Abstract
Food colorants are an important class of food additives that give the first impression to consumers about the quality of food. Ce(IV)-reducing antioxidant capacity assay originally developed in our laboratories was adapted to the determination of synthetic food colorants for the first time. This method allowing for total antioxidant capacity assay of dietary polyphenols, flavonoids, and ascorbic acid in plant extracts is based on the room temperature oxidation of antioxidant compounds with Ce(IV) sulfate in dilute H2SO4 solution and measurement of the absorbance of unreacted Ce(IV) at 320 nm. The results of the proposed method were correlated with high-performance liquid chromatography (HPLC) findings. Individual standard solutions, synthetic mixtures of synthetic colorants, and colorant extracts were identified and quantified with HPLC on a C18 column equipped with a diode array detector, and slight modifications on the existing HPLC method were made to analyze synthetic colorant mixtures. This work proposes Ce(IV)-oxidative spectrophotometry as a complementary technique to HPLC in the analysis of food colorants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food colorants are an important class of food additives attracting the attention of consumers, and give the first impression about the taste and quality of a food product. They are subgrouped as natural and synthetic colorants and controlled by the limitations of European Union (EU) and Federal Food, Drug, and Cosmetic Act (FD&C; EC 1994; FDA 2007.) Synthetic colorants are preferred for their low price and high stability. However, some research studies raise serious doubts about their possible adverse health effects (Kapadia et al. 1998; Eigenmann and Haenggeli 2007). In the light of these risks, it is a necessity to develop rapid, simple, and inexpensive techniques for the analysis of food colorants in relatively less equipped conventional laboratories.
Color in one form or another has been added to our foods for centuries. It is known that Egyptians colored candy and wine dating back to 400 BC (Downham and Collins 2000). Synthetic colorants show several advantages over natural colorants such as high stability to light, oxygen, and pH; color uniformity; low microbiological contamination; and relatively lower production costs (Alves et al. 2008). However, certain researchers raise serious suspects about life-threatening risks of synthetic colorants (Kapadia et al. 1998; Eigenmann and Haenggeli 2007).
Many analytical methods such as molecular spectroscopy (Lachenmeier and Kessler 2008; Altınoz and Toptan 2002; Vallvey et al. 2002); voltammetry (Silva et al. 2007) capillary electrophoresis (Ryvolava et al. 2007); spectrophotometry in combination with chemometrics (Ni et al. 2009) have been developed to identify and determine synthetic food colorants. However, most of these mentioned methods are time-consuming and/or have sensitivity/selectivity problems. Most preferred methods for the determination of synthetic food colorants are still chromatographic techniques coupled with ultraviolet (UV) or diode array detectors (DADs; Serdar and Knezevic 2009; Culzoni et al. 2009; Kirschbaum et al. 2006) There are two main problems with the use of single-wavelength UV detectors. Firstly, different types of food colorants have various UV–visible (UV–Vis) spectra with different maximum absorbance wavelengths. Thus, wavelength switching during the chromatographic run fails as a result of long separation time of several food colorants. The second problem is the possible overlap of colorant peaks or the presence of other organic compounds such as flavors in the sample. Both problems can be solved in the case of DADs. All dyes can be detected near to their maximum wavelength with the aid of multisignal detection capability, and peak identity can be easily confirmed. High-performance liquid chromatography (HPLC) methods have been used with isocratic and gradient elution systems for the determination of colorants in food and beverages.
It is extremely challenging to colorimetrically quantify colorants, thus it may be more selective and accurate to develop an analysis procedure depending on the redox properties of colorants. Such an oxidative estimation can also be carried out by visible spectroscopy using a chromogenic oxidizing agent such as Ce(IV) sulfate, the ceric reducing antioxidant capacity (CERAC) reagent, successful in finding the antioxidant capacity of flavanoids, phenolics, vitamins C and E (Ozyurt et al. 2007, 2010). Since the formal potential of the Ce(IV,III) redox couple in the presence of dilute sulfuric acid containing Na2SO4 is decreased from the standard potential of +1.61 V down to a value less than that of citric acid (Ozyurt et al. 2010), colorants with a redox potential less than this value can easily react with the CERAC reagent and be determined at the maximum absorption wavelength of Ce(IV). The most important advantages of this method are the low cost of reagents and instrumentation, reasonable selectivity of redox spectrophotometry (i.e., citric acid and simple sugars are not oxidized with Ce(IV) under optimal conditions), and the short time of analysis when compared to other analytical techniques.
