Abstract
Olives, the fruit of the Olea europaea tree, are highly appreciated in olive oil and table olives (20 % of crops) not only for their flavor but also for their nutritional properties, especially for antioxidant compounds such as squalling (SQ), α-tocopherol (TH) and β-carotene (BC). This paper presents a new analytical method for simultaneously determining SQ, TH and BC in table olives by using solid phase extraction (SPE) and high performance-liquid chromatography with diode array detection (HPLC-DAD), avoiding the classic saponification process. The correlation coefficients of calibration curves of the analyzed compounds ranged from 0.998 to 0.999, and the recoveries were in the range of 89.4–99.6 %. The validated method was used to analyze 30 table olive samples from Italy for their content of SQ (537–1,583 mg kg−1), TH (21–90 mg kg−1) and BC (0.4–2.6 mg kg−1). Finally, experiments with HPLC-MS were conducted to compare this novel method with the classic saponification procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the Mediterranean area, olive oil and table olives are consumed daily, and form an important agro-alimentary sector in Europe. Mediterranean countries are the largest consumers, producers and exporters of olives and olive oil because of their importance in the Mediterranean diet (Huang and Sumpio 2008). The fruit of the olive tree (Olea europaea) is mainly used for extraction of olive oil, although about 20 % is prepared as table olives (Lopez et al. 2006). In 2009/2010, world production of table olives reached the level of 2,239,000 t (International Olive Oil Council 2010). The olive fruit is highly appreciated for its good flavour, as well as for its nutritional properties, mainly related to the presence of fatty acids (FA) and important antioxidant compounds such as squalene (SQ), α-tocopherol (TH) and β-carotene (BC) (Sakouhi et al. 2008). In fact, unsaturated fatty acids participate in the regulation of blood cholesterol level (Delplanque 1998) and monounsaturated fatty acids stimulate transcription of the RNAm of the LDL cholesterol receptor and reduce breast cancer risks (Sorci et al. 1998). The antioxidants squalene, α-tocopherol and β-carotene are involved in the oxidative stability of olives and olive oil in that they protect their unsaturated fatty acids from oxidation; moreover, these antioxidant compounds have protective roles against cancer and cardiovascular diseases (Lawson 1995; Rader et al. 1997).
SQ, whose structure is reported in Fig. 1, is a triterpenoid compound with six double bonds (C30H50); it is an intermediate in the synthesis of cholesterol and of various sterols in the plant and animal world (Kohno et al. 1995). The concentration of SQ in olive oil ranges between 100–1,200 mg/100 g oil (De Leonardis et al. 1998) and is regarded as partially responsible for the protective effects of olive oil against certain cancers (Psomiadou and Tsimidou 1999). Although the antioxidant activity of SQ is not as significant as that of other vitamins, reports indicate that it is important in maintaining the stability of olive oil; in particular, SQ acts as a quencher of singlet oxygen and plays a vital role in skin lipid protection so that its participation in the photooxidation mechanism of other lipids cannot be excluded a priori (Psomiadou and Tsimidou 2002a).
TH (Fig. 1) is included, together with β-, γ-, and δ -tocopherols and α-, β-, γ-, and δ-tocotrienols, in the generic term of vitamin E, one of the most important antioxidants in vivo, distributed in varying concentrations in vegetables (Psomiadou and Tsimidou 2002b). TH functions as the major radical scavenging antioxidant and efficiently interrupts lipid oxidation chain propagation, thus protecting polyunsaturated fatty acids and low-density lipoproteins from oxidation (Kalman et al. 2003). The level of TH in olive oil ranges from approximately 30 to 100 mg kg−1, depending on the cultivar and the processing method (Sakouhi et al. 2008).
