Abstract
An ultra-performance liquid chromatography (UPLC®) method has been developed for the simultaneous determination of deoxynivalenol (DON) and nivalenol (NIV) in wheat. Ground sample was extracted with water and the filtered extract was cleaned up through an immunoaffinity column containing a monoclonal antibody specific for DON and NIV. Toxins were separated and quantified by UPLC® with photodiode-array detector (λ = 220 nm) in less than 3 min. Mean recoveries from blank wheat samples spiked with DON and NIV at levels of 100–2,000 μg/kg (each toxin) ranged from 85 to 95 % for DON and from 81 to 88 % for NIV, with relative standard deviations less than 7 %. Similar recoveries were observed from spiked samples when methanol/water (80:20, v/v) was used as extraction solvent. However, by using a wheat sample naturally contaminated with DON and NIV, the one-way analysis of variance (Student–Newman–Keuls test) between different extraction solvents and modes showed that water extraction provided a significant increase (P < 0.001) in toxin concentrations (mean values of six replicate analyses) with respect to methanol/water (80:20, v/v). No significant difference was observed between shaking (60 min) and blending (3 min). The limit of detection (LOD) of the method was 30 μg/kg for DON and 20 μg/kg for NIV (signal-to-noise ratio 3:1). The immunoaffinity columns showed saturation of DON/NIV binding sites at levels higher than 2,000 ng in blank wheat extracts spiked with the corresponding amount of mycotoxin, as single mycotoxin or sum of DON and NIV. The range of applicability of the method was from LOD to 4,000 μg/kg, as single mycotoxin or sum of DON and NIV in wheat. The analyses of 20 naturally contaminated wheat samples showed DON contamination in all analyzed samples at level ranging from 30 to 2,700 μg/kg. NIV was detected in two samples at negligible toxin levels (up to 46 μg/kg). This is the first UPLC® method using immunoaffinity column cleanup for the simultaneous and sensitive determination of DON and NIV in wheat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deoxynivalenol (DON) and nivalenol (NIV), two type B trichothecenes produced mainly by Fusarium graminearum and Fusarium culmorum (NIV is produced also by Fusarium poae and Fusarium crookwellense), are common natural co-occurring contaminants of wheat and other small cereals in temperate regions of America, Europe, and Asia (Placinta et al. 1999; Turner 2010). Incidence and levels of contamination vary depending on the geographic origin, environmental conditions, fungal inoculum, and plant stress. DON contamination is generally more frequent than NIV. In the European Union, within an ad hoc Scientific Cooperation (SCOOP) project aimed to evaluate the risk of exposure to Fusarium toxins by the population of EU members states, it was shown that 57 % of 11,022 cereal samples, including wheat, maize, barley, oats, and rye, were contaminated with DON and 16 % of 4,166 samples were contaminated with NIV. Similar contamination percentages were found for wheat with levels of contamination higher for DON (up to 50,000 μg/kg, mean level of 205 μg/kg) than NIV (up to 440 μg/kg, mean level of 24 μg/kg) (SCOOP 2003; Turner 2010). Cases in which NIV levels were higher than DON have been observed in Japanese wheat (Tanaka et al. 1988). The Joint FAO/WHO Expert Committee on Food Additives (JECFA) carried out a more global assessment of DON, but not of NIV, confirming that DON was a common contaminant worldwide of cereals and their derivative products (Bulder et al. 2011).