Materials and Methods
Reagents and Solutions
Analytical reagent-grade chemicals and milli-Q water were used in all experiments. The colorants, tartrazine (E102), sunset yellow FCF (E110), erythrosine (E127) were purchased from Aldrich. Ponceau 4R (E124) and indigo carmine (E132) were obtained from E. Merck, along with the chemicals: ammonium acetate, cerium(IV) sulfate tetrahydrate, sodium sulfate, concentrated sulfuric acid, and HPLC-grade methanol. The chemical structures, common names, and European Community numbers (E numbers) of colorants are shown in Table 1. Stock solutions of colorants were prepared at a concentration of 1,000 mg l-1 in water.
For CERAC spectrophotometric determinations, Ce(IV) sulfate solution at 2.0 × 10−3 M concentration was prepared by dissolving 0.0808 g Ce(SO4)2·4H2O in water, transferring to a 100 ml volumetric flask, adding 17 ml of concentrated H2SO4 to prevent ceric ion hydrolysis, and diluting to the mark with H2O. Na2SO4 at 1.0 M was prepared by dissolving 35.50 g of the salt in water and diluting to 250 ml with H2O.
Apparatus
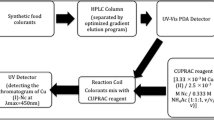
Reversed-phase liquid chromatography (RPLC) experiments were carried out using Hitachi HPLC system consisting of a pump (Hitachi L-2130 HPLC pump), a stainless steel column packed with C18 sorbent (250 mm × 4.6 mm; Hitachi), and UV–PDA detector system (Hitachi L-2455). The UV–Vis spectra and absorption measurements were recorded using a Varian Cary UV–Vis spectrophotometer. For the homogenization of the prepared stock solutions, solutions were kept in BANDELIN RK100H model ultrasonic bath for 30 min.
Chromatographic Conditions
For the separation of colorants, reversed phase C18 column system was used. The mobile phase consisted of 0.13 M ammonium acetate solution (mobile phase A) and HPLC-grade methanol solution (mobile phase B). Gradient elution program was chosen to accurately separate and identify colorants. In order to achieve full resolution of all colorants, a variety of gradient elution programs were tested, using different mobile phases and changing retention times. But in all optimization experiments, the flow rate and injection volume were kept constant as 1 ml min−1 and 20 μl, respectively. The optimized gradient program is shown in Table 2. The total length of time required for separation was 35 min.
For the detection of colorants, DAD system was set to a range of 350–800 nm, monitoring each colorant at its appropriate absorbance wavelength. Column conditioning was maintained by passing through the mobile phase before injection, and a mixture of water–methanol (1:1, v/v) after injection for a minimal time period of 1 h.
Preparation of Standard and Sample Solutions
All stock colorant solutions were prepared by dissolving 100 mg of colorant in 100 ml milli-Q water. The solutions were kept in the dark, and by appropriate dilution of stock solutions, standard solutions in the concentration range between 0.5 and 50 mg l−1 were prepared. The content of individual food colorants in the mixed standard solutions (synthetic mixture solutions) were also in the same concentration range.
Six different real samples were purchased from the local market and analysed for their colorant contents. For energy drinks, dilution was not required. Powder beverage samples such as aromatic tea and fruit juices were accurately weighed as 1 g and dissolved in 50 ml of milli-Q water. Sample solutions were degassed and kept in an ultrasonic bath for 30 min to achieve complete homogenization. The sample solutions were injected to the chromatographic system after filtering through 0.45μm disposable syringe filters.