BC (Fig. 1) belongs to the family of carotenoids, effective inhibitors of photooxidation by quenching singlet oxygen and triplet excited states of photosensitizers. It has been reported to have not only antioxidant but also pro-oxidant effects in purified olive oil models containing high or low BC levels, stored at various conditions of light exposure (Psomiadou and Tsimidou 2002a). BC was found in oil derived from olives in a concentration 1–2 mg kg−1 (Gimeno et al. 2000).
Given these findings, it is easy to understand why analytical determination of these three antioxidant molecules in table olives could add valuable information to what is already known about the properties of this Mediterranean food. The literature offers numerous articles reporting analyses of one of these antioxidant compounds in olive oil, but to our knowledge, few articles concern table olives and none simultaneously determine SQ, TH, and BC in table olives. Moreover, most authors used alkaline saponification for isolating these three natural antioxidant compounds, a chemical step that is often time consuming and can interfere with these target compounds by causing their oxidation. Solid phase extraction (SPE), currently used for food sample preparation, is a faster and easier technique. Manzi and collaborators (Manzi et al. 1998) described the determination of SQ, TH, and BC in six different olive cultivars and in 15 virgin olive oils produced in the Molise region of Italy; they employed high performance-liquid chromatography with diode array detection (HPLC-DAD) on saponified samples. Gimeno and collaborators (Gimeno et al. 2000) analyzed TH and BC in olive oils by saponification with potassium hydroxide, extraction with hexane-ethyl acetate, and instrumental analysis by HPLC-DAD. Grigoriadou and coworkers (Grigoriadou et al. 2007) proposed an SPE–HPLC-DAD procedure to determine SQ and TH in olive oil samples, noting that SPE afforded considerable advantages over other, longer procedures for sample preparations and suggested that their validated method be used for official quality control of virgin olive oils. The aim of this work was to develop a new analytical method for simultaneously determining SQ, TH, and BC in table olives by using SPE–HPLC-DAD. Moreover, the classic method that includes saponification process was tested on the olive samples, and the compounds formed during the process were identified using high performance-liquid chromatography coupled to mass spectrometry system (HPLC-MS) having an ion trap as mass analyzer. The validated method was applied to analyze 30 table olives samples produced in Italy to determine the levels of SQ, TH, and BC.
Materials and Methods
Materials and Standards
Squalene (C30H50, molecular weight 410.73, 98 % minimum, CAS no. 111-02-4), α-tocopherol (C29H50O2, molecular weight 430.72, >96 % HPLC, CAS no. 10191-41-0), β-carotene (C40H56, molecular weight 536.90, CAS no. 7235-40-7) were supplied by Sigma-Aldrich (Milano, Italy). Individual stock solutions were prepared by dissolving 10 mg of squalene in 1 ml of n-hexane, 10 mg of α-tocopherol in 10 ml of n-hexane, and 10 mg of β-carotene in 100 ml of an n-hexane/chloroform mixture (9/1). Each standard solution was stored in glass-stoppered bottles at 4 °C and protected from the light with aluminum foil. Standard working solutions, at various concentrations, were prepared by appropriate dilution with n-hexane of stock solution aliquots.
HPLC-grade methanol, ethanol, i-propanol, acetonitrile, n-hexane, and ethyl acetate were supplied by Sigma-Aldrich (Milano, Italy), tetrahydrofuran 99–100 % by J.T. Baker B.V. (Deventer, Holland). Deionized water (>8 MΩ cm resistivity) was obtained from the Milli-Q SP Reagent Water System (Millipore, Bedford, MA, USA). All the solvents and solutions were filtered through a 0.45-μm PTFE filter from Supelco (Bellefonte, PA, USA) before use. Cartridges Strata SI-1 Silica SPE (6 ml, 1 g), were purchased from Phenomenex (Bologna, Italy). Potassium hydroxide and sodium chloride analytical reagents were purchased from PROLABO VWR (Milano, Italy). L-(+)-ascorbic acid 99 % was purchased from Sigma-Aldrich (Milano, Italy).