Toxicologic studies on animals showed that both these mycotoxins inhibit DNA, RNA, and protein synthesis and cause neurotoxic and immunotoxic effects in mammals. Acute exposure to DON and NIV induces emesis in pigs, whereas chronic exposure can cause growth retardation and immunotoxicity (Pestka 2010). Potential human health concerns of DON have been reviewed by Pestka and Smolinski (2005). In particular, data from Chinese epidemiological studies strongly suggest that DON causes gastrointestinal symptoms and vomiting in humans; however, most of wheat and barley samples from families reporting disorders were also contaminated by NIV (Li et al. 1999, 2002). In order to protect human and animal health, at least 37 countries have established regulatory limits or guidance levels for DON in foods and feeds (van Egmond and Jonker 2004), whereas no guidelines or regulatory limits have been set for NIV, although this mycotoxin frequently contaminates cereals and commonly co-occurs with DON (Placinta et al. 1999; Tanaka et al. 1988; Turner 2010). However, based on risk assessment studies, the Scientific Committee on Food of the European Commission has established a temporary tolerable daily intake (t-TDI) of 0.7 μg/kg b.w. per day for NIV and a TDI of 1 μg/kg b.w. per day for DON (SCF 2002). Therefore, the development of sensitive, rapid, and reliable methods for determining simultaneously DON and NIV in cereals, in particular wheat, is a high priority in order to properly assess and prevent the possible risk of exposure to both mycotoxins for humans and animals.
Different methods have been proposed for the determination of DON and NIV in cereals (Lattanzio et al. 2009; Meneely et al. 2011). Screening methods, including immunochemical assays such as enzyme-linked immunosorbent assays (ELISAs), fluorescence polarization immunoassays (FPIAs), lateral flow devices (LFDs), or surface plasmon resonance (SPR)-based biosensor assays have been developed for the determination of DON alone (Lattanzio et al. 2009; Meneely et al. 2011), whereas no immunoassay specific for NIV has been yet developed. Recently, Maragos et al. developed a monoclonal antibody that cross-reacts with both NIV and DON (Maragos et al. 2006) that was used for developing a SPR immunoassay for rapid detection of the sum of DON and NIV in wheat (Kadota et al. 2010).
With respect to traditional methods, gas-chromatographic (GC) methods based on electron-capture detection (ECD) and mass spectrometric (MS) detection after multifunctional MycoSep column cleanup have been widely used for quantitative and simultaneous determination of both type B (including DON and NIV) and type A trichothecenes after derivatization with specific labeling reagents. However, these methods have been shown to suffer from poor accuracy and precision of the measurements. The main problem derived from matrix interferences that induced enhancement of the trichothecene response (Krska et al. 2001; Petterson and Langseth 2002). High-performance liquid chromatographic methods (HPLC) with UV or diode array detection are commonly used for the determination of DON in cereals and derivative products. Moreover, HPLC methods based on immunoaffinity column (IAC) cleanup and UV detection have been validated by interlaboratory studies (MacDonald et al. 2005; Neumann et al. 2009) and adopted as European standards for the determination of DON in cereals (grain and flour), cereal products, and cereal-based foods for infants and young children (CEN 2010). HPLC/UV methods for the simultaneous determination of DON and NIV in cereals and food products using MycoSep column cleanup are unreliable due to low sensitivity and matrix interferences. Recently, a new multifunctional cleanup column (Autoprep MF-T 1500, Showa Denko K.K., Japan) has been used in a Japanese interlaboratory study for the simultaneous determination of DON and NIV in wheat. Although the study showed good results in terms of recoveries and precision, the worldwide provision of these multifunctional columns may prove problematic (Aoyama et al. 2012). Since HPLC with fluorescence detection (FLD) allow both high sensitivity and good selectivity, coumarin-3-carbonyl chloride has been proposed as derivatizing reagent for the analysis of type A and type B trichothecenes. The limit of this method was the low conversion rate for NIV due to the presence of a high number of hydroxylated groups that gave rise to incomplete and unreproducible derivatization (Dall’Asta et al. 2004; Mateo et al. 2001). Recently, a HPLC/FLD method with on-line chemical post-column derivatization (reaction with sodium hydroxide, methyl acetoacetate, and ammonium acetate) has been developed for the simultaneous determination of DON and NIV in cereals after solid phase extraction (SPE) cleanup. The method ensured high sensitivity and reproducibility of results (Muscarella et al. 2012). To date, LC-MS/MS is the most widely used method for multi-mycotoxin determination, including type B trichothecenes, due to its high sensitivity and due to the fact that extracts do not require derivatization. However, LC-MS equipments are very expensive and require skilled personnel for their use (Kadota et al. 2011; Lattanzio et al. 2008; Plattner and Maragos 2003; Toth et al. 2011).
