Abstract
Over the past years to ensure food safety and particular for food that intend to be consumed by infants and young children, the European Union has adopted specific legislation concerning the control of pesticide residue levels in that kind of food. In this paper, a liquid chromatography tandem quadrupole mass spectrometry (LC–MS/MS) multiresidue method for the simultaneous analysis of 23 pesticides and metabolites chosen according to the Commission Directives 2006/141/EC, 2006/125/EC, and 96 multiclass pesticides and metabolites chosen according to their physicochemical properties is presented and validated. The extraction procedure is based on three modifications of the quick, easy, cheap, effective, rugged, and safe method according to the analyte. The analytical performance was demonstrated by the analysis of extracts from cereal-based infant foods, spiked at two concentration levels for each pesticide or metabolite. Good sensitivity and selectivity of the method were obtained with limits of quantification at 10 or 3 μg/kg, depending on the analyte. All pesticides and metabolites, except six cases, gave recoveries in the range of 60.4–125.4%, with relative standard deviations less than 29.7%, for both validation levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The protection of food crops against pests and diseases by various pesticides is a common approach in conventional farming. Because of the potential health risk for consumers resulting from acute and/or chronic dietary exposure, maximum residue levels for many pesticide/commodity combinations have been established in the European Union (Stepan et al. 2005).
Infants and children, comparing to adults, are heavily exposed to pesticides and biologically are more vulnerable to them, due to high food consumption rate per kilogram of body weight. Commission Directive 2006/125/EC codifies and replaces the previous Directive (European Commission 2006a, b) which had been amended a number of times. It sets the rules on the composition and labeling of processed cereal-based foods for particular nutritional use for infants and young children in good health and is intended for use by infants when they are being weaned and as a supplement to the diet of young children. The mentioned directive also establishes the principle on the prohibition of the use of certain pesticides for the production of agricultural products, intended for processed cereal-based food and baby food. However, this prohibition does not necessarily guarantee that products are free from such pesticides, since some pesticides may contaminate the environment and their residues may be found in the products concerned. It also requires that processed cereal-based food and baby food must not contain residues of individual pesticides at levels exceeding 0.01 mg/kg, except for substances for which specific levels have been set in Annex VI and VII of the Directive, in which case-specific levels apply (0.003 mg/kg for disulfoton, terbufos, fensulfothion and their metabolites, fentin, haloxyfop and its esters, heptachlor, hexachlorobenzene, nitrofen, omethoate, aldrin, dieldrin, and endrin; 0.004 mg/kg for fipronil and fipronil-desulfinyl; 0.006 mg/kg for propineb/propylenethiourea, cadusafos, demeton-S-methyl, demeton-S-methyl sulfone, and oxydemeton-methyl; or 0.008 mg/kg for ethoprophos) (European Commission 2006a, b).
Since the 1st January 2008, Commission Directive 2006/141/EC replaced Directive 91/321/EEC and its successive amendments. This new Directive updated the requirements for the composition and labeling of infant formulae and follow-on formulae and also encompasses specific rules on the presence of pesticides residues in infant and follow-on formulae. It requires that baby food contains no detectable levels of pesticide residues, i.e., meaning less than 0.01 mg/kg. This Directive also prohibits the use of certain pesticides in the production of infant and follow-on formulae and establishes levels lower than the general maximum level of 0.01 mg/kg for a few pesticides.
Considering the low concentration levels needed for successful monitoring of pesticide residues in infant foods, sensitive and reliable confirmation and quantification methods are required (Hercegova et al. 2007). The progress in sample preparation of pesticide residue analysis in baby food follows the trends valid for methods for food analysis in general. Liquid extraction still represents the keystone extraction technique; alternative techniques such as supercritical fluid extraction (SFE), matrix solid-phase dispersion, solid-phase microextraction, and stir bar sorptive extraction, excellent in specific aspects, did not reach the widespread utilization in baby food analysis (Hercegova et al. 2007). A critical aspect of pesticide residues analysis is the cleanup of the crude extract which is required to reduce the matrix effect, and it is essential for the sufficient column sample capacity and the satisfactory long-term performance of the chromatographic system during the analysis of a range of samples (Hercegova et al. 2007). Among them, solid-phase extraction and gel permeation chromatography (GPC) are the most widely used cleanup techniques for baby food analysis. The differences in validation parameters, related to many sample preparation methods published in the last few years, are not significant; therefore, the trend is the employment of an easy and fast procedure (Hercegova et al. 2007).