CERAC Assay of Total Antioxidant Capacity Applied to the Determination of Total Colorant Content
Add 8 ml CERAC reagent [1 ml of 2.0 × 10−3 M Ce(IV) solution + 7 ml of 1 M Na2SO4 solution] + x ml of food colorant solution into a test tube; dilute to 10 ml with H2O [since the Ce(IV) solution originally contains sulfuric acid, the final H2SO4 molarity is normally 0.3 M; however, adjust the final sulfuric acid molarity to 0.3 M if necessary]. Let the mixture stand for 30 min at room temperature and measure the 320 nm absorbance (A 320 nm) due to Ce(IV) (Ozyurt et al. 2010). Construct the calibration curves and titration graphs for each colorant under the conditions described above.
Spectrophotometric Titration of a Fixed Ce(IV) Solution with Food Colorants
As described in the standard CERAC method, 8 ml CERAC reagent [1 ml of 2.0 × 10−3 M Ce(IV) solution + 7 ml of 1 M Na2SO4 solution] was taken, and titrated with increasing volumes of the food colorant solution, and the absorbance at 320 nm was recorded as a function of colorant concentration (exemplified with sunset yellow FCF), the total volume being kept at 10 ml with appropriate dilution.
For controlling the parallellism of titration curves, a fixed volume (0.5 ml) of beverage (exemplified with orange powder beverage containing sunset yellow FCF) was added to 8 ml CERAC reagent, and different volumes of the colorant (changing between 100 and 1,500 μl) were added as titrant, the total volume being kept at 10 ml with appropriate dilution.
Results and Discussion
Optimization of the Chromatographic Separation Method
A simple and fast chromatographic method for the determination of five synthetic food colorants was developed, involving RP-HPLC consisting of a nonpolar or slightly polar stationary phase and a strongly polar mobile phase such as acetonitrile, methanol or water. The accurate analysis of food colorants is based on the appropriate conditions. The most important property in maintaining the right conditions is hydrophobic properties of assayed colorants and the acidic groups present in their molecules. Hydrophobicity of azo-dyes and dyes with naphtelene ring is generally higher than that of the rest of dye molecules. Therefore, for improving separation and shortening time of analysis, inorganic electrolytes are added to the mobile phase. The suitable modificator is often an ammonium acetate buffer at optimal concentration above 0.1 M (Kucharska and Grabka 2010). Higher concentrations of buffer solution cause lengthening of retention time as a result of salting-out effect. In the chromatographic analysis of food colorants, 0.13 M of ammonium acetate buffer solution was chosen to be the most suitable eluent for mobile phase A. Earlier studies in literature recommended a mixture of methanol and acetonitrile for mobile phase B (Minioti et al. 2007) since acetonitrile could improve the asymmetry of the peaks. Nevertheless, in the analysis of five synthetic colorants in this work, the presence of acetonitrile was neither required for the symmetry of peaks nor for eliminating resolution problems.
For the optimization of gradient elution program, a standard mixture solution of five synthetic colorants at 5 mg l−1 concentration value was used. In the first 2 min, mobile phase A was passed through the column for the removal of inorganic impurities. Between the 30th and 32nd min, mobile phase B was passed to remove organic impurities. The last 2 min were necessary to prepare the column for the next run.
Under these optimized conditions, five food colorants in admixture were analyzed (chromatogram shown in Fig. 1) by scanning the wavelength range from 350 nm to 800 nm. The software used to process data enables the calculation of the retention time (t R) and peak area. The flow rate was fixed to 1 ml min−1, since at higher flow rates, high-pressure build-up in the chromatographic column was observed.
Validation of the Chromatographic Method
Maximum absorbance wavelengths and retention times for the five colorants are shown in Table 3 with their calibration line equations, calibration coefficients (as R 2), retention times (t R), linear concentration range, and limits of detection (LOD). Calibration graphs were constructed using peak area (A) versus concentration (mg l−1) of each colorant.