The demand of high samples throughput in a short time has recently given rise to fast liquid chromatography that uses innovative instrumentation and column technology (particle size <2 μm). This new technology allows to speed up the chromatographic run compared to conventional liquid chromatography, while improving chromatographic efficiency and resolution. Several applications have been reported either using UV or fluorescence detection, although most of the applications in food analysis uses mass spectrometric detection in order to guarantee confirmation of the target analyte (Núñez et al. 2012).
The aim of this work was to develop a sensitive, accurate, and reliable method for the simultaneous determination of DON and NIV in unprocessed wheat using a new commercial immunoaffinity column containing a monoclonal antibody specific for DON and NIV for extract cleanup and ultra-performance liquid chromatography (UPLC®) with photodiode array (PDA) for toxin detection. Performances and validation of the UPLC®-PDA method are discussed.
Materials and Methods
Chemicals and Materials
Acetonitrile and methanol (HPLC grade) were purchased from Carlo Erba Reagents (Milan, Italy). Ultrapure water was produced by a Milli-Q system (Millipore, Bedford, MA, USA). Deoxynivalenol (lot no. 040M4062, purity ≥98 %) and nivalenol (lot no. 073K4054, purity ≥98 %) analytical standard grade were purchased from Sigma-Aldrich (Milan, Italy). DON-NIV™ WB immunoaffinity columns were provided from VICAM, A Waters Business (Milford, MA, USA); glass microfibre filters (Whatman GF/A), and paper filters (Whatman No. 4) from Whatman (Maidstone, UK). Wheat flour reference material (European Reference Material ERM®-BC600) was purchased from BAM Federal Institute for Materials Research and Testing (Berlin, Germany).
Wheat sample used for protocol optimization was obtained by thoroughly mixing a durum wheat sample naturally contaminated with DON at levels of 4,000 μg/kg with a durum wheat sample inoculated with a toxigenic strain of F. poae (ITEM 9211 from the ISPA-CNR culture collection, www.ispa.cnr.it/Collection) and incubated for 10 days at 25 °C. The strain was previously demonstrated to produce NIV in vitro when grown on autoclaved wheat (Somma et al. 2010).
Naturally contaminated samples of durum wheat from Northern Italy were provided by the Agricultural Research Council—Research Unit for Cereal Quality (CRA-QCE), Rome, Italy.
Preparation of Standard Solutions
Individual DON and NIV stock solutions at the concentration of approximately 25 μg/mL were prepared by dissolving DON and NIV solid commercial toxins in acetonitrile (HPLC grade). The exact concentrations of the stock solutions were spectrophotometrically determined by using molar absorption coefficients of 6,805 and 6,955 L/mol cm for DON and NIV, respectively (Krska et al. 2007).
Mixed DON and NIV standard solutions (100 μg/mL each) for spiking purposes were prepared by mixing and diluting adequate amounts of the stock solutions with acetonitrile. Standard solutions for UPLC® calibration curve (range 0.1 to 5 μg/mL) were prepared by re-dissolving aliquots of the 100 μg/mL solution in acetonitrile, previously evaporated to dryness under nitrogen stream, with water/methanol (85:15, v/v).
Apparatus
The apparatus consisted of a Waters Acquity UPLC® system (Milford, MA, USA) equipped with a binary solvent manager, a sample manager, a column heater, and a photodiode-array (PDA) detector. The chromatographic separation was performed isocratically on an Acquity UPLC® BEH C18 column (2.1 × 100 mm, 1.7 μm) preceded by an Acquity UPLC® in-line filter (0.2 μm) with a mobile phase of water/methanol (85:15, v/v) at a flow rate of 0.4 mL/min. After toxin elution, methanol was increased to 80 % in 0.5 min and kept constant for 2.0 min to clean the column, then returned to the initial conditions in 0.5 min. The column was equilibrated for 2 min prior to the next sample injection.
The column was kept at a temperature of 35 °C; the detector was set at 220-nm wavelength (sampling rate of 10 Hz). Data acquisition and instrument control were performed by the Empower™ 2 Software (Waters).