Liquid chromatography–mass spectrometry (LC–MS) allows the rapid and efficient determination of many compounds that have been scarcely investigated in food until now, or determined with some difficulties by using laborious and time-consuming GC or conventional LC procedures (Hernandez et al. 2006). Selectivity and sensitivity are notably improved, the sample pre-treatment steps can be minimized, and reliable quantitation and confirmation can be easily achieved at the low concentration levels required (Hernandez et al. 2006). In the last years, some LC–MS/MS methods have been developed for the determination of more than 50 multiclass pesticides in baby foods. Ethyl acetate used as an extraction solvent combined with SFE (Hercegova et al. 2007) or GPC (Cajka and Hajslova 2004) has been shown to be applicable to pesticide residue analysis of baby food matrices and has been used to extract more than 98 multiclass pesticides. Acetonitrile as an extraction solvent has the disadvantage of being both more expensive and more toxic than ethyl acetate. However, because of its higher polarity, much less lipophilic material, such as oils and chlorophyll and to a lesser extent waxes, are co-extracted with the pesticides (Barcelo and Fernandez-Alba 2005). Quick, easy, cheap, effective, rugged, and safe (QuEChERS) method is an extraction procedure for pesticide multiresidue analysis that was developed between 2000 and 2002 and first reported in 2003 (Anastassiades et al. 2003). The QuEChERS procedure involves an initial extraction with acetonitrile followed by an extraction/partitioning step after the addition of a salt mixture. An aliquot of the raw extract is then cleaned up by dispersive solid-phase extraction. The final extract in acetonitrile is directly amenable to determinative analysis based on LC and/or GC (Patel et al. 2004). A lot of publications already deal with this method in its original form or variations of it for the determination of multiclass pesticides in several types of baby food matrices (fruit, potato and cereal-based baby foods, fruit and rice, fish and pasta, potato and pork-based baby foods, apple-based baby foods, romaine lettuce, and orange-based baby foods) (Wang et al. 2005).
The purpose of this paper is to present and validate two variations of the QuEChERS method for the determination of 120 pesticides and metabolites in cereal-based infant foods. Because of the different physicochemical properties of the compounds, several modifications either in the sample preparation step or in the determination step are required as to have better method performance. Therefore, a rapid multiresidue method by LC–MS/MS with electrospray interface (ESI), using the extraction method based on QuEChERS procedure for the determination of 97 multiclass pesticides and metabolites and 23 priority pesticides and metabolites included in the Commission Directives 2006/141/EC and 2006/125/EC, was validated. Also a similar method based on a QuEChERS extraction procedure for the determination of fentin and another one for the determination of haloxyfop, haloxyfop-ethoxyethylester, and haloxyfop-methoxyethylester were also validated.
Materials and Methods
Chemicals and Apparatus
In this work, 120 pesticides and metabolites were used (obtained from Dr Ehrenstorfer Laboratories GmbH Germany), 23 of which are included in the Commission directives 2006/141 and 2006/125 and are marked with an asterisk: acephate, acetamiprid, aldicarb, aldicarb sulfone, aldicarb sulfoxide, ametryn, atrazine, azimsulfuron, azinphos methyl, azoxystrobin, benalaxyl, bensulfuron methyl, boscalid, bromuconazole, buprofezin, cadusafos*, carbaryl, carbofuran, carbofuran 3-hydroxy, carbosulfan, chlorotoluron, clofentezine, cyanazine, cymoxanil, demeton-S-methyl*, demeton-S-methyl sulfone*, demeton-S-methyl sulfoxide*, diazinon, dichlorvos, diethofencarb, difenoconazole, dimethoate, dimethomorph, disulfoton*, disulfoton-sulfone*, disulfoton-sulfoxide*, epoxiconazole, ethofumesate, ethoprophos*, etoxazole, famoxadone, fenamidone, fenbuconazole, fenhexamid, fenoxycarb, fenpropimorph, fenpyroximate, fensulfothion*, fensulfothion sulfone*, fensulfothion-oxon*, fensulfothion-oxon-sulfone*, fenthion, fentin*, fipronil*, fipronil-desulfinyl*, flutriafol, fosthiazate, furathiocarb, haloxyfop*, haloxyfop-ethoxyethylester*, haloxyfop-methoxyethylester*, hexaconazole, hexythiazox, imidacloprid, indoxacarb, iprovalicarb, kresoxim methyl, linuron, metalaxyl M, metconazole, methamidophos, methiocarb, methiocarb sulfone, methiocarb sulfoxide, methomyl, methoxyfenozide, metoxuron, monocrotophos, monolinuron, myclobutanil, nicosulfuron, omethoate*, oxamyl, phosalone, phosmet, pirimicarb, pirimiphos methyl, primisulfuron methyl, procloraz, profenofos, propamocarb, propargite, pymetrozine, pyraclostrobin, pyridaben, pyrifenox, pyrimethanil, pyriproxyfen, quinoxyfen, simazine, spinosad (A), spiroxamine, tebuconazole, tebufenozid, tebufenpyrad, terbufos*, terbufos-sulfone*, terbufos-sulfoxide*, terbuthylazine, tetraconazole, thiacloprid, thiamethoxam, thiodicarb, thiophanate methyl, tolylfluanid, triadimefon, triadimenol, triazophos, trifloxystrobin, and vamidothion.