Sample Collection
The 30 samples of table olives were obtained from Camerino supermarkets and grocery stores and directly from Italian producers in central Italy. In particular (Table 1), 13 are Italian commercial items from different locations, 11 “Tenera Ascolana” variety samples were purchased from different producers, and six stuffed olive samples were acquired from a local olive-producing firm.
Sample Preparation
Five table olives were taken from each packaged sample, all residual brine was dried off, the stone was removed, and 2.5 g of each sample were weighed in a 50-ml centrifuge tube and homogenized with Ultraturrax for 1 min with 10 ml hexane. Each sample was then centrifuged at 4,000 rpm for 5 min, and the supernatant organic solution was purified on a Strata SI-1 Silica cartridge (6 ml, 1 g). Firstly, the cartridge was conditioned with 3 × 3 ml of n-hexane, then the n-hexanic solution was loaded onto the cartridge at a flow rate lower than 0.5 ml min−1. Next, the elution was performed using 6 × 4 ml of an n-hexane/ethyl acetate mixture (9/1) at a flow rate lower than 0.5 ml min−1. The eluate was totally evaporated under a nitrogen stream and the residue dissolved in 10 ml of i-propanol/ tetrahydrofuran (7/3) mixture before the HPLC analysis.
The classic method that includes saponification process was tested on table olives by applying the procedure published by Gimeno (Gimeno et al. 2000) that uses ascorbic acid to prevent oxidation of antioxidant target compounds (i.e. SQ, TH, and BC). The n-hexane/ethyl acetate extract was totally evaporated under vacuum at 40 °C and the residue dissolved in 10 ml of i-propanol/ tetrahydrofuran (7/3) mixture before the HPLC analysis.
HPLC-DAD and HPLC-MS Analyses
The separation of antioxidant compounds, i.e., SQ, TH, and BC, was achieved using a Chromolith RP-18e analytical column (100 × 3-mm I.D., macropore size 2 μm, mesopore size 13 nm) from Merck (Darmstadt, Germany). The mobile phase for HPLC-DAD and HPLC-MS analyses was: (a) acetonitrile/methanol (7/3), (b) i-propanol, flowing at 0.8 ml min−1. The solvent composition varied as follows: 0–6 min, 90:10 A/B (v/v); 6–8 min, 70:30 A/B (v/v); 8–17 min, 70:30 A/B (v/v); 17–20 min, 90:10 A/B (v/v); 20–30 min, 90:10 A/B (v/v).
HPLC-DAD and HPLC-MS studies were performed using an Agilent 1100 series instrument (Santa Clara, CA, USA), made of an autosampler, a quaternary solvent pump, with a DAD and MSD Trap SL equipped with an ESI source operating in negative ionization mode. HPLC-DAD analysis was performed monitoring three different wavelengths: 210 nm for SQ, 252 nm for TH, and 450 nm for BC. The mass spectrometer was tuned for each compound, optimizing ionization source parameters, voltages of the lenses and trap conditions in the ExpertTune mode of the Daltonic Esquire Control software while infusing a standard solution (100 μg ml−1) via a syringe pump at a flow rate of 5 μl min−1, which was mixed with the mobile phase at 0.5 ml min−1 by means of a T piece. The optimized parameters of the ESI interface were: vaporizer temperature, 325 °C; nebulizer gas (nitrogen) pressure, 60 psi; drying gas (nitrogen) flow rate, 13 ml min−1; temperature, 350 °C; capillary voltage, 3,500 V. The mass spectrometer was run in full scan. The trap parameters were set in ion charge control using rolling averaging set at 2 with a target of 20,000 and maximum accumulation time of 50 ms at m/z range from 50 to 1,500 u.
Statistical Analysis
Analysis of variance (ANOVA) was performed using the SPSS (v. 13.0) software package for Windows (SPSS, Inc., Chicago, IL, USA). Values of p < 0.05 were considered as statistically significant.