Sample Extraction and Cleanup
Twenty-five grams of wheat finely ground (particle size ≤1.0 mm) by a Cyclone sample mill (PBI International, Milan, Italy) after addition of 100 mL of water were extracted by shaking at 250 rpm for 60 min (KS 4000i, IKA Werke GmbH & Co. KG., Staufen, Germany). The extract was filtered twice through filter paper (Whatman no. 4) and glass microfiber filter (Whatman GF/A). Two milliliters of the filtered extract (equivalent to 0.5 g sample) were passed through the immunoaffinity column at a flow rate of about 1 drop/s; subsequently, the column was washed with 10 mL of distilled water (2 × 5 mL) at a flow rate of 1–2 drops/s. DON and NIV were eluted from the column with methanol (2 × 0.75 mL) at a flow rate of 1 drop/s. Cleaned up extract was collected in a 4-mL screw cap vial and dried under air stream at 50 °C in a heating block. The dried residue was reconstituted with 250 μL of water/methanol (85:15, v/v) and 10 μL were injected into the UPLC® apparatus by full loop injection system.
The following procedure was followed when methanol/water was used as extraction solvent. Twenty-five grams of ground wheat were extracted with 100 mL methanol/water (80:20, v/v) by shaking at 250 rpm for 60 min. The mixture extract was filtered through filter paper (Whatman no. 4), then 10 ml of filtrate were collected and mixed with 40 mL of distilled water. The diluted extract was filtered through a glass microfibre filter (Whatman GF/A) and the filtrate collected. Ten milliliters of filtrate (equivalent to 0.5 g sample) were passed through the immunoaffinity column at a flow rate of about 1 drop/s. Washing step, toxin elution, and UPLC® analysis were carried out as reported above.
Immunoaffinity Column Capacity
The capacity of the immunoaffinity column was determined for both DON and NIV by comparing the amount of toxin added to the immunoaffinity column with the respective bound amount. Different amounts of DON and NIV, from 500 to 4,000 ng, were added as single mycotoxin or sum (mass ratio of 50:50, w/w) by loading onto the immunoaffinity column 2 mL of extract of blank wheat spiked with the corresponding amount of DON and NIV.
In-House Method Validation
Recovery experiments were performed in quadruplicate by spiking blank wheat samples with DON and NIV at levels of 100, 500, 1,000, 1,750, and 2,000 μg/kg of each toxin. Spiked samples were left 1 h at room temperature to allow solvent evaporation prior to extraction with water.
The trueness of the method was determined by analyzing a wheat flour certified reference material (ERM®-BC600) containing 102 μg/kg of DON (uncertainty 11 μg/kg) and 1,000 μg/kg of NIV (uncertainty 130 μg/kg) on five different days.
Statistical Analysis
Toxin concentrations of a naturally contaminated sample determined by using different extraction solvents and modes were processed by one-way analysis of variance (ANOVA) at P = 0.001 to indicate statistically significant differences between means (Student-Newman-Keuls test). The Sigma Plot® 11 statistical software (Systat Software Inc, London, UK) was used.
The comparison of the measured DON and NIV values of the certified reference material with the certified values was performed according to the procedure described in “Comparison of a measurement result with the certified value”, ERM Application Note 1, January 2010 (http://www.erm-crm.org). The procedure is described here in brief: (1) calculate the absolute difference between mean measured value and the certified value (Δ m); (2) combine measurement uncertainty (u meas) with the uncertainty of the certified value (u CRM): u Δ = (u 2 meas + u 2 CRM)1/2; (3) calculate the expanded uncertainty (U Δ ) from the combined uncertainty (u Δ ) using a coverage factor of two (k = 2), corresponding to a confidence interval of approximately 95 %. If Δ m ≤ U Δ then there is no significant difference between the measurement result and the certified value, at a confidence level of about 95 %.