LC–MS grade acetonitrile, methanol, and water were used. All solvents were obtained from Lab Scan (Ireland). Ammonium formate, magnesium sulfate anhydrous, and disodium hydrogencitrate sesquihydrate were obtained from Fluka (Buchs, Switzerland). Sodium chloride (ACS reagent grade ≥99.0%) and trisodium citrate dihydrate were obtained by Sigma-Aldrich (Madrid, Spain). Primary secondary amine (PSA; Bondesil-PSA 40 μm) were obtained by Varian Inc. Sulfuric acid and sodium hydroxide solutions concentrate 1.0 N TITRISOL Volumetric Solutions were obtained by Merck & Co. Inc.
Preparation of Standard Solutions
Stock standard solutions at 500 mg/L were prepared in methanol for fentin, haloxyfop, haloxyfop-ethoxyethylester, and haloxyfop-methoxyethylester and at 1,000 mg/L in acetone for the other 116 analytes. The stock standard solutions were stored at −20 °C. A single composite standard was prepared by combining aliquots of each stock solution and diluting in methanol to obtain a final concentration of 1 μg/mL. Matrix-matched calibration standard solutions for measurements were prepared in the extract of cereal-based infant food, previously analyzed for the absence of peaks interfering with the peaks of the analytes. The product used as blank composed of 19.7% of milk powder, 17.5% of wheat flour, sucrose, corn stark, whey in powdered form, prebiotic fibers, lactose, galactose, vanillin, glucose, and vitamins.
Preparation of Calibration Standards
The sample extraction procedure was followed for the preparation of matrix-matched standard solutions. At the final step, the blank extract was diluted in 3 mL of methanol. An aliquot of 2 mL is evaporated to dryness by a stream of N2, and 1 mL of a standard solution, of the desired concentration, prepared in methanol/water (30:70, v/v) was added. Before the injection in the chromatographic system, the final solution was filtered through a disposable PTFE syringe filters, 0.45 μm.