CERAC Assay of Total Colorant Determination
CERAC method was first developed by Ozyurt et al. (2007) and later modified in by Ozyurt et al. (2010) to improve selectivity for the determination of total antioxidant capacity. The method is based on the oxidation of phenolic antioxidants with tetravalent cerium at room temperature. The advantage of the method is its simplicity, low cost, and applicability to conventional laboratories without high-level instrumentation (Ozyurt et al. 2007). The redox potential of the Ce(IV) oxidant in the modified assay (Ozyurt et al. 2010) was fine-tuned in 0.3 M H2SO4 + 0.7 M Na2SO4 aqueous medium so as to selectively oxidize true antioxidants but not citric acid, simple sugars, and other pharmaceutical ingredients, constituting the basic novelty of this work to antioxidant research. This main idea of the CERAC method was applied to the determination of food colorants by constructing their calibration curves (as Ce(IV) absorbance at 320 nm versus colorant concentration) and calculating their indirect absorptivities (with respect to their Ce(IV) reducing power) from the slopes of these lines. Standard calibration and/or spectrophotometric titration curves of five synthetic food colorants were redrawn both in water and in a real sample matrix so as to observe the parallelism between the calibration lines. This parallelism indicated the absence of chemical deviations from Beer’s law in the determinations. The absorption coefficients (indirect molar absorptivities) and linear concentration ranges of the tested colorants are given in Table 4.
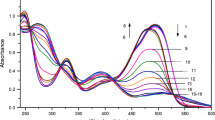
The spectrophotometric titration data of the tested colorants showed that the total colorant content of the real sample solution may be precisely calculated without chemical interactions among constituents (i.e., the observed absorbances were additive). Sunset yellow in orange powder beverage was chosen as a representative sample to verify the parallelism between the left arms of the spectrophotometric titration curves (Fig. 2) of a fixed Ce(IV) solution with the analyte colorant alone and analyte in beverage, indicating the absence of chemical deviations from Beer’s law in the selected spectrophotometric procedure. Before the equivalence point was reached, absorbance at 320 nm (A320) decreased with colorant concentration due to Ce(IV) consumption, and beyond the equivalence point, A320 increased with concentration due to the inherent color of the colorant.
Spectrophotometric titration curves of a fixed Ce(IV) solution with Sunset Yellow FCF alone and with the same colorant in orange powder beverage. a ■ Titration curve with Sunset Yellow FCF [1 ml of 2.0 × 10−3 M Ce(IV) solution + 7 ml of 1 M Na2SO4 solution + x ml colorant solution + (2.0-x) ml water]. b ▲ Titration curve with sunset yellow FCF in orange powder beverage [1 ml of 2.0 × 10−3 M Ce(IV) solution + 7 ml of 1 M Na2SO4 solution + 0.5 ml beverage + x ml colorant solution + (1.5-x) ml water]
Results of Beverage Sample Analyses
The beverage samples were prepared and analyzed as described in “Preparation of standard and sample solutions.” The individual contents of the analyzed beverage samples were found with the aid of the calibration curves drawn as HPLC peak area vs. concentration (Table 3). The sum of the individual concentration values found for each colorant was compared with the colorant content (on a molar basis) achieved by the modified CERAC method of spectrophotometric titration (Table 5).
Conclusions
In this study, determination of five synthetic food colorants was investigated using chromatographic and spectrophotometric methods. HPLC analysis of colorants was performed using PDA detector system, monitoring each colorant at its maximum absorbance wavelength. The selected mobile phase for the gradient elution program enabled the shortening of total analysis time when compared to other chromatographic methods. Furthermore, since acetonitrile as the conventional solvent was not used in the eluent, solution costs were minimized. Food colorants are regulated by the legislations issued by EU, FD&C, or by the own directives of a country. These regulations limit the usage of colorants during the production process of foods or beverages by controlling the quantities of each colorant. However, the total colorant content may be useful in some cases for rapid monitoring of colorants. By adapting the novel spectrophotometric CERAC assay of total antioxidant capacity to the determination of total food colorant content, certain beverage or food samples were easily and accurately analyzed. The total colorant content was found at low reagent and instrumentation costs with the use of a UV–vis spectrophotometer easily found in a conventional laboratory equipped with simple instruments. Moreover, simple sugars and citric acid, which are not colorants, are not oxidized in the CERAC method and therefore do not yield errors in colorant content calculations. For future prospects of this study, HPLC technique and CERAC method are planned to work together in an online post-column system, enabling the monitoring of colorant content in food and beverage samples as fingerprints. In such a system, HPLC column effluents are first detected at a fixed-wavelength UV–Vis detector, and the colorants may be further reacted with the CERAC reagent in a post-column reactor coil to reach a fluorescence detector for measuring the emerged Ce(III) or a UV–Vis absorbance detector for measuring Ce(IV) consumption.
