Abstract
Mycotoxins are secondary metabolites produced by fungi, such as Fusarium, Penicillium, and Aspergillus, which are toxic to humans with high risk factors and pose a significant threat to human health. This study was focused mostly on well-known mycotoxins, such as aflatoxins (AFB1, AFB2, AFG1 and AFG2), fumonisin (FB1, FB2), deoxynivalenol (DON), zearalenone (ZON), ochratoxin A, T-2 and HT-2, in grains. The multi-mycotoxin methods developed in this study utilise an analysis of mycotoxin through liquid chromatography tandem mass spectrometry (LC-MS/MS), which can significantly improve sample analysis efficiency. The Myco6in1™ immunoaffinity column was used for purification to reduce interference from the substrate. Gradient separation to obtain the best peak shift was conducted using solvent with 0.1 % formic acid in deionised water and methanol, and gradient separation was performed on an ACQUITY BEH C18 column chromatograph. The recovery rate test for each toxin using substrates such as rice, peanut, wheat and maize mostly indicated good average recovery rates between 70 % and 120 % and the coefficient of variation mostly under 15 %. The limits of quantification (LOQ) identified by this method are less than 5 ng/g in most toxins, except for 20 ng/g in FB1and FB2. This method can rapidly and simultaneously analyse 11 mycotoxins in 9 min. It can be applied for the practical examination of mycotoxins in food to protect public health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
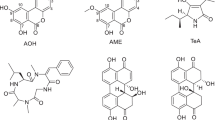
Mycotoxins are the secondary metabolism product from fungi that primarily contaminate grains. Foods harvested with improper processes or inadequate temperature controls during storage are prone to mycotoxin contamination. Mycotoxin-contaminated food consumption can cause liver toxicity, kidney toxicity, and abnormal foetus development and cancer, requiring the need to take proper precautions (Miller 1997; Huang 2010). Aflatoxin (AF) belongs to a group of secondary metabolism products with similar structure, high toxicity and stability and is carcinogenic. In foods, these toxins have been identified as aflatoxin B1(AFB1), B2(AFB2), G1(AFG1), G2 (AFG2), M1(AFM1) and M2(AFM2); AFM1 and AFM2 are metabolites of AFB1 and AFB2, respectively. The most common aflatoxin is AFB1, which also has the highest toxicity (Hesseltine 1967) (the chemical structure is depicted in Fig. 1). The LD50 of AFB1 for animals fed contaminated grain is between 0.5 and 10 mg/kg b.w. (Maurice 2002). Ochratoxin is a natural mycotoxin, which has four different strains (A, B, C and D), among which Ochratoxin A (OTA) has the highest toxicity. OTA is the secondary metabolite from Aspergillus and Penicillium (Bayman and Baker 2006; Pena et al. 2006; Leong et al. 2006). Zinedine et al. (2007) recently collected cases that contaminated zearalenone (ZON) in food and fodder in dozens of countries from around the world including Europe, Africa, South America, North America, Asia and the Atlantic Ocean. Maize and wheat are the foods primarily contaminated by ZON. Some contamination was found in barley, oats, rice and dehydrated vegetables and fruits. The level of ZON contamination is quite broad (some are less than 10 ppb), and contamination can reach as high as several dozen ppm (Zinedine et al. 2007). Fumonisin is primarily derived from Fusarium verticillioides and F. proliferatum. It can be categorised as Fumonisin B1 (FB1), B2 (FB2) and B3 (FB3), based on different carbon positions in the functional base. FB1 is mostly found in maize and maize-based raw materials for fodder and food for humans (Dall’Asta et al. 2008). Deoxynivalenol (DON) is one of the most widely distributed trichothecene compounds. DON is the most hazardous grain toxin in the world, involved not only in the contamination of raw agricultural products, but also in processed grain products. When humans or animals consume DON-contaminated foods, their health will be seriously endangered. Fusarium graminearum and F. culmorum are the primary strains that produce the DON toxin (Birzele et al. 2000; Homdark et al. 2000). T-2 is the secondary metabolite from several Fusarium spp. that belong to the family of trichothecenes. It can be categorised into four classes (A, B, C and D) based on a different functional base, where T-2 and HT-2 belongs to class A. HT-2 is the primary degradation of T-2 and commonly coexists on contaminated grains (European Commission 2001).