Sample Preparation
Extraction of 116 Multiclass Pesticides and Metabolites
For the extraction 116 of the analytes (excluding fentin, haloxyfop, and its esters), the protocol of QuEChERS method concerning commodities with high fat content was followed (Anastassiades et al. 2007). For products with a water content <25% (e.g., wheat flour), water had to be added. The water should be at low temperature (<4 °C) to compensate the heat development caused by the addition of the salts (QuEChERS): According to this method, a 5-g portion of cereal-based infant food was weighted in a 50-mL PTFE centrifuge tube, and 10 mL of water (<4 °C) was added. A short vibration using a Vortex mixer (K-550-GE, Scientific Industries Inc., Bohemia, NY, USA) helped to disperse solvent and pesticides well through the sample. For the extraction of the pesticides, 10 mL of acetonitrile was added and the tube was vigorously shaken for 1 min. A mixture of 1 g of NaCl, 4 g of MgSO4, 1 g of trisodium citrate dehydrate, and 0.5 g of disodium hydrogencitrate sesquihydrate was added, and the tube was vigorously shaken for 1 or more minutes to prevent coagulation of MgSO4. By adding the citrate buffering salts, most samples obtain pH values between 5 and 5.5. This pH range is a compromise, at which both the quantitative extraction of sour herbicides and the protection of alkali labile and acid labile compounds are sufficiently achieved (QuEChERS). The sample was then centrifuged at 4,000 rpm for 5 min. An aliquot of 8 mL of the supernatant acetonitrile phase was then taken and transferred to a 15-mL centrifuge tube and stored for at least 2 h in the freezer. Freezing out helps to partly remove some additional co-extractives with limited solubility in acetonitrile while the major part of fat and waxes solidify and precipitate. An aliquot of 6 mL of the still cold acetonitrile phase was transferred into a 15-mL centrifuge tube containing 150 mg of PSA and 900 mg of MgSO4, and the tube was shaken vigorously for 30 s and centrifuged for 5 min at 4,000 rpm. An aliquot of 5 mL of the cleaned up extract was transferred into a screw cup storage vial, taking care to avoid sorbent particles of being carried over. The extract was slightly acidified by adding 50 μL of a 5% formic acid solution in acetonitrile. An aliquot of 2 mL of the extract was evaporated near to dryness and reconstituted in 1 mL of methanol/water (30:70, v/v), added in the following order: 0.3 mL of methanol was added in the flask, the extract was placed in an ultrasonic bath for 30 s, and then 0.7 mL of water was added. The final extract was placed again in an ultrasonic bath for 30 s. Before the injection in the chromatographic system, the final extract was filtered through a 0.45-μm disposable PTFE syringe filters. Following this extraction procedure, the concentration C (milligrams per kilogram) of the analytes in the sample corresponds to C (micrograms per milliliter) of the analytes in the extract.
Extraction of Fentin
The difference for the extraction procedure of fentin was at the final step of the procedure. An aliquot of 2 mL of the extract was evaporated near to dryness and reconstituted to 1 mL methanol. The final extract was placed in an ultrasonic bath for 30 s. Before the injection in the chromatographic system, the final extract was filtered through a 0.45-μm disposable PTFE syringe filters. Following this extraction procedure, the concentration C (milligrams per kilogram) of the analytes in the sample corresponds to C (micrograms per milliliter) of the analytes in the extract.
Extraction of Haloxyfop and Its Ethoxyethyl and Methoxyethyl Esters
For the extraction of haloxyfop and its ethoxyethyl and methoxyethyl esters, a different variation of the QuEChERS method for acidic pesticides was adopted (CRL for Single Residue Methods 2007): 5 g of cereal-based infant food was weighted in a 50-mL PTFE centrifuge tube, and 10 mL of water (<4 °C) was added. A short vibration using a Vortex mixer helped to disperse solvent and pesticides well through the sample. Then 300 μL of 5 mol/m3 NaOH solution was added to adjust the pH to 12. The tube was shaken vigorously for 1 min, and the mixture was left to stand for 30 min, occasionally shaken every 10 min. Then 300 μL of 5 mol/m3 H2SO4 solution and 10 mL of acetonitrile were added, and the tube was vigorously shaken for 1 min. A mixture of 1 g of NaCl, 4 g of MgSO4, 1 g of trisodium citrate dehydrate, and 0.5 g of disodium hydrogencitrate sesquihydrate was added, and the tube was vigorously shaken for 1 or more minutes to prevent coagulation of MgSO4. The sample was then centrifuged (4,000 rpm) for 5 min. An aliquot of 7 mL of the supernatant acetonitrile phase was then taken and transferred to a 15-mL centrifuge tube and stored for at least 2 h in the freezer. An aliquot of 5 mL of the still cold acetonitrile phase was transferred into a screw cup storage vial, taking care to avoid sorbent particles of being carried over. An aliquot of 2 mL of the extract was evaporated near to dryness and reconstituted in 1 mL of methanol. Before the injection in the chromatographic system, the final extract was filtered through a 0.45-μm disposable PTFE syringe filters. Following this extraction procedure, the concentration C (milligrams per kilogram) of the analytes in the sample corresponds to C(micrograms per milliliter) of the analytes in the extract.
Preparation of Fortification Samples
The recovery and repeatability experiments were conducted in two levels, the limit of quantification (LOQ) and 10× LOQ, with five replicates at each level. Working standard mixture solution was prepared in methanol at 100× LOQ; 5 g of cereal-based infant food, previously analyzed for the absence of pesticides, was weighted and spiked with 50 μL for the LOQ and 500 μL for the 10× LOQ of the working standard mixture solution.
