were prepared using double-distilled water.
Apparatus
A CHI 660C Electrochemical Workstation (Chen-hua, Shanghai, China) consisting of a working electrode (a bare GCE or a PGA/GCE, 3.8 mm in diameter) a counter electrode (a platinum wire electrode), and a reference electrode (a Ag/AgCl, 3.0 M KCl solution) was used for both the fabrication of PGA/GCE and the cyclic voltammetry (CV) experiments. A field emission SEM Sirion 200 (FEI, America) was used for recording the scanning electron microscope (SEM) image of the polymer film at the surface of the modified electrode. Acidity was measured by a PHS-3B Precision pH Meter (Shanghai, China) and sonication was performed on a KQ-100 Ultrasonic Cleaner (Kunshan, China).
Fabrication of PGA/GCE
A bare GCE (3.8 mm in diameter) was polished successively with a piece of grit 1,000 gold sand paper and 0.05 μm alumina powder slurry to mirror-like smoothness, the bare GCE was rinsed thoroughly with doubly distilled water after each polishing step. Then, the electrode was subjected successively to 1:1 nitric acid, absolute ethanol, and doubly distilled water in ultrasonic bath for 5 min, respectively. After being cleaned again with double-distilled water, the polished bare GCE was immersed in a polymerization solution prepared by mixing 3.00 mL of 2.5 × 10−3 mol L−1 glutamic acid, 10.00 mL of pH 7.0 PBS, and 7.00 mL of double-distilled water. Using the polished bare GCE as a working electrode, a Ag/AgCl electrode as a reference electrode and a platinum as a counter electrode, continuous cyclic scan of nine cycles was performed in a potential range from −0.8 to 2.4 V at 120 mV s−1. After polymerization, the modified electrode was rinsed with double-distilled water and dried in air to give a PGA/GCE.
Real Sample Preparation
Bean Paste Sample
Ten grams of accurately weighed bean paste was transferred into a pulverizer. Then, about 30 mL of double-distilled water was added to make a paste, which was then filtered under vacuum. The isolated solid was slurried twice with small amounts of water and filtered under vacuum. All the filtrates were collected into a 100.00 mL volumetric flask, then 5.00 mL of 0.3 mol L−1 EDTA and 5.00 mL of 1 % sodium sulfite were added into the flask, diluted to volume with double-distilled water, and mixed.
Rice Wine Sample
Twenty-five grams of accurately weighed rice wine was transferred into a 100.00-mL volumetric flask, then 2.00 mL of 0.3 mol L−1 EDTA, and 2.00 mL of 1 % sodium sulfite were added into the flask, diluted to volume with double-distilled water, and mixed.
General Procedure for Determination of Kojic Acid
Under optimal conditions, using a PGA/GCE as working electrode, a Ag/AgCl electrode as reference electrode and a platinum as counter electrode, cyclic voltammetry was used in the electrochemical measurements with a scan rate of 120 mV s−1. CVs were obtained by immersing the PGA/GCE in a series of solutions containing different concentrations of kojic acid in pH 4.6 HAc–NaAc and scanning in the potential range from 0.4 to 1.2 V. Upon completion of each scan, the modified electrode was placed in a blank buffer solution and cyclic scan was continued until no peak occurs anymore. The electrode recovery process requires five to ten cycles depending on the concentration of sample solution. Then, the electrode was washed with water and dried with filter paper for reuse.
Results and Discussion
Electropolymerization of Glutamic Acid at the GCE Surface
Electrocatalytic oxidation of kojic acid at the surface of the PGA/GCE can be greatly affected by its fabrication parameters. In view of this point, the influence of the electrode fabrication parameters on the oxidation peak current of kojic acid was investigated.
The range of the polymerization potential was studied by keeping a lower potential at −0.8 V, while changing the upper from 2.2 to 2.8 V, then, by keeping an upper switch potential at 2.4 V and changing the lower from −1.6 to −0.8 V. Based on peak response of kojic acid at the fabricated electrode, the optimum range of the potential was from −0.8 to 2.4 V.