Previous research has successfully established several mycotoxin examination methods for aflatoxins, ochratoxin A, and fumonisin. Most of the analytical methods used in mycological studies such as TLC (Freire et al. 2000), HPLC-DAD (Larsen et al. 2002; Anderson and Frisvad 2004) had been chosen for the determination single-target analysis. However, current trends for determination of mycotoxin in food are focused on the simultaneous detection of multiple mycotoxins via liquid chromatography tandem mass spectrometry (LC-MS/MS). Compared to a traditional HPLC method, this approach is faster, more user-friendly, consumes less solvent, and can analyse multi-mycotoxin simultaneously. It had been demonstrated to be a powerful analytical tool for the determination multi-mycotoxin contaminations in cereal. Several LC-MS/MS multi-mycotoxin methods have been developed for different groups of mycotoxins in food, while the potential of MS/MS to enable low detection limits had been developed for complex matrices (Lattanzio et al. 2007; Stecher et al. 2007; Antonello et al. 2009; Michel et al. 2010; Lattanzio et al. 2011). Recently, the Orbitrap™ mass analyser had been introduced (Nakagawa et al. 2011) to obtain more information on product fragment.
However, it can often be challenging to develop a multi-mycotoxin method because of two issues: choosing an extraction solvent and avoiding a matrix effect from complex matrices’ compositions. Methods that are able to detect multi-mycotoxin are less costly and time-consuming because they can detect several drugs in a single run.
The objective of this study is to develop simultaneous multi-methods to detect 11 mycotoxins, including aflatoxins, ochratoxin A, zearalenone, fumonisin, deoxynivalenol, T-2 and HT-2, to significantly increase the efficiency of detection for mycotoxin cases. In addition, this approach was used to investigate the contamination of grains such as rice, maize, peanut, and wheat and can serve as a reference for developing regulatory standards by the Taiwanese government.
Experimental
Samples
Rice, peanut, wheat and maize were purchased from wholesale clubs and supermarkets from areas around Taipei.
Chemicals and Reagents
AFB1, AFB2, AFG1, AFG2, FB1, FB2, DON, ZON, OTA, T-2 and HT-2 mycotoxin reference materials were purchased from Fermentek Ltd. (Jerusalem, Israel) in 1-mg packages. The Myco6in1™ immunoaffinity column (IAC) was purchased from Vicam (Watertown, MA, USA). HPLC-grade acetonitrile, methanol and formic acid (purity>99 %) were purchased from Merck Ltd. (Darmstadt, Germany). Ultrapure water was obtained from a Milli-Q Gradient water system (Millipore, Bedford, MA, USA). Potassium chloride, potassium phosphate, disodium hydrogen phosphate, and sodium chloride were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nylon syringe filtration (0.22 μm) materials were purchased from Micro Separations Inc. (Massachusetts, USA).
Phosphate-buffered saline (PBS, pH 7.4) consisted of 0.2 g potassium chloride, 0.2 g potassium phosphate, 2.92 g disodium hydrogen phosphate and 8 g sodium chloride in 900 ml deionised water. The pH was adjusted to 7.4 using 0.1 M HCl or 0.1 M NaOH, and the extraction solution was adjusted to 1,000 ml. The analytes were separated with a mobile phase consisting of 0.1 % formic acid in water (solution A) and methanol (solution B) at a flow rate of 0.3 ml/min. Both were filtered through a 0.22-μm membrane and ultrasonically degassed prior to application.
Equipment
-
(a)
Ultra performance liquid chromatography/mass spectrometer — Chromatographic analyses were performed using an ACQUITY UPLC system (Waters Corp., Milford, MA) coupled to a Waters Zevo quadrupole tandem mass spectrometer (Waters, Manchester, UK).
-
(b)
UPLC column — ACQUITY BEH C18 (2.1 × 100 mm, 1.7 μm particle size) (Waters Corp.).
-
(c)
Centrifuges — Allegra 25R centrifuge (Beckman Coulter Inc., CA).