Determination with Liquid Chromatography Tandem Mass Spectrometry
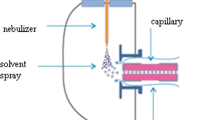
The LC system used consisted of two Varian Prostar 210 pumps. Detection was achieved using a triple quadrupole mass spectrometer (Varian model 1200 L) equipped with an electrospray ionization interface operating in the positive or negative mode. Typical source parameters were as follows: Cone voltage and collision energy varied depending on the precursor ion as shown in Table 1, and source temperature was set at 250 °C and drying gas temperature at 250 °C. Drying gas and nebulizing gas was nitrogen generated from a high purity generator, and their pressures were set at 18 and 55 psi, respectively. For the operation in MS/MS mode, Argon 99.999% was used as collision gas with a pressure of 0.2 Pa. The multiple reaction monitoring experiments were conducted with a dwell time of 100–250 ms, depending at the analyte. For instrument control, data acquisition, and processing, Varian MS Workstation software version 6.8 was used.
Determination of 119 Multiclass Pesticides and Metabolites Included Haloxyfop and Its Ethoxyethyl and Methoxyethyl Esters
Chromatographic separation was achieved using a Polaris C18 5-μm particle size, 50 × 2-mm analytical column from Varian, at a flow rate of 250 μL/min with a mobile phase consisting of water/methanol (90:10, v/v)–1 mmol/L ammonium formate (solvent A) and methanol/water (90:10, v/v)–1 mmol/L ammonium formate (solvent B). A gradient program was used consisting of 90% of solvent A and 10% of solvent B, ramped linearly over the course of 14 min to 100% of solvent B. This composition was held for a further 6 min before returning to the initial condition. The column was re-equilibrated for 10 min at the initial mobile phase composition. The total run time was 30 min. The injection volume was 20 μL. In order to avoid carry-over, the autosampler was purged with a mixture of methanol/water (50:50, v/v) before sample injection.
Determination of Fentin
Chromatographic separation was achieved using a Zorbax Eclipse XDB-C18, 50 × 2.1 mm, 5 μm analytical column at a flow rate of 250 μL/min with a mobile phases consisting of water 5 mmol ammonium formate with 1% formic acid and methanol 5 mmol ammonium formate with 1% formic acid (Michelangelo Anastassiades, personal communication, 2009). The gradient program and the injection volume were the same with the one described in the previous section.
Confirmation
For screening purposes, a first injection was performed by using a method with only one transition of each compound. The chosen transition for screening was that of the quantifier because it is more sensitive than that of the qualifier and minimizes the possibility of false negative results.
Individual MS/MS methods including two transitions for each analyte were developed and used for confirmation purposes. Then, an independent confirmation injection is performed for every positive sample. Confirmation includes comparison of retention times and acceptable tolerances of the ion ratios of qualifier and quantifier ions of the analyte in the matrix-matched calibration standard and the sample extract. The retention time of the analyte in the sample extract must match that of the matrix-matched calibration standard with a tolerance of ±2.5%, and the acceptable tolerances of the ion ratios must match those mentioned in Document SANCO/10684/2009 (European Commission 2009).
Results and Discussion
Selection of Final Solvent
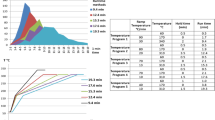
The evaporation of acetonitrile and the reconstruction in methanol/water (30:70, v/v) is a one extra step in the method, but the use of methanol/water (30:70, v/v) as the final solvent proved to have its advantages, mostly for the more polar compounds that elute early. In order to determine the differences between acetonitrile and methanol/water (30:70, v/v), a standard solution of the 116 compounds at 10 μg/mL was prepared in acetonitrile and one in methanol/water (30:70, v/v). Five injections of each standard solution were performed at the same day as to have statistical results. The time distribution of the compounds is shown in Fig. 1. The 57.3% of the analytes elute between the range of 0 and 25 min, and the largest number of analytes is distributed between two 5-min time periods from 20 to 25 and 25 to 30, at the middle of the chromatographic program. As shown in Fig. 2, the standard solution in methanol/water (30:70, v/v) gave higher signal-to-noise (S/N) ratio for the compounds that elute at the early. This percentage was at 100% for the compounds that elute before the first 10 min, 90.9% for those that elute at the next 10 min, and 56.7% for those that elute between 20 and 25 min. The percentages are altered in favor of the acetonitrile for the compounds that elute after the 25 min. Also a main advantage of the methanol/water (30:70, v/v) as final solvent is that it mixes better with the mobile phase because they are prepared from the same solvents, methanol, and water, and the ratio between the mobile phases at the starting point of the chromatographic program (80% water, 20% methanol) and the final solvent of methanol/water (30:70, v/v) are almost the same; therefore, we achieve better chromatographic peaks, especially for the more polar compounds that elute early, as shown in Fig. 3.