Results and Discussion
SPE–HPLC-DAD Method
The method presented in this work offers various advantages over those reported in the literature. First of all, it is able to simultaneously detect the three antioxidant compounds SQ, TH, and BC in a complex matrix (table olives), while the methods of other authors only enabled them to analyze SQ and TH in olive oils (Grigoriadou et al. 2007). The developed method that includes SPE and no saponification process instead of the classic method (including saponification process) (Gimeno et al. 2000; Manzi et al. 1998) provides better performance for three reasons. First, its simplicity and speed makes it possible to work with a high number of samples. Second, it is not necessary to have samples under an inert atmosphere, and the sample is not subjected to oxidative reactives and high temperatures, which can degrade the analytes. Third, although the triglycerides were not completely eliminated in the final eluate, the chlorophylls, which can alter the sample during the saponification process, were totally removed.
Some authors have reported HPLC-DAD analysis of SQ and TH using different instrumental conditions (columns, solvents) for each kind of molecule (Grigoriadou et al. 2007). In our study, the mobile phase chosen for HPLC-DAD and HPLC-ESI-MS analyses, i.e., (a) acetonitrile/methanol (7/3) and (b) i-propanol, permit to analyze all three antioxidant compounds in the same chromatographic step. Moreover, in the same procedure some authors use various detectors for monitoring antioxidant compounds (TH and SQ) such as a fluorescence detector and refracting index detector (Grigoriadou et al. 2007). In our study, an HPLC method using the same detector (DAD) was developed and, in order to increase sensitivity, the optimal wavelength of absorbance for each compound, i.e., 210 nm for SQ, 252 nm for TH and 450 nm for BC, was selected. In addition, in HPLC-DAD analysis, the choice of a Chromolith RP-18e monolithic column proved very advantageous. I-propanol, a very viscous solvent that produces significant back pressure at high flow rates, had to be used as a component of the mobile phase, but the Chromolith column drastically reduces the backpressure normally produced by such solvents, enabling to work at much lower pressures than normally required.
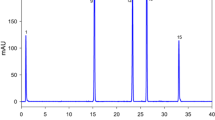
Figure 2 shows a typical HPLC-DAD chromatogram of the monitored antioxidant compounds at three different concentrations and wavelengths, i.e., 5 mg kg−1 and 252 nm (TH), 125 mg kg−1 and 210 nm (SQ), 0.5 mg kg−1 and 450 nm (BC). Table 2 reports the percent recovery and repeatability of the method evaluated in HPLC-DAD on table olives at two spiked levels (n = 5).
Method Validation
Calibration curves of the analyzed compounds were constructed by injecting into the HPLC-DAD 20 μL of standard solutions at five different concentrations for each compound, i.e., SQ 10, 50, 100, 250, 500 mg L−1; TH 0.5, 1, 5, 5, 10, 50 mg L−1; and BC 0.05, 0.1, 1, 5, 10 mg L−1, depending on their relative concentrations in real samples of table olives. The correlation coefficients of calibration curves of the analyzed compounds ranged from 0.998 to 0.999. Five replicates for each concentration were performed during 10 days, and the relative standard deviations (RSDs) ranged from 0.1 % to 0.9 % for run-to-run precision, and from 1.9 % to 6.9 % for day-to-day precision. The highest value of RSDs (6.9 %) relative to BC could be due to the fact that this molecule was monitored at the specific wavelength of 450 nm (by using a diode array detector), without wavelength reference. The recoveries obtained by spiking the olive samples with a standard mixture of antioxidants at two different levels, i.e. the first 500 mg kg−1 (SQ), 20 mg kg−1 (TH), 2 mg kg−1 (BC) and the second 1,000 mg kg−1 (SQ), 40 mg kg−1 (TH), 4 mg kg−1 (BC), are reported in Table 2. For all compounds, they were in the range 96.3–99.6 % at the first fortification level and 89.4–99.4 % at the second fortification level. The repeatability of the method, evaluated five times, was expressed by % RSDs, which proved lower than 17.6 % in all cases. All recovery data were normalized taking into account the percentage of recovery. Limits of detection (LODs) and limits of quantification (LOQs) were estimated on the basis of 3:1 and 10:1 signal-to-noise ratios obtained with standards containing the compounds of interest at low concentration levels. The HPLC-DAD analysis provided the following LODs and LOQs of the three studied compounds, expressed in milligrams per kilogram: 0.1 and 0.5 for SQ, 0.3 and 1 for TH, 0.03 and 1 for BC, respectively. Moreover, the repeatability interday was evaluated by one-way ANOVA using Fisher test. F test was used to evaluate the mean of the peak areas of each compound. The analysis showed no significative differences between measurements relative to three marked compounds, obtaining F crit. of 3.15. The obtained results suggest that the proposed method is reliable.