Results and Discussion
Optimization of the Method
Significant improvements in terms of sensitivity, resolution, and speed can be achieved by ultra-high-performance liquid chromatographic systems with respect to traditional HPLC. The availability of new IACs containing an antibody specific for DON and NIV led us to investigate the use of an UPLC®-PDA system and IAC cleanup of extracts for developing a rapid and sensitive method for the simultaneous determination of DON and NIV at levels naturally occurring in wheat.
Since the presence of low levels of acetonitrile in the extraction solvent can cause denaturation of the IAC antibody, water, and the mixture methanol-water (80:20, v/v) were chosen as extraction solvents. Preliminary recovery experiments (three replicates) at 1,000 μg/kg spiking level of each toxin showed no significant difference at P < 0.001 (SNK test) between mean recoveries obtained with water (DON 93.9 %, relative standard deviation (RSD) 2.9 %; NIV 89.0 %, CV 3.6 %) and methanol–water (DON 92.0 %, RSD 2.1 %; NIV 87.6 %, CV 2.3 %) as extraction solvent. On the contrary, the comparison between different extraction solvents and modes by using a wheat sample obtained by thoroughly mixing a durum wheat sample naturally contaminated by DON with a durum wheat sample inoculated with a toxigenic strain of F. poae showed that water extraction provided a significant increase in DON and NIV concentrations (P < 0.001, mean values of 6 replicate analyses) with respect to methanol/water (80:20, v/v). No significant difference was observed between shaking (1 h) and blending (3 min) when water was used (Table 1). A similar result was obtained by analyzing the certified reference material ERM®-BC600 (certified values: 102 ± 11 μg/kg for DON, 1,000 ± 130 μg/kg for NIV). Extraction with water provided a significant increase of DON and NIV values (DON = 110 ± 3 μg/kg, NIV = 1,082 ± 38 μg/kg, n = 6) with respect to the mixture methanol/water (DON = 49 ± 9 μg/kg, NIV = 605 ± 17 μg/kg, n = 6). The use of water as the best extraction solvent in terms of DON recoveries was recently reported also by Muscarella et al. (2012); however, authors carried out their experiments only with spiked samples. Our experiments, carried out with spiked samples, naturally contaminated samples and wheat reference material, clearly show that the use of naturally contaminated samples and/or certified reference materials is essential to demonstrate the effectiveness of the extraction solvent and the accuracy of a new method. Recovery experiments based only on spiked samples could lead to wrong conclusions.
In the optimized conditions, limits of detection of the method, based on a signal-to-noise ratio of 3, were 30 and 20 μg/kg for DON and NIV, respectively. Although limits of detection (LODs) of the optimized method are two times lower than those reported by Muscarella et al. (2012) (i.e., 14 μg/kg for DON and 11 μg/kg for NIV), the limit of quantification (LOQ) of the proposed method for DON (i.e., 100 μg/kg) fulfill the performance criteria established by the European Committee for Standardization (CEN) for the acceptance of the quantification limit for single-laboratory-validated method for the determination of mycotoxins (CEN 2010), i.e., for levels higher or equal to 100 μg/kg LOQ should be equal or less than 1/5 × maximum legal limit (that are 1,750 μg/kg for unprocessed durum wheat or 1,250 μg/kg for unprocessed cereals other than durum wheat). A similar comment is not possible for NIV because no legal limits have been fixed by the European Commission.
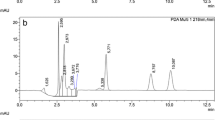
Typical UPLC®-PDA chromatograms of a blank wheat sample, and wheat samples artificially or naturally contaminated with DON and NIV are shown in Fig. 1.
UPLC®-PDA chromatograms of wheat samples: a blank sample (<20 μg/kg NIV and <30 μg/kg DON), b sample spiked with 1,750 μg/kg of NIV and 1,750 μg/kg of DON, c certified reference material ERM®-BC600 (NIV found 1,124 μg/kg; DON found 98 μg/kg). Chromatographic conditions are reported in “Apparatus” section
In-House Method Validation and Application to Naturally Contaminated Wheat Samples
The DON-NIV™ WB immunoaffinity columns showed saturation of DON/NIV binding sites at levels higher than 2,000 ng in blank wheat extracts spiked with the corresponding amount of mycotoxins, as single mycotoxin or sum of DON and NIV (Fig. 2). The range of applicability of the method was from 30 μg/kg (DON) and 20 μg/kg (NIV) to 4,000 μg/kg, as single mycotoxin or sum of DON and NIV in wheat. The method resulted linear over the toxin applicability range. Considering the column capacity, a dilution of the extract before loading on the immunoaffinity column or a lower volume of extract should be loaded on the immunoaffinity columns when the sum of DON and NIV content in wheat exceeds 4,000 μg/kg. These levels of contamination are quite unusual in naturally contaminated samples.