Comparison of signal-to-noise ratios of the 116 analytes at 10 μg/mL in acetonitrile and methanol/water (3:7, v/v) for each time cluster. The red column represents the percentage of the analytes with higher signal-to-noise ratio with methanol/water (3:7, v/v) as final solvent and the blue one with the acetonitrile
MS Optimization
The ionization of the pesticides and metabolites in positive and negative electrospray ion source was studied. Table 1 shows the precursor ions used for data acquisition, the transition used for quantification and confirmation (Federal Institutor of Risk Assessment 2009), the cone voltage and collision energy for each transition, the retention times of the analytes, and the time segment in which the transitions are monitored. Pesticides are ionized in the forms of [M + H] + or [M + NH4] + ions. Tandem mass spectrometry provides a powerful confirmatory tool for pesticide residue analysis because it discriminates efficiently between the analyte and the matrix signal. Individual standard solutions at 100 μg/mL were prepared in methanol/water (30:70, v/v) for optimization of the system by multiple injections at different cone voltage and collision energy.
The source optimization of each pesticide was tuned by introducing each analyte into the mass spectrometer through direct infusion via a syringe pump at a flow rate of 250 μL/min. MS–MS spectra were acquired to obtain information about the maximum number of transitions available for each compound. Typically, the quantification transition was selected to achieve maximum sensitivity. The optimum cone voltage varies between 6 and 85 kV depending on the analyte. Product ion mass spectra for the pesticides were obtained in the positive and negative mode of electrospray ionization using collision-induced dissociation. Variation of the collision energy influences both sensitivity and fragmentation. The collision energy was optimized for two selective product ions of each precursor ion. The optimized values acquired are listed in Table 1.
The time-scheduled data acquisition sequence involved 18 overlapping segments of 1 min each. In each segment, 2 to 35 transitions are monitored. By this technique, we create an artificial window maximum ± 2 min from the retention time of each compound. Therefore, the instrument consumes the ideal amount of time as to have a successful acquisition with less time shifts that can easily cause loss of a peak and sufficient dwell and scan time, without stacking a lot of transitions in one time segment. In our study, 116 transitions are acquired in each run, but considering the low detection levels and the different sensitivities of the analytes, this technique was proved to be very useful. This technique was also proved useful in the development of multiresidue methods with a large number of transitions in one run. With dwell times of 0.1 s, the average scan cycle time for the segments varies between 0.2 and 1 s.
Validation
The validation study was performed according to the European SANCO guidelines 10684/2009 (European Commission 2009). Analytical characteristics evaluated were sensitivity, mean recovery (as a measure of trueness), and repeatability (as a measure of precision).
Linearity
Calibration curves were constructed from injections of matrix-matched calibration standards in blank cereal-based infant food extract in methanol/water (30:70, v/v) for the 116 analytes and in blank cereal-based infant food extract in methanol for fentin, haloxyfop, and its esters. The linearity was estimated at eight concentrations levels 1.5–3.0–5.0–7.5–10–15–20 and 30 μg/mL for the 23 analytes of Appendix IV of the Commission Directives 2006/141/EC and 2006/125/EC and 10–15–23–34–50–76–110 μg/kg for the rest 97 analytes. These calibration curves are used to obtain the predicted concentration C (milligrams per kilogram) of the analyte from a sample which produces an observed response y by the equation:
In Table 2, the basic calibration line parameters for the analytes are presented, including the uncertainty S u on the estimated concentration C. According to EURACHEM/CITAC Guide (2000), there are four sources of uncertainty to consider in arriving at an uncertainty on the estimated concentration C. The most significant of them for normal practice are due to variability in the peak area y. The uncertainty S u of C due to variability in y can be estimated from the calibration data, by the following equation:
where \( {S_{{y/C}}} = \sqrt {{\frac{{\sum\limits_i {{{\left( {{y_i} - \overline y } \right)}^2}} }}{{n - 2}},\left( {{y_i} - \overline y } \right)}} \) is the residual for the ith point and b the slope of the regression line and n is the number of the data points in the calibration.