-
(d)
Other laboratory equipment — Vortex mixer (Vortex-Genie 2, Scientific Industries, Bohemia, NY, USA), Oscillator Recipro shaker (Taitec Co., Ltd., Japan), adjustable pipettors (Gilson Pipetman, Middleton, WI), and nitrogen evaporator with a thermostated water bath (N-EVAP; Organomation Associates, Inc., Massachusetts) or equivalent.
Stock and Working Standard Preparation
The standard solutions were dissolved in 100 % acetonitrile, except for fumonisin, which was dissolved in 50 % aqueous acetonitrile for stock standards. All standard solutions were diluted to 10 ml with a concentration of 100 ppm and stored at −18 °C. A working solution was prepared by diluting the stock standards with 20 % aqueous acetonitrile. When making standard curves (with the exception of fumonisin), all 100 ppm standard toxins were diluted five times with deionised water to make 20 ppm 20 % ACN solutions, while the fumonisin samples were diluted 2.5 times into 40 ppm 20 % ACN solutions. In all, 250 μl of the 40 ppm fumonisin standard and 500 μl of the 20 ppm other toxin standards were combined with the 20 % ACN solution to create 10-ml volumes at 1 ppm mixed toxin standards.
Sample Preparation
Extraction
A 5-g amount of homogenised grain was weighed into a polypropylene centrifuge tube. Samples were added to 25 ml PBS buffer, and the mixture was shaken 60 min and then centrifuged 10 min at 3,000 × g. A 17.5-ml portion of PBS-extracted supernatant was then filtered with glass fibre filter paper (Whatman GF/A) as extraction solution A for purification. Next, 17.5 ml methanol was added into the remaining PBS-extracted supernatant, followed by shaking for 60 min, centrifugation for 10 min at 3,000 × g, and filtering with filter paper. Ten millilitres of this extracted solution was added to 90 ml PBS buffer to fix the volume at 100 ml. The extracts were filtered through filter paper as extraction solution B for the following clean-up procedure.
Purification
Fifty millilitres of extraction B was gradually injected into an immunoaffinity column (1–2 drops/s) and rinsed with 20 ml PBS until no methanol residue remained; the effluent was discarded. Five millilitres of extraction A was placed into the column (1–2 drops/s) and rinsed with 10 ml of deionised water; the effluent was discarded. The column was then washed with 3 ml of 80 % methanol with 0.5 % acetic acid (1–2 drops/s), and the effluent was collected. After 5 min, the column was washed with 3 ml of methanol (1–2 drops/s), and the combined effluents were added together. The effluents were than air-dried with nitrogen gas at 40 °C, and the residue was dissolved with 20 % aqueous acetonitrile to 1 ml and passed through the filter membrane. The effluent was collected for examination, as presented in Fig. 1.
Chromatographic and MS/MS Conditions
LC-MS/MS was conducted using an ACQUITY BEH C18 UPLC column (1.7 μm, 2.1 × 100 mm) at 30 °C and an injection volume of 10 μl. The gradient profile began with 95 % to 15 % solution A from start to 5.5 min, and then decreased to 0 % solution A within 0.3 min. This composition was held for an additional 1.1 min before increasing to 95 % solution A from 6.9 to 7.0 min. The 95 % condition was then held for an additional 2 min before returning to the initial conditions.
The following formula was used to calculate the level of toxin in each sample (ppb):
where C is the mycotoxin concentration in the sample from the standard curve (ng/ml), V is the final sample volume (ml) and M is the sample weight (g)
The analysis was conducted using a Waters Zevo triple quadrupole tandem mass spectrometer. Analysis parameters for the mass spectrograph were maximised using a syringe pump to directly inject 1.0 μg/ml of each toxin standard sample at 10 μl/min. Positive electrospray ionisation (ESI) [M+H]+ was utilised to identify the best analysis parameters for each toxin and transition ions under MRM mode. For precursor ion and cone voltage (CV), MS scanning mode was set to one-time MS scanning to select precursor ions with greater sensitivity for each toxin, and the cone voltage (5–30 V) was adjusted to observe the signal strength of precursor ions. The best cone voltage was selected by the highest signal strength for the precursor ion. To measure product ion and collision energy, once the precursor ion and cone voltage were set, parameters such as argon (3.5 × 10−3 mbar) were turned on, the daughter scan spectrum of precursor ion was observed, and product ions most feasible for MS analysis parameters and MRM mode were identified. The instrument was operated using an ESI source in positive mode. The mass parameters were as follows: capillary voltage 2.5 kV, extractor voltage 2.5 V, source temperature 150 °C, desolvation temperature 500 °C, desolvation gas (nitrogen) flow at 1,000 l/h. The specific parameters of each antibiotic are shown in Table 1. Data acquisition was performed using MassLynx 4.1 software with the QuanLynx program.