Results of recovery experiments of the full analytical procedure carried out with blank wheat samples spiked with DON and NIV at different levels are reported in Table 2. Within the spiking range 100–2,000 μg/kg (each toxin), mean recoveries ranged from 85 to 95 % for DON, with RSDs less than 7.0 % and from 81 to 88 % for NIV, with RSDs less than 6.7 %. Recovery and repeatability values fulfill the performance criteria established by the European Union and the European Committee for Standardization (CEN) for the acceptance of an analytical method for DON (Commission of the European Communities 2006), and NIV (CEN 1999) for the official control of mycotoxin levels in foodstuffs.
Trueness of results obtained with the UPLC®-PDA method was shown by analyzing a certified reference material (ERM®-BC600) in five consecutive days. Results obtained with the UPLC® method were always within the certified values ± uncertainty (certified value 102 μg/kg, uncertainty 11 μg/kg for DON; certified value 1,000 μg/kg, uncertainty 130 μg/kg for NIV). In particular, mean values obtained (after correction for recovery) of 99 μg/kg (RSD 5.0 %, n = 5) for DON and 1,127 μg/kg (RSD 3.1 %, n = 5) for NIV, showed no significant difference from the certified values for the two mycotoxins at a confidence level of about 95 %, demonstrating the good accuracy and precision of the developed UPLC®-PDA method based on immunoaffinity column cleanup.
The method was applied to the analysis of 20 samples of durum wheat originated from Northern Italy, 2012 crop. Results revealed the occurrence of DON in all tested samples at levels up to 2,698 μg/kg (mean value of 560 μg/kg), whereas NIV was detected only in two samples at levels up to 46 μg/kg. The high incidence of DON contamination of durum wheat is not surprising in Northern Italy, as similar results depending on the growing season have been reported in previous surveys (Pascale et al. 2002). On the contrary, the low levels and frequency of contamination of NIV in wheat need further investigation due to the poor availability of occurrence data in Italy.
Conclusions
The use of naturally contaminated materials or certified reference materials, if available, is highly recommended in development and validation of a new method in order to ensure accuracy and reliability of measurement results.
The use of immunoaffinity columns containing antibody specific for DON and NIV has allowed the development of a sensitive and reliable method for the simultaneous determination of these mycotoxins at levels that can occur in wheat. In addition, the use of an UPLC® apparatus allowed rapid chromatographic runs leading to a higher sample throughput and reducing at the same time the consumption of hazardous solvents.
Performance parameters (LOD, accuracy, precision) of the method are comparable to those of other published methods for the simultaneous determination of DON and NIV (Kadota et al. 2011; Lattanzio et al. 2008; Muscarella et al. 2012; Plattner and Maragos 2003; Toth et al. 2011). The proposed method appears to be a good alternative to more expensive LC-MS(MS) methods or to the HPLC/FLD method that use chemical post-column derivatization for generating reliable data on the co-occurrence of these toxic trichothecenes in wheat that can be of concern for animal and human health.