Good linearity was achieved in all cases with correlation coefficients better than 0.990 in most cases, 0.98 in the cases of diethofencarb, difenoconazole, ethoprophos, fenhexamid, fentin, haloxyfop-ethoxyethylester, haloxyfop-methoxyethylester, methiocarb sulfone, profenofos, quinoxyfen, terbufos, tetraconazole, and 0.944–0.955 in the cases of hexaconazole, tebuconazole, tolylfluanid.
Trueness, Precision
Recoveries and repeatability of the method were established in order to evaluate the methods’ trueness and precision, respectively. Mean recoveries of 70–120% with relative standard deviations (SDr) ≤ 20% are acceptable, while in certain cases, typically with multiresidue methods, recoveries outside this range may be acceptable. In routine analysis, the acceptable recoveries are in the range of the mean recovery ±2 SDr. (European Commission 2009). The mean recoveries were determined from spiked cereal-based infant food samples, at two concentration levels. The lowest fortification level was 3 μg/kg for the analytes of Appendix IV of the Commission Directives 2006/141/EC and 2006/125/EC and 10 μg/kg for the rest of the analytes. The second fortification level was ten times higher (30 or 100 μg/kg). The recoveries were calculated using matrix-matched, calibration standards.
As shown in Table 3, the recoveries at the lowest level for 114 of the 120 analytes ranged from 60.5% to 125.4% (91 of the 114 compounds gave recoveries within 70–120%) with SDr less than 29.7% and 56.2–123.1% (108 of the 114 compounds gave recoveries within 70–120%) with SDr less than 26.1% for the highest. The majority of the analytes (91 analytes) gave recoveries and SDr values within the accepted values. In the cases of 23 analytes, as shown in Table 3, the recovery values at the lowest concentration level was below 70% (16 analytes) or over 120% (seven analytes), but consistent (low SDr values) and are considered acceptable (European Commission 2009). Therefore, the method still is able to serve as a semiquantitative method to detect and confirm their presence in samples.
The analytes acephate, aldicarb sulfoxide, metoxuron, phosmet, tetraconazole, tolyfluanid, and omethoate gave recovery and SDr values outside the acceptable ranges, so for these analytes, further investigation is required.
Limit of Quantification
The LOQ was established as the lowest concentration tested for which recovery and SDr values were satisfactory in accordance with the criteria established for analysis of pesticide residues in foods (European Commission 2009) and with S/N ratio higher than 10. Therefore, as LOQ, the lowest validated level with acceptable accuracy and precision results was selected. In Table 3, the S/N ratio at the LOQ is presented. As shown in Fig. 4, the analytes with concentration at the LOQ gave good peak shape with S/N >10.
Reconstructed ion chromatogram by LC–MS–MS for cereal-based infant food extract spiked at 3 μg/kg using the MRM transitions for omethoate, dimethoate, demeton-S-methyl sulfoxide, demeton-S-methyl sulfone, demeton-S-methyl, disulfoton-sulfoxide, disulfoton-sulfone, disulfoton, fensulfothion, fensulfothion sulfone, fensulfothion-oxon, fensulfothion-oxon-sulfone, terbufos, terbufos-sulfone, terbufos-sulfoxide, cadusafos, fipronil, and fipronil-desulfinyl
Analysis of Real Samples
The proposed methodology was applied for the analysis of real baby food samples. Sixteen cereal-based infant food samples were analyzed (rice cereal, farine lactée, biscuit purée, cereal purée, and baby food desserts). In one sample, traces of the pesticide pirimicarb were found at concentrations below the LOQ of the method (2 μg/kg). The quantification of pirimicarb was conducted by single-level calibration. Single-level calibration may provide more accurate results than multi-level calibration if the detector response is variable with time. For the comparison, the sample response should be within ±50% of the calibration standard response. Matrix-matched analytical standards in blank extract from cereal-based baby food, previously analyzed for the absence of peaks interfering with the peaks of the analytes, were used. Figure 5 shows the chromatogram of the baby food sample that contained pirimicarb at a concentration of 2 μg/kg.