Calibration and Quantification Limits
A standard dilution series was made by adding various concentrations (0.05, 0.1, 0.2, 0.5, 1, 5, 10, 25, 50, 100, 250 and 500 ng/g) of mixed toxins to a 20 % aqueous acetonitrile solution. Ten microlitres of the standard solution was injected into the UPLC-MS/MS system under optimised conditions, and the calculation was made using the quantitative ion peak area (Y axis) and the concentration of the standards (X axis) to obtain a standard curve for each toxin. Blank homogenised grain samples were extracted following the same procedure described above to provide matrix-based calibration curves, after spiking the grains sample at five concentration levels (2.5–125 ng/g) following extraction.
The instrument’s response to each analyte was measured by integrating the ion chromatogram peak area. The calibration curves were constructed by calculating the ratio of each peak area relative to an IS. The limit of detection (LOD) and the limit of quantification (LOQ) are often used to estimate method performance in terms of sensitivity, and estimated as the amount of compound injected that produced a signal/noise ratio (peak to peak area) of less than 3 and 10, respectively, and were determined by spiking each standard solution before extraction.
Validation and Data Acceptability
The recovery of each analyte was calculated by comparing the standard calibration curve. Standard solutions of AFB1, AFB2, AFG1, AFG2, FB1, FB2, DON, ZON, OTA, T-2 and HT-2 were added to samples with different concentrations — 0.5, 1 and 10 ng/g of AFB1, AFB2, AFG1; 20, 50 and 100 ng/g of AFG2, FB1 and FB2; DON and 5, 10 and 100 ng/g of ZON; 5, 10 and 50 ng/g in T-2 and HT-2; and 1, 2 and 20 ng/g of OTA, respectively. To evaluate the quantification results, values were obtained from a matrix-matched curve, and the average recovery was calculated by taking three duplicate analyses.
The precision was described as the CV value (intra-day and inter-day) at each spiking level. Under the intra-day test, three duplicate extractions were conducted on rice, peanut, wheat or maize during the same day. Standard solutions were then added with low, medium and high toxin concentrations, and the coefficient of variation (CV) was calculated. For the inter-day test, extractions were taken from substrates on three different days, and 11 mixed standard solutions were spiked with 10 ng/g to calculate the CV values. Microsoft Excel 2003 software was used to perform statistical analyses including average, standard deviation and variation coefficient. Data acquisition was performed with the MassLynx 4.1 software with the QuanLynx program.
Results and Discussion
Sample Preparation
A few extraction solvents were chosen and tested for their suitability. Initially, 70 % methanol was selected as an extraction solvent and was compared to the standard solution, but the recovery rate of FB1, FB2, DON and HT-2 in 100 ng/g shows low recovery rate (26.2 %, 32.5 %, 4.9 % and 13.5 % in rice, respectively; 31.6 %, 4.4 %, 2.5 % and 40.9 % in maize, respectively) (data not shown). Therefore, 70 % methanol was not a suitable extraction solvent. This can be attributed to the fact that during extraction with 70 % methanol, lipid or other endogenous substances including organic or inorganic molecules present in a sample and still present in the final extract are co-extracted with the analytes, which may cause suppression.
For the multi-toxin analysis, the sample preparation methods play a key process in whole experimental design and a suitable extraction solvent with universal properties had to be found that could repeatedly extract as many toxins as possible. According to our original design, we had tried to contain more mycotoxins belonged in different types and groups in one test and in same sample preparation process. One extraction solvent cannot fit these requirements and achieve the desired result in a different matrix according to our proposed experiments. These mycotoxins (11 toxins) are the most important and widely used in Taiwan and also in other countries. A large amount of cereal products, special imported peanuts from Vietnam and rice from Thailand may have been exposed to the mytotoxins’ contamination. Furthermore, immunoaffinity columns that were chosen in order to avoid the matrix effect which could retain the instrument during daily operation and to obtain more specialized results had been proven effectively although at a higher cost.