Analysis of Real Samples of Table Olives
The proposed method for determining the content of SQ, TH, and BC by SPE–HPLC-DAD was applied to the analysis of 30 table olive samples of Italian origin. The results are reported in Table 1 together with the origin and type of samples. Commercial samples of table olives (numbers 1–13) showed a content of SQ and BC ranging from 626 to 1,494 mg kg−1 and from 0.4 to 2 mg kg−1, respectively. The content of TH, ranging from 25 to 90 mg kg−1, was comparable to that reported in literature by Sakouhi and collaborators (Sakouhi et al. 2008).
The “Tenera Ascolana” variety, a valued Italian cultivar for table olives (Gambella et al. 2000), is cultivated in the south of Marches Region and is the main ingredient of a typical fried food called “Olive all’Ascolana”, in which the pitted olive is stuffed with a meat mixture, breaded, and fried. An investigation of the content of TH and carotenes in olive oil from the ‘Tenera Ascolana’ variety has been published (Dabbou et al. 2009), but no data are available about the content of SQ, TH, and BC in “Tenera Ascolana” table olives. In the 11 analyzed samples (numbers 14–24), SQ content ranged from 537 to 1,583 mg kg−1, BC ranged from 0.9 to 2.6 mg kg−1, and TH ranged from 21 to 45 mg kg−1. The highest content of SQ (1,583 mg kg−1) and BC (2.6 mg kg−1) was found in sample number 20, a 7-year-old table olive preserved in brine. In particular, in these olives, the bitter taste was not removed by the classic system of alkaline hydrolysis (NaOH 3 %), which normally causes the breakdown of the oleuropeins; it is possible that the omission of this process allowed the high values of the antioxidant molecules. In this sample, TH also showed one of the highest values (42 mg kg−1) of the list.
Finally, six samples of stuffed olives (numbers 25–30), purchased from a local farmer, were analyzed for the content of the three antioxidant compounds. In these samples, SQ ranged from 539 to 763 mg kg−1, TH from 25 to 32 mg kg−1 and BC from 0.8 to 1.3 mg kg−1.
One of the most important elements in table olive processing is the removal of its bitter taste by hydrolysis of oleuropein (Gambella et al. 2000). Normally, this process is realized by treating the olives with 6 % NaOH solution for 8–10 h. The de-bittering process was used on some of the samples of Table 1 table olives to investigate whether the concentration of SQ, TH, and BC was influenced by the alkaline treatment. Results showed that the starting concentration of the three antioxidants in the olives decreased only slightly after the de-bittering process, and thus concluded that this treatment does not significantly influence the concentration of SQ, TH, and BC in the table olives.