References
Aoyama K, Akashi H, Mochizuki N, Ito Y, Miyashita T, Lee S, Ogiso M, Maeda M, Kai S, Tanaka H, Noriduki H, Hiraoka H, Tanaka T, Ishikuro E, Itoh Y, Nagayama T, Nakajima M, Naito S, Sugita-Konishi Y (2012) Inter laboratory study of LC-UV and LC-MS methods for the simultaneous determination of deoxynivalenol and nivalenol in wheat. Food Hyg Saf Sci 53:152–156
Bulder AS, DiNovi M, Kpodo KA, Leblanc J-C, Resnik S, Shephard GS, Slob W, Walker R, Wolterink G (2011) Deoxynivalenol, In: Safety evaluation of certain contaminants in food. WHO Food Additives Series: 63, FAO JECFA Monographs 8. WHO Press, Geneva, pp. 317–485
CEN (1999) European Committee for Standardization, Food analysis—biotoxins—criteria for analytical methods of mycotoxins (CEN Report 13505:1999)
CEN (2010) European Committee for Standardization, Foodstuffs—determination of deoxynivalenol in cereals, cereal products and cereal based foods for infants and young children: HPLC method with immunoaffinity column cleanup and UV detection (EN 15891:2010)
Commission of the European Communities (2006) Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off J Eur Union L70:12–34
Dall’Asta C, Galaverna G, Biancardi A, Gasparini M, Sforza S, Dossena A, Marchelli R (2004) Simultaneous liquid chromatography–fluorescence analysis of type A and type B trichothecenes as fluorescent derivatives via reaction with coumarin-3-carbonyl chloride. J Chromatogr A 1047:241–247
Kadota T, Takezawa Y, Hirano S, Tajima O, Maragos CM, Nakajima T, Tanaka T, Kamata Y, Sugita-Konishi Y (2010) Rapid detection of nivalenol and deoxynivalenol in wheat using surface plasmon resonance immunoassay. Anal Chim Acta 673:173–178
Kadota T, Kimura M, Hirano S, Tajima O, Nakajima T, Kamata Y, Sugita-Konishi Y (2011) Development of a simultaneous liquid chromatography/tandem mass spectrometric method for the determination of type B trichothecenes, their derivatives, and precursors in wheat. Rapid Commun Mass Spectrom 25:3481–3490
Krska R, Baumgartner S, Joseph R (2001) The state-of-the-art in the analysis of type-A and -B trichothecene mycotoxins in cereals. Fresenius’ J Anal Chem 371:285–299
Krska R, Schubert-Ullrich P, Josephs RD, Emteborg H, Buttinger G, Petterson H, van Egmond HP, Schothorst RC, MacDonald S, Chan D (2007) Determination of molar absorptivity coefficients for major type-B trichothecenes and certification of calibrators for deoxynivalenol and nivalenol. Anal Bioanal Chem 388:1215–1226
Lattanzio VMT, Solfrizzo M, Visconti A (2008) Determination of trichothecenes in cereals and cereal-based products by liquid chromatography-tandem mass spectrometry. Food Addit Contam Part A 25:320–330
Lattanzio VMT, Pascale M, Visconti A (2009) Current analytical methods for trichothecenes mycotoxins in cereals. TRAC-Trends Anal Chem 28:758–768
Li FQ, Luo XY, Yoshizawa T (1999) Mycotoxins (trichothecenes, zearalenone and fumonisins) in cereals associated with human red-mold intoxications stored since 1989 and 1991 in China. Nat Toxins 7:93–97
Li FQ, Li YW, Luo XY, Yoshizawa T (2002) Fusarium toxins in wheat from an area in Henan province, PR China, with a previous human red mould intoxication episode. Food Addit Contam 19:163–167
MacDonald SJ, Chan D, Brereton P, Damant A, Wood R (2005) Determination of deoxynivalenol in cereals and cereal products by immunoaffinity column cleanup with liquid chromatography: interlaboratory study. J AOAC Int 88:1197–1204
Maragos C, Busman M, Sugita-Konishi Y (2006) Production and characterization of a monoclonal antibody that cross-reacts with the mycotoxins nivalenol and 4-deoxynivalenol. Food Addit Contam 23:816–825
Mateo JJ, Llorens A, Mateo R, Jiménez M (2001) Critical study of and improvements in chromatographic methods for the analysis of type B trichothecenes. J Chromatogr A 918:99–112