The thickness of the polymer film grows with increasing cyclic number of voltammetric scans and the thickness can be controlled by adjusting the cyclic number. It has been found that, at the beginning, the oxidation peak current of kojic acid at the fabricated electrode increased with the increase of cyclic number. After nine cycles of voltammetric scans, the peak currents gradually decreased likely because of a decrease of conductivity of the thicker polymer film. Thus, nine cycles of voltammetric scan were chosen for the preparation of modified film.
Five different scan rates, including 80, 100, 120, 140, and 160 mV s−1, were studied to see its influence on the catalysis. It has been found that the PGA/GCE prepared at a scan rate of 120 mV s−1 exhibited best catalytic effect to the oxidation of kojic acid, thereby, a scan rate of 120 mV s−1 was chosen for the polymerization.
We also studied the pH dependence of the polymer film growth. Electropolymerization was performed in PBS at various solution pH from 2.2 to 8.0. Experimental results show that the polymerization of glutamic acid took place at all tested pH values. PGA/GCE fabricated in a pH 7.0 solution gave the maximum oxidation peak response, thus, pH 7.0 PBS was used for the electrode fabrication.
The cyclic voltammograms of polymerization under the above-optimized conditions are shown in Fig. 1. At the first cycle, a well-defined oxidation peak at 1.7 V appeared, which may be attributed to the oxidation of amino group of glutamic acid to its corresponding cation free radical. The glutamic acid monomer could be linked to the electrode surface by carbon–nitrogen linkage and then formed a poly(glutamic acid) film (Scheme 1). From the second cycle, the peak at 1.7 V disappeared and a new broad peak of poly(glutamic acid) appeared. The peak current of the new peak increased continuously with the increase of cyclic number, indicating a continuous growth of the polymer at the electrode surface. When the polymerization was completed, a uniform adherent blue polymer film was formed on the GCE surface. Figure 2 shows the SEM image of the modified electrode surface, indicating that glutamic acid was successfully polymerized onto the surface of the GCE.
Electrochemical Behavior of Kojic Acid at the PGA/GCE
Cyclic voltammograms of kojic acid at the bare GCE and the PGA/GCE are shown in Fig. 3. From the figure, we can see that the intensity of the oxidation peak current of kojic acid at the PGA/GCE (curve 2, Fig. 3) was sharply increased in contrast to the poor response at the bare GCE (curve 1, Fig. 3), suggesting that the poly(glutamic acid) film immobilized on the electrode has a high electrocatalytic activity toward the oxidation of kojic. The electrocatalytic activity of the poly(glutamic acid) film towards kojic acid may be attributed to the formation of hydrogen bond between hydroxyl group in kojic acid and the nitrogen atom in poly(glutamic acid). The formation of hydrogen bond can weaken the bond energy between hydrogen and oxygen and the electron transfer is liable to occur via the bond of N–H–O. A similar mechanism was reported for the electrocatalysis of poly(aminosulfonic acid) upon salbutamol (Lijun et al. 2007). Further, no corresponding reduction peak of kojic acid was observed both at the bare GCE and the PGA/GCE, indicating that the electrochemical oxidation of kojic acid was irreversible under this experimental condition. Using kojic acid as an indicator, the property of the poly(glutamic acid) membrane was studied by electrochemical impedance spectroscopy. The obtained Nyquist plot of impedance spectra show that electron transfer resistance value obtained at bare electrode is larger than that obtained at the PGA/GCE, indicating that the electron transfer rate of kojic acid at the PGA/GCE is faster than that at the bare electrode.
Figure 4 shows the cyclic voltammograms of 1.00 × 10−3 mol L−1 kojic acid at the PGA/GCE at various scan rate, from which we can see that the oxidation peak current increases linearly with the square root of scan rate and the linear regression equation can be expressed as i pa = 2.29 × 10−5 + 4.15 × 10−6 v 1/2, r = 0.9992, indicating that the oxidation process of kojic acid at the PGA/GCE is a diffusion-controlled process.