Lattanzio et al. (2007) developed an analytical method for quantification of 11 toxins in maize after multitoxin immunoaffinity cleanup. When selecting the columns for toxin purification, except SPE, most researchers use Mycosep and IAC to purify the extracts. MycoSep contains substances such as activated charcoal, diatomaceous, and ion exchange resins to absorb the substrate of samples with one-step purification. Immunoaffinity columns utilise the specificity of an antibody to rinse off the impurities before recovering pure extractions. Although immunoaffinity columns require longer time for rinsing and washing, they have higher specificity and less substrate interference and are therefore better for purification. Most mycotoxin analyses utilise high percentage methanol or acetonitrile as extraction solutions. Our study utilised the modified extraction methods of Lattanzio et al. (2007) and Vicam Company. This modification included the use of a PBS solution and methanol to conduct secondary extraction of the mycotoxin from samples, which significantly increased the recovery rate of all toxins.
Optimization Conditions for Liquid Chromatography-Mass Spectrometry
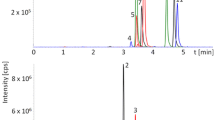
This study used ESI to detect precursor ions, product ions, cone voltages and collision energy from 11 toxins, as listed in Table 1. UPLC was used for samples that can tolerate higher pressure than traditional HPLC (Waters Co. 2006). When matched with the proper column, UPLC can analyse multiple samples in shorter times with a faster analytical pace than HPLC. With regard to the composition of solvents, most references utilise water, acetonitrile and methanol, while formic acid and ammonium acetate were widely used as a dressing agent (Ren et al. 2007; Spanjer et al. 2008). This study used a water and methanol solution with 0.1 % formic acid to conduct a gradient analysis. The results indicated that the high flow rate at 0.3 ml/min is better than that at 0.1–0.2 ml/min because it has a narrower and symmetrical peak and significantly reduces the analysis times. Figure 2 shows the MRM chromatogram for 11 standard mycotoxins.
Precursor ions collide with argon molecules in a collision cell to induce the decomposition and generate product ions. A secondary mass analysis is then used to detect proper product ions and obtain the maximum detection sensitivity. From the collision of precursor ion A and to induce product ion B, two product ions from the debris of precursor ions with the strongest molecular structure, breakage specificity, and signal strength as MRM detection ions are selected. The fragments of precursor ions are verified by injecting the standard sample into MC under full spectrum scanning mode via an injection syringe. The fragments with stronger signals are as follows: AFB1 at m/z 313, AFB2 at m/z 315, AFG1 at m/z 329, AFG2 at m/z 331, FB1 at m/z 722, FB2 at m/z 706, OTA at m/z 404, ZON at m/z 319, DON at m/z 297, T-2 toxin at m/z 489 and HT-2 toxin at m/z 447. Because T-2 and HT-2 have molecular weights of 466 and 424, respectively, they have an additional m/z 23 relative to their precursor ions. We conclude that the precursor ions for these two toxins are [M+Na]+, which is consistent with the report of Ren et al. (2007).
Identification of Standard Curve and Matrix-Matched Calibration Curve
During quantification via LC-MS/MS, the co-eluting matrix compound recovered with the sample will impact the ionisation of samples on the MS and possibly inhibit or enhance the results (which is called the matrix effect). To eliminate the impact of the matrix effect on the quantitative analysis results, a matrix-matched calibration curve or standard sample with an additive approach for quantification can be used. Because this study used an immunoaffinity column for purification, the matrix effect was likely reduced. Table 2 illustrates the slope comparison of the standard curve with the matrix-matched calibration curve, which contains the substrates from rice, maize, wheat and peanut. These curves have a very similar slope, which indicates this method is almost free of the matrix effect. Therefore, using a standard curve alone can be utilised for quantification (Spanjer et al. 2008; Ferrer et al. 2005).