Comparison with Method Including Saponification and HPLC/MS Analysis
The proposed method for analyzing SQ, TH, and BC in table olives by SPE–HPLC-DAD was compared against the classic one that uses the saponification process for hydrolyzing the triglycerides, followed by HPLC-DAD analysis. Though ascorbic acid was used to prevent the oxidation of SQ, TH, and BC during the saponification process (by using 76 % potassium hydroxide solution for 30 min at 70 °C), scarce recoveries were obtained for the three antioxidant compounds, especially for TH. Perfectly in accordance with a previous work published by Czauderna and coworkers (Czauderna and Kowalczyk 2007), alkaline saponification in the presence of ascorbic acid decreases the concentration of tocopherols, especially TH, and the poor recovery of tocopherols is due to the decomposition of tocopherols in other species.
In order to investigate the chemical structure of the molecular species derived from TH and formed during the saponification process, HPLC-ESI-MS experiments were carried out on table olive extracts and directly on TH. In particular, by using an ion trap system as mass analyzer, mass spectra relative to the chromatographic peaks were acquired in full scan and in negative ionization mode. Figure 3 shows HPLC-ESI-MS chromatograms for a sample of TH saponified in presence of ascorbic acid (a) and chromatograms where TH was saponified in absence of ascorbic acid (b). Figure 3a presents an overlapping of two extracted ion chromatograms for the ions at m/z 429 and 445. The ionization of TH produced a mass spectrum where the base peak was the deprotonated molecule [M–H]− at m/z 429, while the peak for the ion at m/z 445 corresponds to one of the major products of oxidation of TH: α-tocopherolquinone (TQ) (Faustman et al. 1999; Damodaran et al. 2008). Figure 3b presents an overlapping of two extracted ion chromatograms for the ions at m/z 429 and 461. One can note that in the absence of ascorbic acid, together with the peak for the ion at m/z 429 (TH), another peak for the ion at m/z 461 appears, corresponding to another product of oxidation: 5,6-epoxy-α-tocopherol-quinone (TQE1) (22). At the same time, the peak for the ion at m/z 445 (chromatogram not shown) disappears. Similar results were obtained when the extracts of table olives containing TH were saponified and then analyzed with HPLC-ESI-MS equipment.
This study demonstrated that the saponification process degrades the original TH quantity present in a food matrix (the table olive), both in the presence and in the absence of ascorbic acid, forming various oxidation products identified and confirmed with HPLC-ESI-MS experiments. For these reasons, a gentler method by using SPE–HPLC-DAD without a saponification process, is more suitable for analyzing such sensible molecules as antioxidants in food.
Finally, the presented method using SPE–HPLC-DAD offers significant improvements over other published methods. It is a quick and reliable analytical procedure and makes it possible to simultaneously monitor the three antioxidant compounds, SQ, TH and BC, in table olives without employing the saponification process. Analysis of such important bioactive molecules as antioxidants in table olives can contribute to valorizing this typical food of Mediterranean diet.
Abbreviations
- SQ:
-
Squalene
- TH:
-
α-Tocopherol
- BC:
-
β-Carotene
- SPE:
-
Solid phase extraction
- HPLC-DAD:
-
High-performance liquid chromatography with diode array detection
- HPLC-ESI-MS:
-
High-performance liquid chromatography with electrospray ionization source and mass spectrometry
References
Czauderna M, Kowalczyk J (2007) Alkaline saponification results in decomposition of tocopherols in milk and ovine blood plasma. J Chromatogr B 858:8
Dabbou S, Issaoui M, Servili M, Taticchi A, Sifi S, Montedoro GF, Hammami M (2009) Characterization of virgin oils from European olive cultivars introduced in Tunisia. Eur J Lipid Sci Technol 111:392