Cyclic voltammograms of 1.00 × 10−3 mol L−1 kojic acid at a PGA/GCE. Each of the numbers from 1 to 8 corresponds to a scan rate of 60, 80, 100, 120, 140, 160, 180, and 200 mV s−1, respectively. Inset is the plot of oxidation peak current of kojic acid versus the square root of scan rate obtained in a potential range of 0.4 to 1.2 V
Figure 5 shows the influence of electrolyte pH on the oxidation reaction of kojic acid at the fabricated PGA/GCE. From the figure, we can see that the oxidation peak shifts negatively with the increase of the pH value of the supporting electrolyte. Because the electrochemical oxidation of kojic acid at the PGA/GCE is irreversible, the value of electrochemical transfer coefficient αn (where α is the transfer coefficient and n is the number of electrons involved) at each pH value can be calculated according to the following equation:
Cyclic voltammograms of 1.00 × 10−3 mol L−1 kojic acid at the PGA/GCE in HAc–NaAc of various pH values. Each of the numbers from 1 to 6 corresponds to a pH of 3.8, 4.2, 4.6, 5.0, 5.4, and 5.8, respectively. Scan rate, 120 mV s−1. Inset is the plot of peak potential of kojic acid versus pH of supporting electrolyte obtained in a potential range of 0.4 to 1.2 V
where E p/2 is the potential in volts that corresponds to i p/2 (Portela et al. 1996). By this equation, the calculated αn value at pH 4.6 is 0.90 V−1. From the plot E p vs. pH (inset of Fig. 5), we can see that E pa and electrolyte pH are linearly proportional in the pH range of 3.8 to 5.8, and the linear equation can be expressed as E pa = −0.066pH + 1.25, r = −0.9902, with a slope close to 59 mV, indicating that the oxidation reaction involves proton and the number of protons and electrons involved in the reaction are equal.
The number of protons involved in the electrode reaction (m) can be calculated according to the following equation (Yang et al. 2010) :
With the above-obtained data, m is calculated to be 1.01, indicating that the electrocatalytic oxidation of kojic acid involves one proton and the oxidation is a one proton–one electron process. A possible reaction mechanism of kojic acid at the PGA/GCE is shown in Scheme 2.
Optimization of Conditions for the Determination of Kojic Acid
The conditions for the determination of kojic acid were optimized by investigating the effects of scan rate, pH of supporting electrolyte, and sample accumulation time. The peak current of kojic acid linearly increases with the scan rate and the scan rate of 120 mV s−1 gave the best peak shape (Fig. 4). In a pH range of 3.8 to 5.8, the oxidation peak current increased as the pH value of supporting electrolyte changed from pH 3.8 to 4.6, and then decreased after pH > 4.6. Maximum peak current in the investigated pH range was obtained in a pH 4.6 supporting electrolyte (Fig. 5). To study the effect of accumulation time, we detected kojic acid of lower, middle, and higher concentrations within the linear range by varying the accumulation time between 0 and 120 s. Results showed that the sensitivity of detection was significantly improved by stirring. The peak currents for all concentrations first increase and reach the maximum value and then stabilize on increasing stirring time. This is because excess potential caused by concentration difference becomes smaller with better mixing, resulting in increased response current. A stirring time of 50 s can effectively eliminate the excess potential for all concentrations. Thereby, a scan rate of 120 mV s−1, a phosphate buffer solution of pH 4.6, and an accumulation time of 50 s were chosen as the determination parameters in this study.
Interference Studies
Potential interference to the determination of kojic acid from some common species present in food products was individually investigated by cyclic voltammetry under the above-optimized conditions. The oxidation peak current of a real rice wine sample containing 3.20 × 10−5 mol L−1 kojic acid was measured when 50-fold concentration of K+, Na+, Ca2+, Cu2+, Mg2+, Fe2+, Zn2+, Al3+, Fe3+, Cl−, SO4 2−, PO4 3−, citric acid, tartaric acid, sucrose, ascorbic acid, and β-cyclodextrin were individually added and equilibrated. Experimental results showed that the measured peak current changes after addition of these species were less than ±3 %, indicating that the PGA/GCE is highly selective towards the determination of kojic acid.