Method Performance
Eleven toxins were spiked in rice, maize, wheat and peanut blank samples in the following amounts: AFB1, AFB2, AFG1 and AFG2: 0.5, 1, 10 ng/g; FB1 and FB2: 20, 50, 100 ng/g; DON and ZON: 5, 20, 100 ng/g; OTA: 0.5, 1, 20 ng/g; and T-2 and HT-2: 1, 5, 50 ng/g. Sample preparation is based on established methods and using a standard curve to calculate toxin concentrations in each sample. Recovery rate and redundancy are then assessed within the same day. Repetition testing was conducted on 3 different days with the following amounts: AFB1, AFB2, AFG1 and AFG2: 10 ng/g; FB1, FB2, DON and ZON: 100 ng/g; OTA: 20 ng/g; and T-2 and HT-2: 50 ng/g.
The average recovery rate for the 11 toxins in rice, maize, wheat and peanut were between 76.4 % and 115.0 %, 70.3–129.9 %, 69.6–97.0 % and 73.4–104.3 %, respectively, except ZON in Table 3, T-2 and HT-2 in wheat and ZON in wheat and peanut. The coefficient of variation all less than 15 %, except ZON in wheat and peanut and HT-2 in peanut (Table 3).
T-2 and HT-2 in wheat have a low recovery and high rate. As mentioned previously, HT-2 is the primary degradation product of T-2; however, under the same extraction conditions, no such situations were presented from rice, maize and peanut samples. It is likely that maize and wheat samples have an enzyme (carboxylesterase) (Ren et al. 2007) that induces the enzymatic hydrolysis of T-2 during the extraction and can cause the de-acetylation on the C4 position of T-2. As a result, HT-2 has a high recovery rate in these samples. However, a similar situation did not occur in maize under higher additive concentrations (50 ng/g). This could be the result of insufficient enzyme function. Wheat should have a relatively high enzyme amount. Therefore, it is reasonable that both recovery rates were close to 200 %. These data were previously presented at a JECFA meeting in 2001, explaining that T-2 and HT-2 are bonded to each other (T-2 and HT-2 Toxins). Furthermore, a European Union meeting in 2006 changed the T-2 daily allowance into a total amount of T-2 and HT-2 (EC Commission Regulation 2006). Although the recovery rates of ZON in the matrix of wheat and peanut are quite low at 22.4 % and 14.3 %, respectively, the recovery rates of the remaining toxins are mostly between 70 % and 120 %, with the coefficient of variation mostly under 15 %. Using the spectrum peak with an S/N ratio larger than 3 as threshold criteria, the LOD for AFB1, AFB2, AFG1, AFG2, FB1, FB2, DON, ZON, OTA, T-2 and HT-2 are 0.05, 0.05, 0.05, 0.05, 1, 0.5, 0.5, 1, 0.5, 0.1 and 0.5 ng/g, respectively. Comparing recovery data, the results indicate that AFB1, AFB2, AFG1, AFG2 and OTA in grains have an LOQ of 0.5 ng/g; for FB1 and FB2, it is 20 ng/g, and for DON and ZON it is 5 ng/g except in wheat and peanut. Additionally, the LOQ for T-2 and HT-2 is 1 ng/g. Using this method (except for ZON, which is only applicable on rice and maize), the results for the remaining ten toxins apply to rice, maize, wheat and peanut substrates. The LOQ for the above-mentioned toxins are all lower than the regulated limits, and the recovery rate and repetition at these concentrations were both very good.
The study method was compared to the traditional HPLC method published by the Department of Health, Executive Yuan (Method of Test for Mycotoxin in Foods). The traditional HPLC approach requires approximately 2 h per toxin sample for pre-preparation. To prepare different toxin samples separately, a total of 11 toxins will take approximately 20 h for sample pre-preparation. A rapid screening method can be used as a tool for evaluation of cereal and feed quality and could be applied when mycotoxicoses caused by contaminated feed are suspected in farm animals (Kokkonen and Jestoi 2009). This study examined the potential of an ultra-high performance liquid chromatography/tandem mass spectrometric (UPLC-MS/MS) multi-mycotoxin method to identify and quantify the mycotoxins produced by pure fungal isolates grown on yeast extract sucrose agar (Van Pamel et al. 2011). Using an LC-MS/MS method to conduct simultaneous analysis, multiple toxins can be processed at the same time, and total pre-preparation requires only 6 h. Using this simultaneous analysis method requires only 9 min for the apparatus to analyse the sample, which is faster than the traditional HPLC method. The detection limits by the UPLC method are all similar or lower than those reported in published HPLC methods. This study provides a quick and reliable method for the simultaneous analysis of important grain mycotoxins and can be used to protect consumer health.