Conclusions
In conclusion, the QuEChERS method for the extraction procedure combined with the LC–ESI–MS–MS was found to be a sensitive method for the determination of 120 pesticides and metabolites according to the Commission Directives 2006/141/EC and 2006/125/EC in cereal-based infant food. Slightly modifications either on the extraction procedure or the LC conditions made possible the confirmation and quantification of pesticides that require special conditions like fentin, haloxyfop, and its esters. Although for some pesticides and metabolites the recovery values were low, still confirmation is feasible and quantification of the analyte can be achieved. The method is simple, fast, and suitable for routine analysis for the determination of pesticides in cereal-based infant food and other products with high protein or high starch content meeting the EU guidelines method performance criteria.
References
Anastassiades M (2009) QuEChERS—a mini-multiresidue method for the analysis of pesticide residues in low-fat products. Available at http://www.quechers.com. Accessed 22 Mar 2009
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) J Assoc Off Anal Chem Int 86:412–431
Anastassiades M, Tasdelen B, Scherbaum E, Stajnbaher D (2007) Recent developments in QuEChERS methodology for pesticide multiresidue analysis. In: Ohkawa H, Miyagawa H, Lee P (eds) Pesticide chemistry: crop protection, public health, environmental safety. WILEY-VCH, Weinheim, pp 439–458. ISBN 978-3-527-31663-2
Barcelo D, Fernandez-Alba A (2005) Comprehensive analytical chemistry: chromatographic–mass spectrometric food analysis for trace determination of pesticide residues, volume XLIII. Wilson & Wilson’s, Amsterdam
Cajka T, Hajslova J (2004) J Chromatogr A 1058:251–261
CRL for Single Residue Methods (2007) Analysis of acidic pesticides in wheat flour samples by LC–MS(/MS) using the QuEChERS method (including optional alkaline hydrolysis to release covalently bound compounds). Available at: http://www.crl-pesticides.eu/library/docs/cf/acidicpesticides_wheat_quechers.pdf. Accessed 2 Jun 2009
EURACHEM/CITAC Guide (2000) Quantifying uncertainty in analytical measurement, 2nd edn. EURACHEM, Lisbon, pp 106–107
European Commission (2006a) Council Directive 2006/125/EC of 5 December 2006 on processed cereal-based foods and baby foods for infants and young children. Off J Eur Union L 339:16–35, 6 Dec 2006
European Commission (2006b) Council Directive 2006/141/EC of 22 December 2006 on infant formulae and follow-on formulae and amending Directive 1999/21/EC. Off J Eur Union L 401:1–33, 30 Dec 2006
European Commission (2009) Document No. SANCO/10684/2009, method validation and quality control procedures for pesticide residues analysis in food and feed. Available at: http://ec.europa.eu/food/plant/protection/resources/qualcontrol_en.pdf. Accessed 11 Jul 2010
Federal Institutor of Risk Assessment (2009) List of pesticides for which methodological data is available at present. Available at: www.bfr.bund.de/cm/289/list_of_pesticides_for_which_methodological_data_ist_available_at_present.pdf. Accessed 26 Jan 2009
Hercegova A, Domotorova M, Matisova E (2007) J Chromatogr A 1153:54–73
Hernandez F, Pozo OJ, Sancho JV, Bijlsma L, Barreda M, Pitarch E (2006) J Chromatogr A 1109:242–252
Patel K, Fussell RJ, Goodall DM, Keely BJ (2004) Food Addit Contam: Part A Chem Anal Control Expo Risk Assess 21:658
Stepan R, Ticha NJ, Hajslova J, Kovalczuk T, Kocourek V (2005) Food Addit Contam: Part A Chem Anal Control Expo Risk Assess 22:1231–1242
Wang J, Cheung W, Grant D (2005) J Agric Food Chem 53:528
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anagnostopoulos, C.J., Aplada Sarli, P., Liapis, K. et al. Validation of Two Variations of the QuEChERS Method for the Determination of Multiclass Pesticide Residues in Cereal-Based Infant Foods by LC–MS/MS. Food Anal. Methods 5, 664–683 (2012). https://doi.org/10.1007/s12161-011-9296-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-011-9296-z