Validation Studies
Linearity Range and Limit of Detection
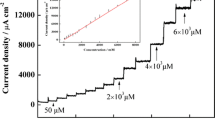
Under the above-optimized conditions, the variation of oxidation peak current with concentration of kojic acid at the PGA/GCE was studied by cyclic voltammetry and the result is shown in Fig. 6. From the figure, we can see that the oxidation peak current of kojic acid at the PGA/GCE was proportional to its concentration in pH 4.6 HAc–NaAc in a range from 8.0 × 10−6 to 6.6 × 10−4 mol L−1. The linear regression equation is i pa = 4.92 × 10−6 + 0.048c (μmol L−1), r = 0.9992. The limit of detection was estimated by gradually decreasing the concentration levels of kojic acid. When the concentration of kojic acid was decreased to 8.0 × 10−7 mol L−1, the oxidation peak can still be observed, but the oxidation peak almost disappeared when the concentration was further decreased. Therefore, the limit of detection was evaluated to be 8.0 × 10−7 mol L−1.
Cyclic voltammograms of kojic acid at various concentrations at the PGA/GCE in pH 4.6 HAc–NaAc. Scan rate, 120 mV s−1. Each of the numbers from 1 to 9 corresponds to a concentration of 8.00 × 10−6, 1.20 × 10−5, 4.80 × 10−5, 7.20 × 10−5, 9.60 × 10−5, 1.20 × 10−4, 2.40 × 10−4, 4.60 × 10−4, and 6.60 × 10−4 mol L−1, respectively. Inset is the plot of oxidation peak current of kojic acid versus its concentration obtained in a potential range of 0.4 to 1.2 V
Accuracy and Repeatability
Accuracy and repeatability of the proposed method was assessed on real bean paste and rice wine samples spiked with three concentration levels. Six replicate determinations for each spiked concentration level were made. Satisfactory recoveries and relative standard deviations (RSD) were obtained and are reported in Table 1. The recoveries obtained confirmed the high accuracy and the relative standard deviations obtained confirmed the good repeatability of the proposed method.
Robustness
The robustness of the proposed method was evaluated by studying the effect of small variations in electrolyte pH (4.4–4.2), scan rate (118–122 mV s−1), and accumulation time (48–52 s) on the recovery of kojic acid. Recovery for kojic acid under all variable conditions is in the range of 96.0–99.7 %. No significant changes were observed when these critical parameters were slightly varied, indicating a very good robustness of the proposed method.
Stability and Reproducibility of the PGA/GCE
Stability of the PGA/GCE was evaluated by storing the electrode in air for 30 days and then the CVs were recorded and compared with the CVs obtained previously. The electrode retained 98.4 % of its initial peak current response for 5.0 × 10−5 mol L−1 kojic acid standard solution, indicating good stability of the immobilized poly(glutamic acid) film on the surface of GCE. In order to study the reproducibility of the electrode preparation procedure, five modified electrodes based on the same procedure were fabricated and used for the determination of 5.0 × 10−5 mol L−1 kojic acid standard solution. The RSD for the between electrode peak currents (average of six determinations on each electrode) was calculated to be 3.2 %, indicating good reproducibility of the electrode preparation procedure.
Analytical Application
Analysis of kojic acid in bean paste and rice wine was performed using the PGA/GCE. First, real samples were analyzed. Then, the analyzed samples were spiked with kojic acid standard solutions and similarly analyzed. The concentration of kojic acid in the electrolytic cell and calculated recoveries are listed in Table 1. Good recoveries demonstrated the applicability of the modified electrode for determination of kojic acid. After calculation, the corresponding concentrations of kojic acid in the analyzed bean paste and rice wine were 1,200.00 and 36.67 mg kg−1, respectively.
Conclusions
A poly(glutamic acid)-modified glassy carbon electrode was fabricated, and a convenient electrochemical method for the determination of kojic acid was developed using the fabricated electrode. The poly(glutamic acid) film immobilized at the electrode surface exhibits remarkable catalytic activity toward the electrochemical reaction of kojic acid. In pH 4.6 HAc–NaAc, the oxidation peak current of kojic acid obtained by cyclic voltammetry is linearly proportional to its concentration in a range from 8.0 × 10−6 to 6.6 × 10−4 mol L−1, with a detection limit of 8.0 × 10−7 mol L−1. The reproducibility of the method shows a RSD of less than 4.0 % and the recovery of the method is between 96.0 and 99.2 %. The method is fast, inexpensive, and does not require any solvents. It can be used for the routine analysis of a wide variety of food products.