Survey Market Sample
This method was used to test 26 grain samples bought from a market in Taiwan (four rice samples, eight maize samples, six wheat samples, and eight peanut samples). Of the samples, the rice products included white and brown rice, the maize products included corn kernel and corn starch, the wheat products were primarily imported Italian noodles and domestic noodles, and the peanut products included groundnut, peanut butter, and peanut powder. The results show that one peanut product had an AFB1 concentration of 1.5 ng/g; one maize product had a FB1 concentration of 51 ng/g; one maize product had a ZON concentration of 14.5 ng/g; one wheat, one maize and five peanut products had T-2 (or HT-2) concentrations between 1 and 9.4 ng/g; and two maize, four wheat and one peanut sample had DON concentrations between 8.8 and 439.7 ng/g. Of the 26 grains, one contained AFB1 and an identification rate of 3.8 %; one had FB1 with an identification rate of 3.8 %; one had ZON and an identification rate of 3.8 %; seven had T-2 and HT-2 and identification rates of 26.9 %; another seven had DON and identification rates of 26.9 %; and the remaining tests had no identification (Table 4). Figure 3 presents an MRM chromatogram using this method to identify DON, ZON and HT-2 from one incurred maize sample.
Conclusions
This study has established a useful method using LC-MS/MS to simultaneously analyse 11 mycotoxins (Aflatoxin B1, B2, G1, G2, fumonisin B1, B2, deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2) in rice, maize, wheat and peanut. The sample preparation process utilised PBS and 70 % methanol to conduct the secondary extraction and resulted in a higher recovery rate for higher hydrophilic toxins, especially for DON. The sample was then purified by passing through a Myco6in1™ immunoaffinity column, followed by LC-MS/MS for qualification and quantification. All relevant coefficients of the standard curve from each toxin were greater than 0.995. The average recovery rates of toxin additives in rice, maize, wheat and peanut were mostly between 70 % and 120 %, with CV of less than 15 % (indicating good replication). The LOQ was between 0.5 and 20 ppb. This method features a simple and user-friendly protocol, requires short analysis time, is very sensitive and reliable, and can simultaneously identify 11 of the most common toxins in grain within 9 min.
References
Anderson B, Frisvad JC (2004) Natural occurrence of fungi and fungal metabolites in moldy tomatoes. J Agric Food Chem 52:7507–7513
Antonello S, Rosalia F, Maria CS, Alejandro A, Alberto R (2009) Multitoxin extraction and detection of trichothecenes in cereals: an improved LC-MS/MS approach. J Sci Food Agric 89:1145–1153
Bayman P, Baker JL (2006) Ochratoxins: a global perspective. Mycopathologia 162:215–223
Birzele B, Prange A, Kramer J (2000) Deoxynivalenil and ochratoxin A in German wheat and changes of level in relation to storage parameters. Food Addit Contam 17(21):1027–1035
Dall’Asta C, Galaverna G, Aureli G, Dossena A, Marchelli R (2008) A LC/MS/MS method for the simultaneous quantification of free and masked fumonisins in maize-based products. World Mycotoxin J 1(3):237–246
EC Commission Regulation No. 1881/2006 (2006) Setting maximum levels for certain contaminants in foodstuffs
EC, European Commission (2001) Reports on tasks for scientific co-operation. Collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU Member States
Ferrer I, García-Reyes JF, Mezcua M, Thurman EM, Fernández-Alba AR (2005) Multi-residue pesticide analysis in fruits and vegetables by liquid chromatography–time-of-flight mass spectrometry. J Chromatogr A 1082:81–90
Freire F, Kozakiewics Z, Paterson R (2000) Mycoflora and mycotoxins in Brazilian black pepper, white pepper and Brazi nuts. Mycopathologia 159:281–289