Meneely JP, Ricci F, van Egmond HP, Elliott CT (2011) Current methods of analysis for the determination of trichothecene mycotoxins in food. TRAC-Trends Anal Chem 30:192–203
Muscarella M, Iammarino M, Nardiello D, Palermo C, Centonze D (2012) Determination of deoxynivalenol and nivalenol by liquid chromatography and fluorometric detection with on-line chemical post-column derivatization. Talanta 97:145–149
Neumann G, Lombaert GA, Kotello S, Fedorowich N (2009) Determination of deoxynivalenol in soft wheat by immunoaffinity column cleanup and LC-UV detection: interlaboratory study. J AOAC Int 92:181–189
Núñez O, Gallart-Ayala H, Martins CPB, Lucci P (2012) New trends in fast liquid chromatography for food and environmental analysis. J Chromatogr A 1228:298–323
Pascale M, Bottalico A, Pancaldi D, Perrrone G, Visconti A (2002) Occurrence of deoxynivalenol in cereals from experimental fields in different Italian regions. Petria 12:123–129
Pestka JJ (2010) Toxicological mechanism and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J 3:323–347
Pestka JJ, Smolinski AT (2005) Deoxynivalenol: toxicology and potential effects on humans. J Toxicol Environ Health Part B 8:39–69
Petterson H, Langseth W (2002) Intercomparison of trichothecenes analysis and feasibility to produce certified calibrants. European Commission BCR Information Project, EU Reports EUR 20285/1 EN (82 pp) and EUR 20285/2 EN (145 pp)
Placinta CM, D’Mello JPF, MacDonald AMC (1999) A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim Feed Sci Technol 78:21–37
Plattner RD, Maragos CM (2003) Determination of deoxynivalenol and nivalenol in corn and wheat by liquid chromatography with electrospray mass spectrometry. J AOAC Int 86:61–65
SCF (2002) Scientific Committee on Food—Opinion of the Scientific Committee on Food on Fusarium toxins. Part 6: group evaluation of T-2 toxin, HT-2 toxin, nivalenol and deoxynivalenol (SCF/CS/CNTM/MYC/27 Final). http://ec.europa.eu/food/fs/sc/scf/out123_en.pdf. Accessed 10 May 2013
SCOOP (2003) Collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states. SCOOP Task 3.2.10 Final report. http://ec.europa.eu/food/fs/scoop/task3210.pdf. Accessed 10 May 2013
Somma S, Alvarez C, Ricci V, Ferracane L, Ritieni A, Logrieco A, Moretti A (2010) Trichothecene and beauvericin mycotoxin production and genetic variability in Fusarium poae isolated from wheat kernels from northern Italy. Food Addit Contam Part A 27:729–737
Tanaka T, Hasegawa A, Yamamoto S, Lee U-S, Sugiura Y, Ueno Y (1988) Worldwide contamination of cereals by the Fusarium mycotoxins, nivalenol, deoxynivalenol and zearalenone. I. A survey on 19 countries. J Agric Food Chem 36:979–983
Toth SB, Jolankai R, Muranyi Z, Dallos A (2011) Analysis of deoxynivalenol, nivalenol, zearalenone in food by LC-APCI-MS. Chromatographia 73:S171–S174
Turner PC (2010) Deoxynivalenol and nivalenol occurrence and exposure assessment. World Mycotoxin J 3:315–321
van Egmond HP, Jonker MA (2004) Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition Paper 81. Food and Agriculture Organization, Rome. ISBN 92-5-105162-3
Acknowledgments
This work was carried out with the financial support of the Italian Ministry of Agricultural, Food and Forestry Policies, MiPAAF (project “MICOPRINCEM”).
Conflict of Interest
Michelangelo Pascale declares that he has no conflict of interest. Giuseppe Panzarini declares that he has no interest. Stephen Powers declares that he has no conflict of interest. Angelo Visconti declares has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pascale, M., Panzarini, G., Powers, S. et al. Determination of Deoxynivalenol and Nivalenol in Wheat by Ultra-Performance Liquid Chromatography/Photodiode-Array Detector and Immunoaffinity Column Cleanup. Food Anal. Methods 7, 555–562 (2014). https://doi.org/10.1007/s12161-013-9653-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9653-1