References
Altınoz S, Toptan S (2002) Determination of tartrazine and Ponceau-4R in various food samples by Vierordt’s method and ratio spectra and first-order derivative UV spectrophotometry. J Food Compos Anal 15:667–683
Alves SP, Brum DM, Branco de Andrade EC, Netto ADP (2008) Determination of synthetic dyes in selected foodstuffs by high performance liquid chromatography with UV-DAD detection. Food Chem 107:489–496
Culzoni MJ, Schenone AV, Llamas NE, Garrido M, Di Nezio MS, Band BS, Goicoechea HC (2009) Fast chromatographic method for the determination of dyes in beverages by using high performance liquid chromatography–diode array detection data and second order algorithms. J Chromatogr A 1216:7063–7070
Downham A, Collins P (2000) Colouring our foods in the last and next millennium. Int J Food Sci Technol 35:5–22
EC (1994) Directive of the European Parliament and of the council 94/36/EC of June 30, 1994 on colors for use in foodstuffs, Official J, L237, 13, 10/9/1994
Eigenmann PA, Haenggeli CA (2007) Food colourings, preservatives, and hyperactivity. Lancet 370:1524–1525
FDA (2007) Summary of color additives for use in United States in foods, drugs, cosmetics and medical devices
Kapadia G, Tokuda H, Sridhar T, Balasubramanian V, Takayasu J, Bu P, Enjo F, Takasaki M, Konoshima T, Nishino H (1998) Cancer chemopreventive activity of synthetic colorants used in foods, pharmaceuticals and cosmetic preparations. Cancer Lett 129:87–95
Kirschbaum J, Krause C, Brückner H (2006) Liquid chromatographic quantification of synthetic colorants in fish roe and caviar. Eur Food Res Technol 222:572–579
Kucharska M, Grabka J (2010) A review of chromatographic methods for determination of synthetic food dyes. Talanta 80:1045–1051
Lachenmeier DW, Kessler W (2008) Multivariate curve resolution of spectrophotometric data for the determination of artificial food colors. J Agric Food Chem 56:5463–5468
Minioti KS, Sakellariou CF, Thomaidis NS (2007) Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Anal Chim Acta 583:1045–1051
Ni Y, Wang W, Kokot S (2009) Simultaneous kinetic spectrophotometric analysis of five synthetic food colorants with the aid of chemometrics. Talanta 78:432–441
Ozyurt D, Demirata B, Apak R (2007) Determination of total antioxidant capacity by a new spectrophotometric method based on Ce(IV) reducing capacity measurement. Talanta 71:1155–1165
Ozyurt D, Demirata B, Apak R (2010) Modified cerium(IV)-based antioxidant capacity (CERAC) assay with selectivity over citric acid and simple sugars. J Food Compos Anal 23:282–288
Ryvolava M, Taborsky P, Vrabel P, Krasensky P, Preisler J (2007) Sensitive determination of erythrosine and other red food colorants using capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr A 1216:7063–7070
Serdar M, Knezevic Z (2009) Simultaneous LC analysis of food dyes in soft drinks. Chromatographia 70:1519–1521
Silva MLS, Garcia MBQ, Lima JLFC, Barrado E (2007) Voltammetric determination of food colorants using a polyallylamine modified tubular electrode in a multicommutated flow system. Talanta 72:282–288
Vallvey LFC, Valencia MC, Nicolas EA (2002) Flow injection analysis with on-line solid phase extraction for spectrophotometric determination of ponceau 4R and its subsidiary unsulfonated dye in sweets and cosmetic products. Microchim Acta 138:69–76
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olgun, F.A.O., Ozturk, B.D. & Apak, R. Determination of Synthetic Food Colorants in Water-Soluble Beverages Individually by HPLC and Totally by Ce(IV)-Oxidative Spectrophotometry. Food Anal. Methods 5, 1335–1341 (2012). https://doi.org/10.1007/s12161-012-9384-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-012-9384-8