Hesseltine CW (1967) Aflatoxina and other mycotoxins. Health Lab Sci 4:222–228
Homdark S, Fehrmann F, Beck R (2000) Influence of different storage conditions on the mycotoxins production and quality of fusarium-infected wheat grain. J Phytopathol 148:7–15
Huang C-C (2010) Risk and control of mycotoxin. Food Ind Mon 42(4):1–3
Kokkonen Meri K, Jestoi Marika N (2009) A Multi-compound LC-MS/MS method for the screening of mycotoxins in grains. Food Anal Methods 2:128–140
Larsen TO, Gareis M, Frisvad JC (2002) Cell cytotoxicity and mycotoxin and secondary metabolite production by common Penicillia on cheese agar. J Agric Food Chem 50:6148–6152
Lattanzio VMT, Solfrizzo M, Powers S, Visconti A (2007) Simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in maize by liquid chromatography/tandem mass spectrometry after multitoxin immunoaffinity cleanup. Rapid Commun Mass Spectrom 21:3253–3261
Lattanzio VMT, Gatta SD, Godula M, Visconti A (2011) Quantitative analysis of mycotoxins in cereal foods by collision cell fragmentation-high-resolution mass spectrometry: performance and comparison with triple-stage quadrupole detection. Food Addit Contam 28:1424–1437
Leong SL, Hocking AD, Pitt JI, Kazi BA, Emmett RW, Scott ES (2006) Australian research on ochratoxigenic fungi and ochratoxin A. Int J Food Microbiol 111:S10–S17
Maurice OM (2002) Risk assessment for aflatoxins in foodstuffs. Int Biodeter Biodegr 50:137–142
Method of Test for Mycotoxin in Foods, www.fda.gov.tw/Bgradation_index.aspx? site_content_sn=42
Michel S, Krska R, Rainer S (2010) Application of an LC-MS/MS based multi-mycotoxin method for the semi-quantitative determination of mycotoxins occurring in different types of food infected by moulds. Food Chem 119:408–416
Miller JD (1997) In: Trenholm HL (ed) Mycotoxin in grain. Compounds other than aflatoxins, 2nd edn. Eagan Press, St Paul, MN
Nakagawa H, Ohmichi K, Sakamoto S, Sago Y, Kushiro M, Nagashima H, Yoshida M, Nakajima T (2011) Detection of a new Fusarium masked mycotoxin in wheat grains by high-resolution LC-Orbitrap™ MS. Food Addit Contam 28:1447–1456
Pena A, Seifrtova M, Lino C, Silveira I, Solich P (2006) Estimation of ochratoxin A in Portuguese population: new data on the occurrence in human urine by high performance liquid chromatography with fluorescence detection. Food Chem Toxicol 44:1449–1454
Ren Y, Zhang Y, Shao S, Cai Z, Feng L, Pan H, Wang Z (2007) Simultaneous determination of multi-component mycotoxin contaminants in food and feed by ultra-performance liquid chromatography tandem mass spectrometry. J Chromatogr A 1143:48–64
Spanjer MC, Rensen PM, Scholten JM (2008) LC-MS/MS multi-method for mycotoxin after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit Contam 25(4):472–489
Stecher G, Jarukamjorn K, Zaborski P, Bakry R, Huck CW, Bonn GK (2007) Evalution of extraction methods for the simultaneous analysis of simple and macrocyclic trichothecenes. Talanta 73:251–257
T-2 and HT-2 Toxins, www.inchem.org/documents/jecfa/jecmono/v47je06.htm
Van Pamel E, Vlaemynck G, Heyndrickx M, Herman L, Verbeken A, Daeseleire E (2011) Mycotoxin production by pure fungal isolates analysed by means of an UHPLC-MS/MS multi-mycotoxin method with possible pitfalls and solutions for patulin-producing isolates. Mycotoxicol Res 27:37–47
Waters Co. (2006) ACQUITY UPLC system quick start guide. Revision C. Waters Corporation, Massachusetts, USA
Zinedine A, Soriano JM, Molto JC, Manes J (2007) Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogen mycotoxin. Food Chem Toxicol 45:1–18
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, Y.Y., Lin, H.Y., Chen, Y.C. et al. Development of a Quantitative Multi-Mycotoxin Method in Rice, Maize, Wheat and Peanut Using UPLC-MS/MS. Food Anal. Methods 6, 727–736 (2013). https://doi.org/10.1007/s12161-012-9473-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-012-9473-8