Damodaran S, Parkin KL, Fennema OR (2008) Fennema’s. Food Chemistry, edition 4. Eds; CRC Press, Taylor and Francis group: Broken Sound Parkway NW, Boca Raton, FL, 7: 461
De Leonardis A, Macciola V, De Felice M (1998) Rapid Determination of squalene in virgin olive oil using gas-liquid chromatography. Ital J Food Sci 10:75
Delplanque B (1998) Intérêt nutritionnel des huiles d’olives. Oleagineux Corps Gras Lipides 6:1
Faustman C, Liebler DC, Burr JA (1999) α-Tocopherol oxidation in beef and in bovine muscle microsomes. J Agric Food Chem 47:1396
Gambella F, Piga A, Agabbio M, Vacca A, D’hallewin G (2000) Effect of different pre-treatments on drying of green table olives (Ascolana tenera var.). Grasas Aceites 51:173
Gimeno E, Calero E, Castellote AI, Lamuela-Raventos RM, De la Torre MC, Lopez-Sabater MC (2000) Simultaneous determination of α-tocopherol and β-carotene in olive oil by reversed-phase high performance liquid chromatography. J Chromatogr A 881:255
Grigoriadou D, Androulaki A, Psomiadou E, Tsimidou MZ (2007) Solid phase extraction in the analysis of squalene and tocopherols in olive oil. Food Chem 105:675
Huang CL, Sumpio BE (2008) Olive Oil, the Mediterranean diet, and cardiovascular health. J Am Coll Surg 207:407
International Olive Oil Council, World Table Olive Figures. http://www.internationaloliveoil.org/estaticos/view/132-world-table-olive-figures. Accessed 1 Nov 2010
Kalman A, Mujahid C, Mottier P, Heudi O (2003) Determination of α-tocopherol in infant foods by liquid chromatography combined with atmospheric pressure chemical ionization mass spectrometry. Rapid Commun Mass Spectrom 17:723
Kohno Y, Egawa Y, Itoh S, Nagaoka S, Takahashi M, Mukai K (1995) Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochim Biophys Acta 1256:52
Lawson H (1995) Food oils and fats-technology, utilization and nutrition. Chapman and Hall, New York, p 184
Lopez A, Montaño A, Garcia P, Garrido A (2006) Fatty acid profile of table olives and its multivariate characterization using unsupervised (PCA) and supervised (DA) Chemometrics. J Agric Food Chem 54:6747
Manzi P, Panfili G, Esti M, Pizzoferrato L (1998) Natural antioxidants in the unsaponifiable fraction of virgin olive oils from different cultivars. J Sci Food Agric 77:115
Psomiadou E, Tsimidou M (1999) On the role of squalene in olive oil stability. J Agric Food Chem 47:4025
Psomiadou E, Tsimidou M (2002a) Stability of virgin oil. 2. Photo-oxidation studies. J Agric Food Chem 50:722
Psomiadou E, Tsimidou M (2002b) Stability of virgin oil. 1. Autoxidation studies. J Agric Food Chem 50:716
Rader JI, Weaver CM, Patrascu LH, Angyal G (1997) α-Tocopherol, total vitamin A and total fat in margarines and margarine-like products. Food Chem 58:373
Sakouhi F, Harrabi S, Absalon C, Sbei K, Boukhchina S, Kallel H (2008) α-Tocopherol and fatty acids contents of some Tunisian table olives (Olea europea L.): changes in their composition during ripening and processing. Food Chem 108:833
Sorci TM, Wilson MDl, Johnson F, Rudeell LL (1998) Studies on the expression of genes encoding apolipoprotein B 100 and the LDL receptor in non human primates. Comparison of dietary fat and cholesterol. J Biol Chem 264:9039
Acknowledgments
The authors would like to thank the local farmers for providing table olive samples of the “Tenera Ascolana” variety and Castellino Ralò srl for providing stuffed olive samples. They would also like to thank Sheila Beatty for her editing of the English usage in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sagratini, G., Allegrini, M., Caprioli, G. et al. Simultaneous Determination of Squalene, α-Tocopherol and β-Carotene in Table Olives by Solid Phase Extraction and High-Performance Liquid Chromatography with Diode Array Detection. Food Anal. Methods 6, 54–60 (2013). https://doi.org/10.1007/s12161-012-9422-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-012-9422-6