References
Bentley R (2006) Nat Prod Rep 23:1046–1062
Burdock GA, Soni MG, Carabin IG (2001) Regul Toxicol Pharmacol 33:80–101
Chusiri Y, Wongpoomchai R, Kakehashi A, Wei M, Wanibuchi H, Vinitketkumnuan U, Fukushima S (2011) Food Chem Toxicol 49:471–476
Fujimoto N, Watanabe H, Nakatani T, Roy G, Ito A (1998) Food Chem Toxicol 36:697–703
Futamura T, Okabe M, Tamura T, Toda K, Matsunobu T, Park YS (2001) J Biosci Bioeng 91:272–276
Higashi Y, Fujii Y (2012) J Cosmet Sci 63:205–212
Huang SC, Lin CC, Huang MC, Wen KC (2004) J Food Drug Anal 12:13–18
Huang J, Liu Y, Ding T, Zhang XY, Chen HL, Shen CY, Wu B, Niu W (2012) Sepu 30:578–583
Januario Correr C, Cordeiro G, Gasparetto J, Peralta-Zamora P, Pontarolo R (2005) Acta Farm Bonaer 24:416–420
Kimura K, Hirokada M, Yasuda K, Nishijima M (2000) Shokuhin Eiseigaku Zasshi 41:70–73
Lijun L, Laibo Y, Hao C, Qifeng C, Fengmin W, Tian C, Xiaoyong Z, Hongxing K, Jianling W (2007) Anal Lett 40:3290–3308
Lin CH, Wu HL, Huang YL (2007a) Anal Chim Acta 581:102–107
Lin YH, Yang YH, Wu SM (2007b) J Pharm Biomed Anal 44:279–282
Liu S (2004) Chin J Chromatogr 22:660
Liu J, Zhou D, Liu X, Wu K, Wan C (2009) Colloids Surf B 70:20–24
Piantavini MS, Stremel DP, Trindade ACLB, Pontarolo R (2010) Lat Am J Pharm 29:1468–1472
Portela MJ, Gomez De Balugera Z, Goicolea MA, Barrio RJ (1996) Anal Chim Acta 327:65–71
Rho HS, Baek HS, You JW, Kim S, Lee JY, Kim DH, Chang IS (2007) Bull Korean Chem Soc 28:471–473
Shih Y (2002) J AOAC Int 84:1045–1049
Shih Y, Zen JM (1999) Electroanalysis 11:229–233
Shih Y, Zen JM, Ke JH, Hsu JC, Chen PC (2007) J Food Drug Anal 15:151–155
Wang Y, Tang J, Luo X, Hu X, Yang C, Xu Q (2011) Talanta 85:2522–2527
Wang Y, Zhang D, Wu J (2012) J Electroanal Chem 664:111–116
Wang L, Qi W, Su R, He Z (2013) Food Anal Methods 1–7
Wei CI, Huang TS, Fernando SY, Chung KT (1991) Toxicol Lett 59:213–220
Yabuta T (1924) J Chem Soc Trans 125:575–587
Yang X, Zhang H (2007) Food Chem 102:1223–1227
Yang D, Zhu L, Jiang X (2010) J Electroanal Chem 640:17–22
Yang Z, Yin Z, Chen F (2011) Electrochim Acta 56:1089–1093
Zhao S, Li Y, Zhao H, Ji R, Li Y (2003) J Hyg Res 32:384–385
Acknowledgments
This work was financially supported by a project of Shandong Province Higher Educational Science and Technology Program (J12LD53) and Heze University Scientific Research Fund (XY12BS07).
Conflict of interest
Xinying Ma declares that she has no conflict of interest. Mingyong Chao declares that she has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, X., Chao, M. Study on the Electrochemical Properties of Kojic Acid at a Poly(glutamic Acid)-Modified Glassy Carbon Electrode and Its Analytical Application. Food Anal. Methods 7, 1458–1464 (2014). https://doi.org/10.1007/s12161-013-9772-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9772-8